Abstract
Pulmonary embolism (PE) represents a potentially life-threatening venous thromboembolic disorder, and prompt treatment is vital to prevent early mortality. However, diagnosis of PE is complicated by the range of signs and symptoms with which it presents. Clinical risk scores, imaging techniques, and laboratory tests are recommended in clinical guidelines to aid diagnosis, and risk stratification strategies can be used to inform treatment decisions. Long-term anticoagulation is key to avoid the risk of later complications of acute PE, such as recurrent venous thromboembolism and chronic thromboembolic pulmonary hypertension. Rivaroxaban is a direct oral anticoagulant that has been approved for the treatment of PE (and deep vein thrombosis) and prevention of recurrent venous thromboembolism; other direct oral anticoagulants have undergone phase III trials for these indications. These agents may provide advantages over traditional anticoagulants, such as vitamin K antagonists, because they are administered at fixed doses and do not require routine coagulation monitoring. These advantages may improve patient adherence and aid general practitioners by simplifying long-term management of PE in daily primary care.
Key message
Rivaroxaban provides a new option for the treatment of pulmonary embolism and the prevention of associated long-term complications.
Introduction
Pulmonary embolism (PE) ranges from asymptomatic, incidentally discovered subsegmental thrombi to massive, life-threatening infarction of pulmonary parenchyma. In recent years the incidence of diagnosed PE has increased. In Japan, the number of cases of acute PE has increased 2.3-fold in the past decade (Citation1). A Chinese study noted an almost 5-fold rise in cases of PE from 1996 to 2005, which was partially attributed to the implementation of a venous thromboembolism (VTE) awareness initiative and better use of diagnostic modalities (Citation2). In non-Asian countries, similar increases have been noted. An 81% increase in the incidence of PE was reported for the US between 1996 and 2003 (Citation3). The increase in PE diagnosis can at least be attributed partially to several non-invasive procedures for diagnosing PE that have become available in recent years (Citation4). Bearing in mind that the traditional method for diagnosing PE is invasive and associated with 0.5% mortality (Citation5), the availability of non-invasive procedures has increased the willingness on the part of physicians to test for PE.
Factors that promote a prothrombotic or hypercoagulable state may increase the risk of PE. Risk factors usually fit into one of three categories described by Virchow's triad: circulatory stasis, a hypercoagulable state, or endothelial injury (Citation5). These factors include immobilization, surgery and trauma, pregnancy, oral contraceptives and oestrogen replacement therapy, cancer, hereditary factors (with the factor V Leiden mutation and prothrombin G20210A mutation being the two most common hereditary risk factors), and acute medical illness. The presence of multiple risk factors has a cumulative impact on the risk of VTE (Citation6). Several predisposing risk factors for PE have also been identified, including increased age, obesity, and history of VTE (Citation7–9). Of particular note, evidence suggests that 50% of untreated proximal deep vein thrombosis (DVT) cases will extend to PE within 3 months (Citation10).
PE can be unprovoked and can be difficult to recognize, especially because the symptoms can be confused with a variety of other disorders. The key to effectively managing PE and preventing secondary complications is making a prompt diagnosis, assessing the risk of early mortality, and administering optimal treatment both initially and over the long term. Anticoagulation is the cornerstone of treatment and prevention of secondary complications, which include recurrent VTE and chronic thromboembolic pulmonary hypertension, which is associated with high mortality and severe morbidity. Without anticoagulant treatment, the risk of VTE recurring after an initial acute PE is high, and is 3.1 times more likely after an initial PE than after an episode of DVT (Citation11). Patients with PE who stop anticoagulant therapy have a cumulative recurrence rate over 5 years of almost 25% for VTE (DVT and PE) and approximately 10% for PE alone (Citation11). Rates of VTE recurrence are significantly higher in patients with unprovoked PE (4.5%/year) compared with patients with provoked PE (2.5%/year; i.e. those with transient risk factors such as surgery), and patients who discontinue treatment have a significantly higher risk of recurrence than patients who continue anticoagulant therapy (Citation12).
Traditional anticoagulation therapy for PE includes initial parenteral anticoagulation with unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux, overlapping with and then followed by a vitamin K antagonist (VKA; e.g. warfarin). This combination is effective but has a number of limitations, including the parenteral administration of UFH, LMWH, and fondaparinux (Citation13) and the regular monitoring, dose adjustments, and food and drug interactions associated with VKAs (Citation14). Non-adherence to therapy is a problem among patients treated with VKAs, resulting in sub-optimal anticoagulation; time in therapeutic range for the international normalized ratio (INR; 2.0–3.0) as low as only 40% has been reported (Citation15,Citation16).
The management and treatment of acute PE has advanced considerably over the last few years, and progress is expected to continue with the advent of better diagnostic techniques and new treatment options. This article focuses on the direct oral anticoagulants as new treatment options for the effective treatment of PE, with a focus on long-term treatment in primary care. Rivaroxaban has been approved in the US and the EU for treatment of acute DVT and PE and prevention of recurrent VTE (Citation17,Citation18). Phase III studies of apixaban, dabigatran, and edoxaban have been undertaken and have shown promising results (Citation19–24). The introduction of the direct oral anticoagulants in this indication is likely to simplify the management of PE and might result in earlier discharge, particularly of low-risk PE patients, thereby shifting more of the responsibility from secondary to primary care.
Guidelines for treating pulmonary embolism
The American College of Chest Physicians (ACCP) guidelines for treating PE divide the duration of anticoagulation therapy into three phases: the initial phase (0–7 days), the long-term phase (7 days–3 months), and the extended phase (≥ 3 months) (Citation25). For the initial treatment phase of acute PE, the ACCP recommends parenteral anticoagulation with LMWH, fondaparinux, or UFH in parallel with and then followed by a VKA at an INR of 2.0–3.0 (target, 2.5), with the parenteral agent discontinued once the INR target has been maintained for at least 24 hours (Citation25). Alternatively, monotherapy with the oral, direct factor Xa inhibitor rivaroxaban is also recommended by the ACCP (Citation25). VKAs, LMWHs, and rivaroxaban are recommended as long-term treatment options. The ACCP recommends treatment for at least 3 months in all patients with PE and extended treatment in patients with active cancer or in patients with unprovoked VTE who have a low to moderate risk of bleeding. Current ACCP guidelines suggest the use of VKA and LMWH therapy over rivaroxaban. Rivaroxaban provides an alternative choice for patients in whom injections or regular monitoring may be inconvenient (Citation25). Dabigatran, which has been assessed after an initial period of parenteral anticoagulant in phase III studies (Citation19–21), is also mentioned as a treatment option but is not yet approved for this indication. Data on other direct oral anticoagulants are emerging rapidly; therefore, the ACCP expects there to be changes to the recommendations.
Additional thrombolytic therapy may be considered in patients who are at risk of developing hypotension or have confirmed hypotension. If thrombolysis has failed or is contraindicated, or if shock may lead to death before thrombolysis can take effect, catheter-assisted thrombus removal or surgical embolectomy may be performed (Citation25).
The European Society of Cardiology (ESC) guidelines on managing PE were last issued in 2008 and so do not include recommendations on direct oral anticoagulants (Citation9). The guidelines are expected to be updated in 2014. Currently, the ESC divides treatment into initial therapy, undertaken to treat the initial PE, and long-term therapy, aimed at preventing the recurrence of VTE. Although this differs from the ACCP categorization, the suggested management strategies are similar.
Risk stratification
Up to 11% of PEs result in death within 1 hour of onset; therefore, rapid treatment is essential (Citation26). Although clinical severity is usually associated with embolus size and the degree of vessel occlusion, the anatomical burden of the PE does not accurately predict the risk of death (Citation27). Therefore, all patients with PE require rapid risk stratification according to the expected PE-related risk of early death to provide guidance to the physician on the most appropriate intervention strategy. The ESC guidelines initially classify PE based on the risk of early death (in-hospital or 30-day mortality), as either high risk or non-high risk; the latter includes intermediate- and low-risk groups () (Citation9). In the US, high-risk PE is classified as massive (presence of sustained hypotension, a weak pulse, or a heart rate of < 40 beats per minute with signs of shock) or submassive (right ventricular dysfunction or myocardial necrosis) PE (Citation28). The US definition of low-risk PE is similar to the EU definition; namely, acute PE without signs of right ventricular dysfunction or myocardial necrosis (Citation28).
Figure 1. Risk stratification of PE and appropriate management, based on European Society of Cardiology 2008 guidelines (Citation9). RV = right ventricular.
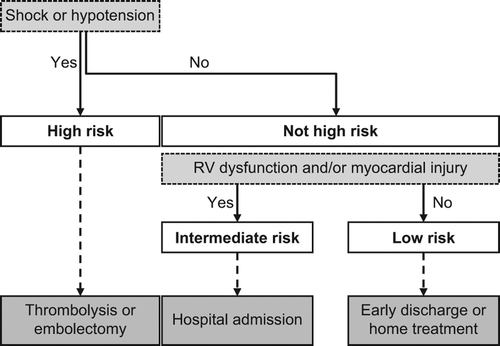
The widely validated, prognostic Pulmonary Embolism Severity Index (PESI) scoring system predicts the 30-day mortality risk. It is based on the presence of 11 variables (), to which points are assigned (Citation29–31). An overall score is determined by adding the patient's age in years to the sum of the points derived from the variables. The original PESI was complex to calculate. However, using a simplified PESI () score, one or more of the following variables equates to a high risk of PE: age > 80 years, male sex, history of cancer or chronic lung disease, low blood pressure, or arterial oxyhaemoglobin saturation < 90% (Citation31).
Table I. Variables used to calculate the Pulmonary Embolism Severity Index (PESI) score (original and simplified PESI) for the assessment of pulmonary embolism probability (X indicates the use of the variable in the respective scoring index) (Citation29–31).
Potential new options for treating pulmonary embolism
Anticoagulants such as VKAs and LMWHs have historically formed the mainstay of VTE treatment and prevention. In contrast, the direct oral anticoagulants—apixaban, dabigatran, edoxaban, and rivaroxaban—target specific factors in the coagulation cascade. Rivaroxaban is approved in the EU and the US for treating acute PE and DVT and preventing recurrent VTE. All of these direct oral agents are approved for other indications. Apixaban, dabigatran, and rivaroxaban are approved for the prevention of stroke in patients with non-valvular atrial fibrillation. These three agents are also approved in the EU for the prevention of VTE after elective hip or knee replacement surgery, rivaroxaban is approved in the US for this indication, and edoxaban is approved for this indication only in Japan.
Pharmacokinetics and pharmacodynamics of direct oral anticoagulants
Dabigatran binds to the active site of factor IIa (thrombin) (Citation32,Citation33), whereas apixaban, edoxaban, and rivaroxaban bind directly to factor Xa () (Citation34–36). The direct oral anticoagulants have predictable pharmacokinetic profiles and a wide therapeutic window and, as such, do not require the regular monitoring associated with VKAs (Citation37,Citation38). Unlike the slower-acting VKAs, direct oral anticoagulants reach peak plasma concentrations rapidly, usually within 4 hours, and their half-life ranges between 5 and 17 hours (Citation39–42).
Table II. Pharmacokinetic and pharmacodynamic properties of the direct oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban compared with warfarin.
Metabolism of direct oral anticoagulants
Known drug–drug interactions are rare for the direct oral anticoagulants. However, knowledge of their metabolism and routes of elimination is key to understanding contraindications, where caution is required, or dose adjustments associated with certain concomitant medications. Apixaban is eliminated via multiple pathways, with 27% of total clearance occurring via the kidneys (Citation43). The oral prodrug, dabigatran etexilate, is converted in the liver to its active form, dabigatran, with the majority of the unchanged drug (85%) eliminated through the kidneys (Citation44). About 65% of edoxaban is eliminated by the liver, with the remainder excreted via the kidneys (Citation42). Rivaroxaban has a dual mode of elimination: two-thirds of the drug is metabolized and eliminated via the hepatobiliary and renal routes equally, and the remaining one-third is excreted as unchanged drug in the urine (Citation45).
Drug–drug interactions of direct oral anticoagulants
Apixaban, edoxaban, and rivaroxaban are substrates for the cell efflux transporter P-glycoprotein (P-gp) and also for isoforms of cytochrome P450 (CYP). Rivaroxaban and apixaban are not recommended in patients receiving concomitant systemic treatment with strong inhibitors of both CYP3A4 and P-gp, such as azole-antimycotics (e.g. ketoconazole, itraconazole, voriconazole, and posaconazole) or HIV protease inhibitors (e.g. ritonavir) (Citation17,Citation46). Strong inducers of both CYP3A4 and P-gp (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital, or St John's wort) should be co-administered with caution in patients receiving apixaban or rivaroxaban (Citation17,Citation46). Dabigatran is dependent on P-gp transporters for its uptake. Therefore, co-medications that induce P-gp (e.g. rifampicin) are expected to reduce dabigatran plasma concentrations. Concomitant medication with any P-gp inhibitors or inducers may require caution or dose adjustments, and ketoconazole and itraconazole are contraindicated with dabigatran therapy (Citation44). Moreover, rivaroxaban, apixaban, and dabigatran should be used with caution in patients who are treated concomitantly with medicinal products affecting haemostasis, such as non-steroidal anti-inflammatory drugs, acetylsalicylic acid (ASA; aspirin), and platelet aggregation inhibitors because these agents typically increase the risk of bleeding (Citation17,Citation44,Citation46).
Coagulation assays
Although the direct oral anticoagulants do not require routine coagulation monitoring, there may be situations in clinical practice when it would be helpful to measure the patient's coagulation status. Such situations include patients presenting with bleeding or thromboembolic events, suspected overdose, preceding emergency surgery, or in patients with deteriorating renal function (Citation47,Citation48). It is important to note that the conventional INR monitoring tests were specifically developed for VKAs and are not suitable for measuring plasma concentrations of the direct oral anticoagulants. All direct oral anticoagulants influence routine coagulation tests (such as prothrombin time and activated partial thromboplastin time), but measurements do not reflect the circulating levels of these drugs and, therefore, are not suitable for quantitative assessment of exposure (Citation46,Citation49–51). If quantitative measurement of rivaroxaban or apixaban is required, standardized anti-factor Xa chromogenic assays or the Rotachrom® anti-FXa assay, respectively, are now commercially available (Citation17,Citation46,Citation52). Coagulation tests to measure dabigatran exposure include measurement of diluted thrombin time (e.g. HEMOCLOT® thrombin inhibitors assay), ecarin clotting time (e.g. HaemoSys-ECA Ecarin Chromogenic Assay®), and activated partial thromboplastin time (Citation44,Citation53,Citation54). However, these tests are not standardized, and, therefore, results should be interpreted with caution (Citation44). It is important to note that any assessment of plasma concentrations must be interpreted in relation to the timing of drug administration, and routine measurement of the drug effect or plasma concentrations is not recommended with any of the direct oral anticoagulants. Moreover, interpretation of results must also consider interindividual variability in the response to a defined dose of drug. Although routine coagulation monitoring is not required for direct oral anticoagulants, in observational studies with rivaroxaban and dabigatran in patients undergoing hip or knee replacement surgery, routine coagulation tests poorly reflected the circulating concentrations of these agents and exhibited high interindividual variability (Citation51). Currently there are no guidelines on how treatment should be adjusted based on the results from coagulation measurement assays, but this may be a subject for future study.
Reversal of direct oral anticoagulants
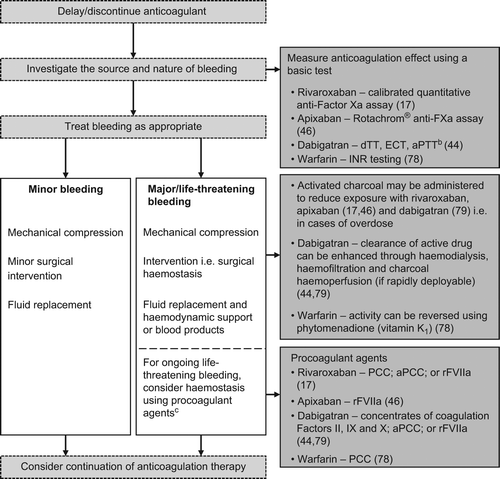
Currently, no clinically approved reversal agents exist for any of the direct oral anticoagulants. However, owing to the relatively short half-life of these agents (Citation17,Citation44,Citation46), reversal may be less of a requirement. Therefore, should bleeding occur with the direct oral anticoagulants, drug discontinuation is recommended, and routine protocols for the management of bleeding should be applied () (Citation17,Citation44,Citation46). Dabigatran can also be dialysed if bleeding occurs, but there is limited clinical experience to demonstrate the utility of this approach (Citation44). In cases of life-threatening bleeding, administration of haemostatic procoagulant agents—prothrombin complex concentrate, activated prothrombin complex concentrate (e.g. factor VIII inhibitor bypassing activity; FEIBA), or recombinant activated factor VII—may be considered. However, data on the usefulness of these agents in the clinical setting and risk of thromboembolism are very limited to date and have shown heterogeneic results (Citation17,Citation44,Citation46,Citation55).
Figure 2. Management of bleeding in patients receiving oral anticoagulants (Citation17,Citation44,Citation46,Citation78,Citation79).
aThere is currently no Summary of Product Characteristics for edoxaban; btests are not standardized, and results should be interpreted with caution; csome reversal agents may interfere with results from anticoagulation tests. aPCC, activated prothrombin complex concentrate; aPTT = activated partial thromboplastin time; dTT = diluted thrombin time; ECT = ecarin clotting time; INR = international normalized ratio; PCC = prothrombin complex concentrate; rFVIIa = recombinant activated factor VII.
Direct oral anticoagulants and hepatic and renal impairment
Apixaban, dabigatran, edoxaban, and rivaroxaban are partly metabolized by the liver, and moderate/severe hepatic impairment is known to affect coagulation (Citation56). The incidence of major bleeding has been reported to be higher in patients with worsening liver function receiving long-term anticoagulation (Citation57); therefore, patients with hepatic impairment should be carefully assessed for safety when administering direct oral anticoagulants. In cases of hepatic disease associated with coagulopathy and clinically relevant bleeding risk, apixaban and rivaroxaban are contraindicated (Citation17,Citation46), and in patients with elevated liver enzymes greater than twice the upper limit of normal, dabigatran is not recommended (Citation44). Patients with renal impairment and the elderly also should be assessed before administering dabigatran, because of the extent of renal clearance and the resulting increase in drug plasma concentrations (Citation44). The direct oral anticoagulants have not been systematically tested in patients with creatinine clearance (CrCl) < 30 mL/min and are not recommended for those with CrCl < 15 mL/min. Dabigatran is contraindicated for patients with CrCl < 30 mL/min. Rivaroxaban does not require dose adjustment in PE patients with renal impairment and should be given in accordance with the standard dosing regimen (15 mg twice daily for 3 weeks, followed by 20 mg once daily for long-term treatment) (Citation17). However, a reduction from rivaroxaban 20 mg once daily to 15 mg once daily should be considered if the patient's assessed risk of bleeding outweighs the risk of recurrent VTE (Citation17).
Clinical trial outcomes of the direct oral anticoagulants
Several phase III trials have investigated apixaban, dabigatran, edoxaban, and rivaroxaban for the treatment of acute DVT and/or PE and the secondary prevention of VTE. Most of these studies have assessed the safety and efficacy of these anticoagulants in mixed populations of patients with DVT and/or PE, including studies with apixaban, dabigatran, and edoxaban (Citation19–24). Therefore, subsequent sub-analyses may not be sufficiently powered to assess the effectiveness of the anticoagulant in patients with PE only. The exception to this is the rivaroxaban EINSTEIN PE study, which included only patients with PE (with or without DVT) (Citation58). Most of the trials were double-blind, with the exception of EINSTEIN DVT and EINSTEIN PE, which were open-label () (Citation58,Citation59). Rivaroxaban and edoxaban were administered once daily in their respective trials (Citation24,Citation58,Citation59), whereas apixaban and dabigatran were administered twice daily (Citation19–23). Another key difference in these trials was that in the dabigatran RE-COVER trials and the edoxaban study (Citation19,Citation21,Citation24), patients in both arms had already received LMWH therapy, whereas rivaroxaban and apixaban have both been evaluated as single-drug approaches from the initiation of treatment (Citation22,Citation58,Citation59). Exclusion criteria for the enrolment in these trials are listed in and were largely similar between trials (Citation19–24,Citation58,Citation59).
Table III. Design of the clinical trial studies (Citation19–24,Citation58,Citation59).
Table IV. Inclusion and exclusion criteria of the clinical trial studies (Citation19–24,Citation58,Citation59).a
Trials evaluating the treatment of acute pulmonary embolism
EINSTEIN PE was designed to assess outcomes in patients with PE with or without DVT. Rivaroxaban was found to be non-inferior compared with enoxaparin/VKA for the treatment of PE and the prevention of recurrent VTE (2.1% versus 1.8%, respectively; P = 0.003) () (Citation58). The risk of major bleeding was reduced by 51% with rivaroxaban (1.1% rivaroxaban versus 2.2% enoxaparin/VKA; hazard ratio (HR) = 0.49; 95% confidence interval (CI) 0.31–0.79; P = 0.003) (). Based on the results of EINSTEIN PE, EINSTEIN DVT, and EINSTEIN EXT, rivaroxaban was approved for the treatment of DVT and PE and the prevention of VTE recurrence.
Table V. Results from the acute VTE studies (Citation19,Citation21,Citation22,Citation24,Citation58,Citation59).
Other direct oral anticoagulants have been evaluated for the treatment of VTE, enrolling mixed DVT and PE populations () (Citation19,Citation21,Citation22,Citation24). The efficacy and safety outcomes of these studies are listed in . Studies with apixaban (Citation22) and edoxaban (Citation24) have published efficacy and safety outcomes for the subgroup of PE patients. Efficacy outcomes for this subgroup have been published in a pooled analysis of the dabigatran studies (Citation21). In PE patients, monotherapy with apixaban was non-inferior to enoxaparin/warfarin for the primary efficacy outcome, defined as the composite of recurrent symptomatic VTE and VTE-related death (2.3% versus 2.6%; HR = 0.9; 95% CI 0.5–1.61) (Citation22). The rate of major bleeding (principal safety outcome) was significantly lower in the apixaban group compared with the enoxaparin/warfarin group (0.4% versus 2.8%; no HR or 95% CI given). In the dual-drug Hokusai-VTE study, heparin/edoxaban was non-inferior to heparin/warfarin for the prevention of recurrent symptomatic VTE (primary efficacy outcome) in patients with PE (2.8% versus 3.9%; HR = 0.73; 95% CI 0.50–1.06) (Citation24). The principal safety outcome, defined as major plus clinically relevant non-major bleeding, occurred at similar rates in both treatment arms (10.1% versus 11.2%; no HR or 95% CI given) (Citation24). In a pooled analysis of the RE-COVER and RE-COVER II trials, the rates of recurrent symptomatic VTE and VTE-related death (primary efficacy outcome) in patients with symptomatic PE were similar in both treatment groups (dabigatran group 2.3% versus warfarin group 2.6%; no HR or 95% CI given) (Citation21). Safety outcomes were not reported for this subgroup.
Patients with active cancer are a subgroup of specific interest to primary care physicians and general practitioners because cancer carries a high risk for VTE, and VKA therapy is associated with an increase in complications in these patients (Citation60). The numbers of patients with active cancer were relatively low in the phase III VTE treatment studies of the direct oral anticoagulants. A pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies allowed a statistical analysis of efficacy and safety outcomes in patients with active cancer (Citation61). Rivaroxaban was associated with lower rates of recurrent VTE (5.1% versus 7.1%; HR = 0.69; 95% CI 0.36–1.33) and lower rates of major bleeding (2.8% versus 5.0%; HR = 0.53; 95% CI 0.23–1.23) when compared with enoxaparin/VKA, although these differences were not significant. With regard to patients with active cancer, Hokusai-VTE reported recurrent VTE in 3.7% of patients in the heparin/edoxaban group and 7.1% of patients in the heparin/warfarin group, with numbers being too low for a statistical analysis. Hokusai-VTE also analysed bleeding events (defined as major and non-major clinically relevant bleeding) in these patients, reporting rates of 18.3% versus 25.3% (odds ratio 0.67; 95% CI 0.34–1.29). In patients with active cancer at baseline, the pooled analysis of RE-COVER and RE-COVER II reported primary efficacy outcomes in 3.5% of patients in the dabigatran group and 4.7% of patients in the warfarin group (no HR or 95% CI given). Safety outcomes were not reported for this subgroup (Citation21). AMPLIFY (approximately 70 patients per treatment group had active cancer at baseline) did not report efficacy or safety outcomes in this subgroup (Citation22).
Some data have also been published on the safety and efficacy of the direct oral anticoagulants in other high-risk subgroups. Rivaroxaban showed similar efficacy compared with enoxaparin/VKA but significant reductions in the incidence of major bleeding events in fragile patients, defined as age > 75 years, calculated CrCl < 50 mL/min, or low body weight (≤ 50 kg) (Citation61). In fragile patients in the Hokusai-VTE study (defined as patients aged ≥ 75 years, body weight ≤ 50 kg, or CrCl ≥ 30–≤ 50 mL/min), heparin/edoxaban treatment was associated with significant reductions in rates of recurrent VTE compared with heparin/warfarin and was associated with similar rates of major plus non-major clinically relevant bleeding (Citation24). In the AMPLIFY study, subgroup analysis demonstrated similar efficacy compared with enoxaparin/warfarin. Rates of major bleeding events were significantly lower in elderly patients (age ≥ 75 years) receiving apixaban compared with enoxaparin/warfarin (Citation22). Similar efficacy outcomes for dabigatran compared with warfarin were reported for subgroups, including elderly patients (age > 75 years), in the pooled analysis of RE-COVER and RE-COVER II; there were no bleeding outcomes data reported in subgroups (Citation21).
Trials evaluating the long-term prevention of recurrence
Apixaban, dabigatran, and rivaroxaban have been evaluated for the long-term prevention of VTE recurrence in several phase III trials (, ). EINSTEIN EXT, AMPLIFY-EXT, and RE-SONATE were placebo-controlled trials, whereas RE-MEDY was an active controlled trial (Citation20,Citation23,Citation59). All of these trials were designed to include patients who had previously received anticoagulation therapy but in whom the clinical decision to continue treatment was not clear (equipoise).
Table VI. Results from the extended VTE studies (Citation20,Citation23,Citation59).
Rivaroxaban, apixaban, and dabigatran were superior to placebo for the primary efficacy outcomes in EINSTEIN EXT, AMPLIFY-EXT, and RE-SONATE, respectively (). In the RE-MEDY study, dabigatran was non-inferior to warfarin for the primary efficacy outcome. Major bleeding was not significantly increased compared with placebo in EINSTEIN EXT, AMPLIFY-EXT, or RE-SONATE and was similar to warfarin in RE-MEDY () (Citation20,Citation23,Citation59). In RE-MEDY, dabigatran was associated with a significant increase in cases of acute coronary syndrome compared with warfarin (0.9% versus 0.2%; P = 0.02) (Citation20).
ASA has also been assessed for extended secondary prevention of VTE in two recent studies (Citation62,Citation63). The Warfarin and Aspirin (WARFASA) study demonstrated that ASA reduced the risk of VTE recurrence (relative risk reduction 37%) in patients with unprovoked VTE after withdrawal of standard oral anticoagulant therapy with warfarin as compared with placebo, with similar rates of major bleeding events in both treatment groups (Citation62). In the Aspirin to Prevent Recurrent Venous Thromboembolism (ASPIRE) study, ASA demonstrated a non-significant trend for reduction in VTE recurrence compared with placebo in patients with a first unprovoked episode of VTE (Citation63). However, apixaban, dabigatran, and rivaroxaban reduced the risk of recurrent VTE by more than 80% compared with placebo in phase III studies, indicating that the reduction in risk of recurrent VTE was considerably lower in the ASA studies (Citation20,Citation23,Citation59,Citation62,Citation63).
Risk of bleeding and potential complications of anticoagulation use
Because of their mode of action, all anticoagulants carry a risk of bleeding. However, as discussed previously, major bleeding events generally occur less often with direct oral anticoagulants compared with standard LMWH/VKA therapy. The incidences of types of bleeding of particular concern to physicians, such as gastrointestinal and intracranial haemorrhage, are in general low. RE-COVER and RE-COVER II reported higher rates of gastrointestinal bleeding with dabigatran than with warfarin (RE-COVER: 53 cases (4.2%) versus 35 cases (2.8%); RE-COVER II: 48 cases (3.8%) versus 33 cases (2.6%)) (Citation19,Citation21). Major intracranial haemorrhage only occurred in the warfarin group (3 cases) in RE-COVER (Citation19). There were 2 cases (0.2%) of major intracranial haemorrhage in each treatment group in RE-COVER II (Citation21). Although there were cases of fatal intracranial haemorrhage in the EINSTEIN PE study, the number was low (2 cases in each group, < 0.1%) (Citation58). Non-fatal intracranial haemorrhage occurred more often in the enoxaparin/VKA group (10 cases, 0.4%), and only 1 case was documented in the rivaroxaban group (< 0.1%) (Citation58). In the AMPLIFY study, major intracranial haemorrhage occurred in 3 cases in the apixaban group (0.1%) and in 6 cases in the enoxaparin/VKA group (0.2%), and gastrointestinal bleeding occurred in 7 and 18 cases, respectively (0.3% versus 0.7%). In Hokusai-VTE, fatal intracranial haemorrhages only occurred in the warfarin group (6 cases, 0.1%), and non-fatal intracranial haemorrhages occurred in 5 (0.1%) and 12 cases (0.3%) in the edoxaban and warfarin groups, respectively. Moreover, fatal gastrointestinal bleeding events occurred in 1 and 2 cases (both < 0.1%), respectively.
To date, no large-scale real-world data have been published regarding bleeding events in patients receiving direct oral anticoagulants for the treatment of acute DVT or PE. A recent literature review identified cases of haemorrhagic complications in clinical practice with dabigatran or rivaroxaban (Citation64). Most of these events were precipitated by at least one risk factor for anticoagulant-associated bleeding, including medication non-adherence, prescriber errors, co-medication with antiplatelet drugs or P-gp inhibitors, impaired renal function, older age, and low body weight. Following the approval of dabigatran for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation, there were several reports of severe haemorrhagic complications that were not observed when the other direct oral anticoagulants were approved. This may in part be because dabigatran was the first drug to market in this indication (US Food and Drug Administration approval of dabigatran in October 2010 and rivaroxaban in November 2011) (Citation64). Future real-world studies will provide additional information on the risk of bleeding with the direct oral anticoagulants and which patient subgroups may be associated with a higher risk of bleeding.
When bleeding requires intervention, management strategies are similar for direct oral anticoagulants (): for example, the discontinuation of treatment and, when necessary, the administration of blood products (Citation14). Although there are no specific reversal agents available to counteract the effects of direct oral anticoagulants, there are several potential candidates in development, including a universal factor Xa inhibitor reversal agent (Citation65) and neutralizing fragment antibodies (Citation66). Prothrombin complex concentrate has also shown potential for reversing the anticoagulant effect of rivaroxaban in healthy subjects (Citation67).
Other problems have also been reported with the long-term use of traditional anticoagulants, including loss of bone mineral density with long-term LMWH use (Citation68) and an increased risk of vertebral and rib fractures with long-term VKA use (Citation69); these problems may also need to be considered.
Adherence
Patient adherence is key to reducing the incidence of secondary events but is affected by ease of administration and patient- perceived satisfaction with a particular therapy (Citation70–74). VKA therapy is associated with many limitations, and it has been reported that approximately 60% of patients receiving VKA treatment are not appropriately anticoagulated (Citation15,Citation16) and are, therefore, at risk of adverse events.
Rivaroxaban does not require routine coagulation monitoring or dose adjustments, has no dietary restrictions, and is dosed once daily for long-term treatment. Once-daily dosing has also been found to improve patient adherence and persistence in the treatment of chronic and cardiovascular diseases (Citation70,Citation71). These features may help improve patient adherence to rivaroxaban regimens. The simplicity of a single-drug approach, along with the ease of long-term, once-daily administration, and the use of easy-to-open blister packs and dispensers, makes rivaroxaban an attractive option for the treatment of PE (if approved for the treatment of PE, apixaban may also offer similar advantages). The link between patient satisfaction and adherence has been established and is becoming more important when making treatment decisions (Citation72–74). In the phase III EINSTEIN DVT and EINSTEIN PE trials, patients reported improved satisfaction and convenience of treatment with rivaroxaban compared with enoxaparin/VKA (Citation75,Citation76). This may contribute to better patient adherence and ultimately lead to improved clinical efficacy and health outcomes.
It is important to recognize the role general practitioners and primary care physicians play in encouraging proper patient adherence. Poor communication is often a cause of non-adherence, especially in the elderly or those with memory disorders, who may have difficulty understanding or following instructions (Citation77). Awareness of a patient's individual daily requirements, especially in the long-term setting of secondary prevention of PE, is necessary to tailor the treatment to the patient's life-style. Health professionals can encourage patients to comply with therapy by fully explaining the cause of PE and highlighting the effectiveness of the treatment, through frequent reinforcing visits and, if necessary, by using laboratory tests to measure anticoagulant activity and monitor adherence. Informing patients of potential side-effects, discussing treatment goals, and encouraging family support are additional strategies that may be used to improve patient adherence (Citation77).
Conclusion
Although the incidence of PE has increased over the last decade, possibly because of better detection methods and risk stratification, developments in patient management have contributed to lower rates of mortality. Management strategies for patients with PE are still improving and include earlier and even better risk stratification of patients. The simplified therapeutic approach provided by the direct oral anticoagulants may further contribute to optimizing patient treatment and outcomes. Among the direct oral anticoagulants, rivaroxaban is the first agent to be approved in the US and the EU as a single-drug approach for treating acute PE and secondary prevention of VTE. A phase IV study in acute DVT, XALIA (NCT01619007), has been initiated to evaluate rivaroxaban in the real-world setting and will aim to provide further support to results from the phase III EINSTEIN studies. Approval of other direct oral anticoagulants, such as apixaban, is likely to follow and may provide additional options for treating PE and preventing the recurrence of VTE.
After an acute PE, effective long-term anticoagulation is paramount for preventing the recurrence of VTE and chronic thromboembolic pulmonary hypertension. General practitioners play a key role in the long-term management of patients with PE, from making treatment decisions to encouraging patient adherence and being vigilant for signs of complications. Rivaroxaban is approved for PE treatment in the EU and the US and can be used in all haemodynamically stable PE patients. Based on clinical trial data, it is likely that other direct oral anticoagulants will be approved in this indication in the future. The introduction of rivaroxaban and potentially other direct oral anticoagulants for the treatment of PE may result in earlier hospital discharge, particularly of low-risk PE patients, and simplify long-term management of PE in daily primary care. Direct oral anticoagulants are likely to provide benefits in a broad range of patients, including patients traditionally considered more challenging to manage, such as patients with cancer or renal impairment, or fragile patients. It is important to educate physicians and patients about how to use these new drugs safely and effectively, including correct dosing regimens, management of adverse events, and switching between anticoagulants. Although regular anticoagulation monitoring may not be required with direct oral anticoagulants, clinical follow-up of patients in primary care will remain vital.
Acknowledgements
The authors would like to acknowledge Claudia Wiedemann, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Declaration of interest: Peter P. Toth does not have any conflicts of interest to declare and did not receive any honoraria for the preparation of this manuscript.
References
- Yamada N, Nakamura M, Ito M. Current status and trends in the treatment of acute pulmonary thromboembolism. Circ J. 2011;75:2731–8.
- Yang Y, Liang L, Zhai Z, He H, Xie W, Peng X, et al. Pulmonary embolism incidence and fatality trends in Chinese hospitals from 1997 to 2008: a multicenter registration study. PLoS One. 2011;6:e26861.
- Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–7.
- Segard T, Macdonald WB. Changing trends in venous thromboembolism-related imaging in Western Australian teaching hospitals, 2002–2010. Med J Aust. 2013;198:100–3.
- Blann AD, Lip GYH. Venous thromboembolism. Br Med J. 2006;332: 215–19.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–8.
- Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–80.
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276–315.
- Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–30.
- Baglin T, Douketis J, Tosetto A, Marcucci M, Cushman M, Kyrle P, et al. Does the clinical presentation and extent of venous thrombosis predict likelihood and type of recurrence? A patient level meta-analysis. J Thromb Haemost. 2010;8:2436–42.
- Agnelli G, Prandoni P, Becattini C, Silingardi M, Taliani MR, Miccio M, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139:19–25.
- Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e24S–43S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–88S.
- Willey VJ, Bullano MF, Hauch O, Reynolds M, Wygant G, Hoffman L, et al. Management patterns and outcomes of patients with venous thromboembolism in the usual community practice setting. Clin Ther. 2004;26:1149–59.
- Deitelzweig SB, Lin J, Kreilick C, Hussein M, Battleman D. Warfarin therapy in patients with venous thromboembolism: patterns of use and predictors of clinical outcomes. Adv Ther. 2010;27:623–33.
- Bayer Pharma AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf (accessed 20 February 2014).
- Janssen Pharmaceuticals Inc. Xarelto® (rivaroxaban) Prescribing Information. 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022406s007lbl.pdf (accessed 28 February 2014).
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52.
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18.
- Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014; 129:764–72.
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708.
- The Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15.
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–94S.
- Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108: 978–81.
- Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4:49–59.
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–830.
- Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–6.
- Aujesky D, Roy PM, Le Manach CP, Verschuren F, Meyer G, Obrosky DS, et al. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J. 2006;27:476–81.
- Aujesky D, Perrier A, Roy PM, Stone RA, Cornuz J, Meyer G, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med. 2007;261:597–604.
- Hauel NH, Nar H, Priepke H, Ries U, Stassen JM, Wienen W. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002;45:1757–66.
- Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–62.
- Pinto DJ, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, et al. Discovery of 1-(4-Methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro- 1H-pyrazolo[3,4-c]pyridine-3-carboxamide (Apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50:5339–56.
- Furugohri T, Isobe K, Honda Y, Kamisato-Matsumoto C, Sugiyama N, Nagahara T, et al. DU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost. 2008;6:1542–9.
- Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 - an oral, direct factor Xa inhibitor. J Thromb Haemost. 2005;3:514–21.
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22.
- Schulman S, Crowther MA. How I treat with anticoagulants in2012: new and old anticoagulants, and when and how to switch.Blood.2012;119:3016–23.
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21.
- Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303.
- Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, et al. Apixaban, an oral, direct factor Xa inhibitor: single-dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–87.
- Ogata K, Mendell-Harary J, Tachibana M, Matsumoto H, Oguma T, Kojima M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50:743–53.
- Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- Boehringer Ingelheim International GmbH. Pradaxa® (dabigatran etexilate) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf (accessed 20 February 2014).
- Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs and humans. Drug Metab Dispos. 2009;37:1056–64.
- Bristol-Myers Squibb, Pfizer EEIG. Eliquis® (apixaban) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf (accessed 20 February 2014).
- Samama MM, Guinet C. Laboratory assessment of new anticoagulants. Clin Chem Lab Med. 2011;49:761–72.
- Turpie AGG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban - an oral, direct factor Xa inhibitor. Thromb Haemost. 2012;108:876–86.
- Gerotziafas GT, Baccouche H, Sassi M, Galea V, Chaari M, Hatmi M, et al. Optimisation of the assays for the measurement of clotting factor activity in the presence of rivaroxaban. Thromb Res. 2012;129:101–3.
- Mani H, Hesse C, Stratmann G, Lindhoff-Last E. Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost. 2011;106:156–64.
- Freyburger G, Macouillard G, Labrouche S, Sztark F. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127:457–65.
- Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673–9.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–43.
- Gosselin R, Hawes E, Moll S, Adcock D. Performance of various laboratory assays in the measurement of dabigatran in patients receiving therapeutic doses: a prospective study based on peak and trough plasma levels. Am J Clin Pathol. 2014;141:262–7.
- Lillo-Le Louët A, Wolf M, Soufir L, Galbois A, Dumenil AS, Offenstadt G, et al. Life-threatening bleeding in four patients with an unusual excessive response to dabigatran: implications for emergency surgery and resuscitation. Thromb Haemost. 2012;108:583–5.
- Graff J, Harder S. Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin Pharmacokinet. 2013;52:243–54.
- Landefeld CS, Cook EF, Flatley M, Weisberg M, Goldman L. Identification and preliminary validation of predictors of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1987;82:703–13.
- The EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510.
- Prandoni P, Lensing AWA, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8.
- Prins MH, Lensing AWA, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11:21.
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–67.
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–87.
- Pfeilschifter W, Luger S, Brunkhorst R, Lindhoff-Last E, Foerch C. The gap between trial data and clinical practice—an analysis of case reports on bleeding complications occurring under dabigatran and rivaroxaban anticoagulation. Cerebrovasc Dis. 2013;36:115–19.
- Hutchaleelaha A, Lu G, DeGuzman FR, Karbarz MJ, Inagaki M, Yau S, et al. Recombinant factor Xa inhibitor antidote (PRT064445) mediates reversal of anticoagulation through reduction of free drug concentration: a common mechanism for direct factor Xa inhibitors. Eur Heart J. 2012;33:496. Abstract 2962.
- van Ryn J, Litzenburger T, Waterman A, Canada K, Hauel N, Sarko C, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol. 2011;57:E1130. Abstract 1142–367.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–9.
- Wawrzynska L, Tomkowski WZ, Przedlacki J, Hajduk B, Torbicki A. Changes in bone density during long-term administration of low-molecular-weight heparins or acenocoumarol for secondary prophylaxis of venous thromboembolism. Pathophysiol Haemost Thromb. 2003;33:64–7.
- Caraballo PJ, Heit JA, Atkinson EJ, Silverstein MD, O’Fallon WM, Castro MR, et al. Long-term use of oral anticoagulants and the risk of fracture. Arch Intern Med. 1999;159:1750–6.
- Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22–33.
- Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28:669–80.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97.
- Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(Suppl 1):S9–24.
- Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36.
- Prins M, Bamber L, Cano S, Wang M, Lensing A, Bauersachs R. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic pulmonary embolism. Blood (ASH Annual Meeting Abstracts). 2012;120. Abstract 1163.
- Bamber L, Wang MY, Prins MH, Ciniglio C, Bauersachs R, Lensing AW, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosis. Thromb Haemost. 2013;110:732–41.
- Vermiere E, Avonts D, Van Royen P, Buntinx F, Denekens J. Context and health outcomes. Lancet. 2001;357:2059–60.
- Bristol-Myers Squibb. Coumadin (warfarin sodium) Prescribing Information. 2011. Available at: http://packageinserts.bms.com/pi/pi_coumadin.pdf (accessed 15 January 2014).
- Makris M, Van Veen JJ, Tait C, Mumford A, Laffan M; on behalf of the British Committee for Standards in Haematology. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol. 2012;160:35–46.
- Wong PC, Crain EJ, Xin B, Wexler RR, Lam PY, Pinto DJ, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6:820–9.
- Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. Effects of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate, on thrombus formation and bleeding time in rats. Thromb Haemost. 2007;98:333–8.
- Raskob G, Cohen AT, Eriksson BI, Puskas D, Shi M, Bocanegra T, et al. Oral direct factor Xa inhibition with edoxaban for thromboprophylaxis after elective total hip replacement. A randomised double-blind dose-response study. Thromb Haemost. 2010;104:642–9.
- Frost C, Yu Z, Nepal S, Bragat A, Moore K, Shenker A, et al. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation. J Clin Pharmacol. 2008; 48:1132. Abstract 142.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–99.
- Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51:549–61.
- Wagner JG, Welling PG, Lee KP, Walker JE. In vivo and in vitro availability of commercial warfarin tablets. J Pharm Sci. 1971;60:666–77.
- Weinz C, Buetehorn U, Daehler HP, Kohlsdorfer C, Pleiss U, Sandmann S, et al. Pharmacokinetics of BAY 59-7939 – an oral, direct factor Xa inhibitor – in rats and dogs. Xenobiotica. 2005;35:891–910.
- Yacobi A, Udall JA, Levy G. Serum protein binding as a determinant of warfarin body clearance and anticoagulant effect. Clin Pharmacol Ther. 1976;19:552–8.
- Mueck W, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–61.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 – an oral, direct factor Xa inhibitor – after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873–80.
- Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24:2757–65.
- Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40:2250–5.
- Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S, et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013; 13:331–42.
- Masumoto H, Yoshigae Y, Watanabe K, Takakusa H, Okazaki O, Izumi T. In vitro metabolism of edoxaban and the enzymes involved in the oxidative metabolism of edoxaban. AAPS J. 2010;12. Abstract W4308.
- Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–66.

