Abstract
The circadian timing system (CTS) controls several critical molecular pathways for cancer processes and treatment effects over the 24 hours, including drug metabolism, cell cycle, apoptosis, and DNA damage repair mechanisms. This results in the circadian time dependency of whole-body and cellular pharmacokinetics and pharmacodynamics of anticancer agents. However, CTS robustness and phase varies among cancer patients, based on circadian monitoring of rest– activity, body temperature, sleep, and/or hormonal secretion rhythms. Circadian disruption has been further found in up to 50% of patients with metastatic cancer. Such disruption was associated with poor outcomes, including fatigue, anorexia, sleep disorders, and short progression-free and overall survival. Novel, minimally invasive devices have enabled continuous CTS assessment in non-hospitalized cancer patients. They revealed up to 12-hour differences in individual circadian phase. Taken together, the data support the personalization of chronotherapy. This treatment method aims at the adjustment of cancer treatment delivery according to circadian rhythms, using programmable-in-time pumps or novel release formulations, in order to increase both efficacy and tolerability. A fixed oxaliplatin, 5-fluorouracil and leucovorin chronotherapy protocol prolonged median overall survival in men with metastatic colorectal cancer by 3.3 months as compared to conventional delivery, according to a meta-analysis (P = 0.009). Further analyses revealed the need for the prevention of circadian disruption or the restoration of robust circadian function in patients on chronotherapy, in order to further optimize treatment effects. The strengthening of external synchronizers could meet such a goal, through programmed exercise, meal timing, light exposure, improved social support, sleep scheduling, and the properly timed administration of drugs that target circadian clocks. Chrono-rehabilitation warrants clinical testing for improving quality of life and survival in cancer patients.
Key messages
The circadian timing system temporally controls several physiological and molecular mechanisms involved in anticancer drugs’ efficacy and safety, providing the rationale for circadian-based anticancer treatment (chronotherapy).
Disruption of the circadian timing system of patients with cancer is associated with several systemic symptoms, including fatigue, appetite loss, and sleep problems, as well as with poor outcomes.
Circadian re-synchronization, through behavioural and pharmacological integrated treatments, could potentially increase the chronotherapeutic index, reduce bothersome systemic symptoms, and prolong overall survival in cancer patients; research is needed to test the effects of circadian re-synchronization on clinical outcomes.
The circadian timing system (CTS)
Circadian rhythms consist of biological oscillations with a period of about 24 hours. The term ‘circadian’ comes from the Latin circa (around) and dies (day). Such rhythms have been found in all living beings, from bacteria to mammals. In rodents and humans, they characterize most physiological parameters, such as sleep–wakefulness, rest–activity, appetite, muscular strength, and performance, as well as hormonal secretions, among the many biological variables that play a role in well-being () (Citation1–6). Circadian rhythms persist in the absence of environmental cycles in living organisms, yet with a period that slightly differs from precisely 24 h (Citation1). This finding has supported the concept of circadian rhythm generation by an endogenous clock or clock system that can be influenced by environmental cycles (Citation7). Indeed, circadian rhythms display precise 24-h periods, following their synchronization with environmental 24-h cycles, such as the regular alternation of light and darkness. Other environmental cues that can strengthen circadian rhythms include timed physical activity and rest, socio-professional and familial interactions, and feeding patterns () (Citation8–11). Such circadian co-ordination and adjustment to environmental cues mostly operate through the suprachiasmatic nuclei (SCN), hypothalamic pacemakers located above the optic chiasm (Citation12). The SCN integrate the light–dark information transmitted from the retinal ganglion cells in the eye and generate multilevel redundant rhythmic signals that affect neuromediator trafficking, body temperature, cytokines, and hormonal secretions () (Citation13,Citation14). Thus SCN ablation suppresses circadian rhythms including rest–activity cycle and profoundly alters or suppresses that of core body temperature in mice (Citation15). Similar alterations result from frequent shifts in light–dark exposure schedule which mimic chronic jet lag (Citation16–18). SCN-dependent rhythmic signals co-ordinate genetic molecular clocks that reside within each mammalian cell, resulting in a hierarchical clocks network that constitutes the circadian timing system (CTS) (Citation1–4,Citation19).
Figure 1. Schematic representation of the circadian timing system (CTS). Anatomical location on an axial plane from a nuclear magnetic resonance image of the central pacemaker, the SCN (suprachiasmatic nuclei). Included are also light and social entrainment cues, signalling the SCN through direct or indirect neural connections, four circadian biomarkers of CTS function, and the peripheral molecular clocks, controlling tissue-specific temporal variations in gene expression, through molecular regulation of transcription and post-transcription processes. Melatonin and rest–activity rhythms re-enforce the co-ordination of the central pacemaker, whereas cortisol and core body temperature rhythms provide endogenous resetting signals to peripheral molecular clocks.
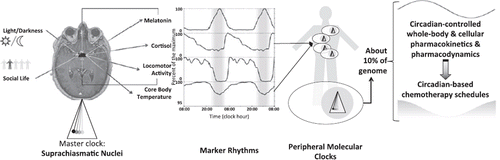
These genetic clocks rhythmically control proliferation (Citation20–24), DNA repair (Citation25), and apoptosis among other functions that are critical for cancer processes and treatment effects (Citation26–28). Circadian oscillations are generated within each cell through three interwoven transcriptional–translational feedback loops involving at least 15 genes that constitute the circadian molecular clock (Citation29–33). The feedback loops ensure clock robustness against perturbations within each cell, thus contributing to accurate circadian time-keeping at the tissue level. The feedback loops further help generate phase delays in circadian transcription that result in the adjustment of clock gene expression patterns to local physiology changes (Citation19,Citation34). The molecular circadian clock rhythmically controls the mRNA expression of approximately 10% of the transcriptome over the 24-h cycle. However, those genes that are rhythmically controlled by the clock differ according to tissue function, so that a large part of the genome is under circadian clock control with tissue specificity (Citation35–37). As a result of its multilevel organization, the CTS rhythmically moderates metabolism and proliferation in peripheral organs or tissues, such as liver, heart, lung, kidney, intestine, pancreas, muscle, breast, brown fat, and skin throughout the 24-h cycle. For example, in the mouse liver, about 5000 proteins preferentially accumulate in the morning or during the night. Among them, several hundred showed robust diurnal oscillations related to core hepatic physiological functions (Citation38). Moreover, ∼15% of all identified metabolites, such as fatty acids in plasma and amino-acids in saliva, display circadian oscillations (Citation39).
The molecular circadian clock is also involved in genotoxic stress response and cell cycle checkpoint control, i.e. in the operation that verifies whether the processes at each phase of the cell cycle have been accurately completed before progression into the next phase. The genes actively involved in the cell cycle at the mitotic peak, including WEE1, CDC2, and P21, show circadian oscillations (Citation24,Citation40). Moreover, genes whose transcription is under circadian control and therefore vary over 24 hours include genes involved in the biotransformation, detoxification, or elimination of chemotherapy drugs, as well as those that are direct targets of cytotoxic or targeted drugs (Citation1,Citation6,Citation9,Citation28,Citation31,Citation41,Citation42). Thus, the molecular circadian clock cyclically regulates most processes constituting the hallmarks of cancer (cell cycle gating, apoptosis, genome stability, cellular energetics, neo-angiogenesis, inflammation, and immune response) as well as several pharmacokinetic and pharmacodynamic determinants of anticancer drugs (Citation1,Citation6,Citation9,Citation28,Citation31,Citation41,Citation42). Accounting for these biological rhythms in order to increase chemotherapy therapeutic index with concomitant improvement of its activity and safety constitutes the rationale for circadian-based drug administration, or chronotherapy (Citation6,Citation43,Citation44).
CTS assessment through circadian biomarkers determination
Twenty-four-hour changes in circulating melatonin and cortisol concentrations, as well as body temperature and rest–activity rhythms, have been used as CTS biomarkers in order to assess the clinical relevance of the circadian clock network in cancer patients, since these circadian rhythms are controlled by the central pacemaker in the SCN (Citation1).
Circulating melatonin and cortisol
Melatonin is a hormone usually secreted by the pineal gland during darkness in mammals, irrespective of whether they are nocturnal or diurnal animals. Thus light exposure inhibits melatonin synthesis, through SCN-dependent pathways (Citation45). However other minor sources of production exist, especially in the intestine (Citation46). Indeed melatonin secretion is an endogenous rhythm as well since it persists in constant darkness in most rodents, and in blind people as well (Citation45). Its susceptibility to light exposure can differ according to genetic background, since it is secreted predominantly during the late light span in B6D2F1 mice (Citation47). In humans, melatonin secretion usually starts near 18.00, peaks at 02.00, and returns to near zero level at 10.00. However, peak melatonin plasma concentration differed by up to 8 h among healthy human subjects studied during their usual routine, a finding that discloses differences in endogenous timing, circadian synchronization, and/or light at night exposure in humans (Citation1). Indeed artificial light at night, for instance with an intensity of 2500 lux from 02.00 to 04.00, rapidly and transiently suppresses nocturnal melatonin production in humans (Citation45,Citation48). Light exposure for several consecutive nights, however, shifts peak melatonin secretion to the early morning (Citation45). Melatonin and its 6-sulfatoxy metabolite can be measured in saliva, plasma, or urine. Thus, their rhythmic patterns can be reliably determined and used as a circadian biomarker that contributes to CTS robustness. Melatonin also reinforces the nocturnal decrease of central temperature, an event which facilitates sleep propensity (Citation45). Melatonin further inhibits or slows progression of some experimental breast and prostate cancer types, both in vitro and in vivo (Citation49,Citation50), whereas suppression of melatonin via constant light exposure increases tumour growth in a dose-dependent manner in light-sensitive experimental models (Citation51). There is now ample evidence that prolonged night-time shift-work is associated with an increased risk of developing cancer (Citation52), so that shift-work has been classified as a probable carcinogen by the International Agency for Research on Cancer (Citation49). However, it is far from being clear yet whether this results from melatonin suppression or circadian disruption (Citation50,Citation53). In cancer patients, melatonin circadian pattern is not systematically disrupted, and the rhythms of melatonin, cortisol, lymphocytes, and rest–activity likely reflect different components of the circadian system, which may be altered differently during cancer processes (Citation54).
Cortisol is a steroid hormone that is secreted with a robust circadian rhythm by the adrenal cortex, under the control of the hypothalamic–pituitary–adrenal axis (HPA) involving hypothalamic corticotropin-releasing factor (CRF) and pituitary adrenocorticotropic hormone (ACTH) secretions and their multiple feedback circuits (Citation55). On average, plasma cortisol secretion usually starts rising around 04.00, when melatonin begins to decline, then reaches a peak near awakening, when melatonin comes close to its nadir levels. Cortisol secretion then decreases throughout the day and reaches its nadir value around 02.00, coincident with the melatonin peak () (Citation56,Citation57). Although both hormonal rhythms appear to be 8–12 h out of phase in healthy humans, there are many reported examples where both rhythms coincide, despite apparently similar synchronization (Citation1). This finding supports complex interactions between CTS and HPA regulation. Glucocorticoids effectively reset molecular clocks and clock-controlled pathways both in vitro and in vivo in experimental models, a finding which supports the contribution of cortisol circadian rhythms to CTS robustness (Citation2). However, cortisol secretory response can also be moderated by stress (Citation58,Citation59). Cortisol dysregulation can adversely impact physiological processes controlling inflammation, depression, post-traumatic stress disorder, cardiovascular disease, type II diabetes, or stroke (Citation58,Citation60,Citation61). An altered pattern of circadian cortisol secretion reflecting flattening or even reversal of the normal diurnal variation predicts poor survival in patients with metastatic or advanced breast, ovarian, kidney, or lung cancer independently of other known prognostic factors (Citation62–65). A similar trend yet without any statistical significance was found between cortisol rhythm and overall survival in patients with metastatic colorectal cancer (Citation66). In patients with this disease, the rest–activity rhythm held robust prognostic value for overall survival and progression-free survival (Citation67,Citation68).
Body temperature
Central body temperature is tightly regulated in mammals so it usually varies from ∼36.5°C near 02.00 at night to ∼37.5°C around 18.00 in the late afternoon in human beings () (Citation69). Core body temperature gradually declines during bedtime, as a result of vasodilation-associated heat loss that occurs at the extremities and at skin surface level and can be influenced by physical activity (Citation61,Citation62,Citation63). The central temperature decrease that occurs early at night has been considered to play a critical role in triggering sleep onset (Citation72). Environmental temperature usually displays day–night variations, so that external temperature cycles may also contribute to mammalian CTS synchronization (Citation73). Moreover, the application of external temperature cycles entrains rhythmic clock gene expression patterns both in cultured Rat-1 fibroblasts and primary glial cells, and in peripheral organs of rodents (Citation74,Citation75). External temperature cycles further phase-shift circadian clocks in cultured chick pineal neurons and mammalian SCN neurons and cells from other organs such as liver, adrenal, and cortical glia (Citation74,Citation76).
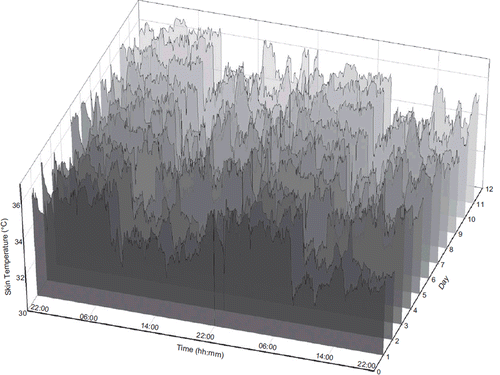
Body temperature has been measured at various sites, including the rectum, axilla, oral cavity, external ear canal, nasopharynx, oesophagus, and vagina, since the development of the first thermometer for human use in the late nineteenth century. For research purposes, single temperature measurements at these sites were either uncomfortable or unreliable, or both. While the rectal or external ear canal have been recommended for precise and reliable body temperature measurements, none of them are amenable to continuous monitoring beyond a few days (Citation69). A recently developed teletransmitting temperature pill makes it now possible to monitor closely the internal body temperature of humans for about 24 h (Core Body Temperature Capsule Ingestable, Jonah™, VitalSense®, Philips Respironics, MA, Norwell, USA) (Citation77). New thermal sensor technology with great measurement sensitivity (0.01°C) further enables continuous precise skin surface temperature monitoring at multiple sites during the usual routine activities of the subject for several days (VitalSense®, Philips Respironics, MA, USA). The thermal patches can be placed on the anterior chest (Citation70,Citation78). Their use has recently demonstrated that circadian rhythms in skin surface temperature vary among cancer patients, sites of measurements, and with chemotherapy delivery. Indeed, in about 80% of the time series obtained at four thoracic sites a circadian rhythm in skin surface temperature was validated, yet with acrophases that could differ by up to 12 h among patients with metastatic cancer. These rhythms remained robust during and after the delivery of chronomodulated chemotherapy in ∼50% of the patients, while transient or sustained alterations occurred in the other 50%. These data support further evaluation of skin surface temperature as a clinically relevant CTS biomarker in cancer patients ().
Figure 2. Continuous skin temperature monitoring. Double-plot example of fully ambulatory, non-invasive continuous monitoring of skin temperature using dermal patches over 12 days in a patient with advanced colorectal cancer (WHO PS = 1). Baseline recording before treatment start was obtained from day 1 to day 4. Chronomodulated chemotherapy (chronoIFLO4; (Citation230)) was administered from day 5 to day 8. No disruption of peripheral temperature circadian rhythm was observed either during or after treatment. Data from (Citation70).
Rest–activity rhythm
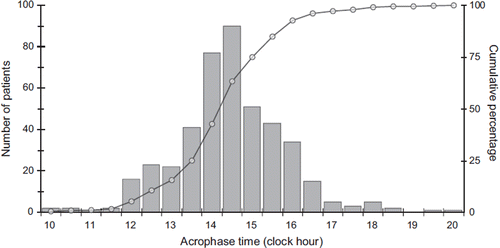
The rest–activity rhythm has been widely assessed using wrist actimetry in healthy and diseased humans in various conditions (Citation79,Citation80). Actimetry is a non-invasive rhythm assessment method that involves the use of an accelerometer within a wrist watch-like device to be worn on the non-dominant arm. Actimetry provides information on the rest–activity patterns and some sleep parameters in healthy subjects and patients. The relevance of the rest–activity rhythm as a CTS biomarker was first established in experimental models, since this rhythm was suppressed following SCN destruction, chronic jet lag exposure, or selected clock gene mutations combined with external synchronizer removal (Citation18). Indeed, the rest–activity circadian phenotype led to the discovery of most clock genes in Drosophila and rodents, and their subsequent identification in humans (Citation81). Actimetry time series allow the identification of circadian and other periodic components, period lengths with corresponding amplitudes, and acrophases, as well as non-parametric criteria such as the dichotomy index I < O and the autocorrelation coefficient at 24 h (r24). I < O represents the percentage of activity in-bed that is less than the median activity out-of-bed (Citation82). I < O can range from 0% to 100%, with highest values indicating a robust rhythm with high day-time activity and low activity at night, that is consistent both with adequate circadian synchronization and undisturbed sleep (Citation83,Citation84). The lower the I < O value, the worse the circadian disruption. A median value of 97.5% has been used to discriminate practically between clinically relevant circadian disruption (lower values) and circadian robustness (higher values) (Citation67,Citation68,Citation85). However, recent studies reveal that the prognostic value of I < O for cancer patient outcomes is continuous (Citation67,Citation68). The autocorrelation coefficient (r24) can range from − 1 to + 1. It estimates the reproducibility of the 24-h pattern from one day to the next over the recording span. Robust and stable circadian rhythms in activity are indicated by an r24 value which exceeds 0.5 (Citation83,Citation84). Wrist actimetry also provides some information regarding the phase of locomotor activity, even though this parameter is masked by voluntary physical activity (Citation69,Citation86). In a large sample of 436 patients with metastatic colorectal cancer, 2-day actimetry recording during the patient routine revealed large interindividual variability in acrophase timing, which spanned over 10 hours (unpublished results, ). These and other data support the concept of personalized chemotherapy timing for optimizing treatment effects in cancer patients.
Figure 3. Variability in acrophase timing. Temporal distribution of the circadian acrophases in rest–activity rhythms from 436 patients with metastatic colorectal cancer undergoing 2–3-day monitoring with a wrist-watch accelerometer. Data from (Citation67,Citation68).
Wrist actimetry also provides data that are amenable to selected sleep parameter estimation, besides non-invasive quantitative CTS assessment (Citation79). This method is less expensive and non-intrusive, as compared to polysomnography. Moreover, it can record minute activity counts for weeks or months at the patients home or during his or her usual routine. This makes it possible to monitor the dynamic changes in rest–activity and sleep patterns throughout cancer treatment programmes in order to gather quantitative measurements or estimates of the CTS, as well as sleep, physical activity, and fatigue (). Indeed, rest–activity monitoring provides relevant information regarding CTS disruption in patients with sleep or mood disorders, cardiovascular diseases, obesity, diabetes, hip fractures, or cancer, as well as shift-workers (Citation87).
Table I. Clinical studies in patients with cancer involving concomitant rest–activity monitoring through wrist actimetry and subjectively rated fatigue assessment.
CTS disruption and clinical outcomes
Studies have shown that patients with insomnia, depression, or schizophrenia have low melatonin secretion (Citation45). Evening melatonin dosing can improve sleep quality, blood pressure, metabolism, and mood, thus helping prevent the earlier morning cortisol secretion peak that indicates CTS phase advance (Citation45). Experimental data further show that CTS disruption also facilitates cancer development during or after host exposure to a carcinogen. For example, both diethylnitrosamine-induced liver carcinogenesis and radiation-induced lymphoma formation were increased several-fold in mice with CTS disruption resulting from chronic experimental jet lag, as well as from alteration of molecular clock genes, such as Per2 mutation or double Cry1/Cry2 deficiency. In these experimental models, CTS disruption increased the number of mice with tumours, the number of tumours per mouse, and the size of the largest tumour (Citation88–91). The increased carcinogenic potential of exposure to the same carcinogen dose and schedule likely results from the up-regulation of c-Myc, the down-regulation of p53, and the increased cytokine production caused by CTS disruption (Citation16,Citation90). These consequences of clock deregulation, respectively, favour cellular proliferation, genomic instability, and inflammation, three established hallmarks of cancer (Citation92,Citation93). These experimental findings may explain the increased cancer risk in shift-workers with prolonged exposure to the most circadian disruptive night-shifts (Citation94–96).
Circadian disruption and fatigue
Fatigue is a subjective symptom which is experienced by individuals as a state of weariness, with a decrease in physical and/or mental abilities, interfering with physical or intellectual efforts. In healthy individuals, fatigue is normal at the end of a work-day or after a physical or mental activity. Although this type of fatigue can be at times annoying, it has little impact on daily life, and it responds to a good night's sleep.
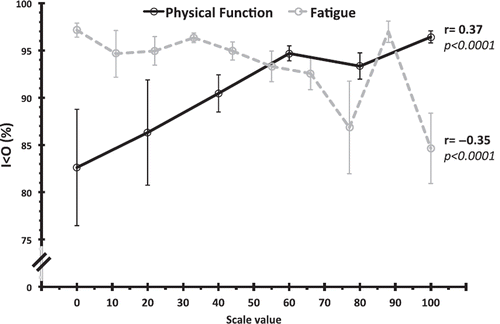
Cancer-related fatigue is significantly more intense and troublesome. Both the disease and its treatment can induce fatigue (Citation97,Citation98). Nearly all cancer patients undergoing chemotherapy report cancer-related fatigue, and 30% to 40% of cancer survivors report moderate to severe fatigue up to 5 years after cancer treatment (Citation99,Citation100). The precise causes of cancer-related fatigue are not entirely known, but disrupted rest–activity rhythms (Citation101), flattened diurnal cortisol rhythms (Citation102), and pro-inflammatory cytokines could play a role (Citation103–105). In contrast, physical activity and exercise decreased self-rated fatigue both during and after treatment in cancer patients (Citation106,Citation107). Such self-rated fatigue, based on internationally validated scales or questionnaires, was significantly associated with CTS disruption and poor sleep, as assessed with both wrist actimetry parameters I< O and r24 in 245 patients with metastatic colorectal cancer () () (Citation83,Citation84,Citation101,Citation108–110).
Figure 4. Associations between rest–activity circadian rhythm and subjective ratings of physical function and fatigue. Correlation between the circadian rest–activity rhythm parameter I< O (y-axis) computed from wrist actimetry records and physical function (black solid line) or fatigue (grey dotted line) items derived from the EORTC QLQ-C30 quality of life questionnaire (Citation231) in 245 patients with metastatic colorectal cancer. The x-axis values correspond to fatigue and quality of life scores, ranging from 0% to 100%. Displayed fatigue and physical quality of life scores are mean ± SEM of individual ratings computed according to the recommended procedure (236). Correlation coefficients calculated using Spearman's rank-order test. Note higher variance in patients with severe fatigue or low physical function, whereas patients with low fatigue or high physical function consistently display high I< O values, indicating robust circadian patterns. Redrawn with merged data from (Citation101) and (Citation84).
Circadian disruption and sleep problems
Subjective complaints of sleep problems are exceedingly prevalent in both cancer patients and survivors. Nearly 80% of cancer patients who have received chemotherapy experience difficulty with falling or staying asleep, earlier-than-intended awakening, or/and day-time fatigue (Citation111,Citation112). Although cancer diagnosis and treatment might precipitate their development, these sleep problems often continue long after the treatments are over. Up to two-thirds of all cancer survivors experience problems with sleep for several years after treatment is completed (Citation113). For some patients, sleep problems even begin or become worse before the diagnosis of cancer. Sleep alterations are frequently associated with circadian disruption as evidenced by flattened cortisol rhythms (Citation114) or altered rest–activity patterns () (Citation83,Citation84). Circadian and sleep disruption create multiple adverse health consequences via several pathways including increasing inflammation and oxidative stress and HPA dysregulation and can be biomarkers of early disease progression (Citation115–117).
Circadian disruption and overall survival
Cancer patients with CTS disruption, as assessed with either wrist actigraphy or salivary cortisol, have shorter overall survival as compared with patients with robust circadian rhythms (). This phenomenon has been observed in various clinical settings, is independent of other clinical prognostic factors, and persists many years after circadian assessment. Thus, flattened diurnal cortisol rhythm has been found to predict shorter survival in patients with breast, lung, ovarian, and kidney cancers () (Citation64,Citation65,Citation118). Similarly, low day-time activity and poor night-time rest, estimated with an I < O value lower than 97.5% (median value) either before treatment start or while on chemotherapy, are associated with poor survival in patients with metastatic colorectal cancer () (Citation67,Citation68,Citation85). These clinical results are supported by experimental data showing accelerated tumour progression in mice with CTS disruption through anatomical or functional ablation of the central pacemaker, or clock gene mutations (Citation1,Citation18,Citation91).
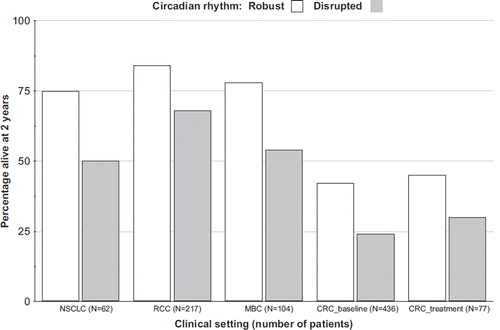
Figure 5. Detrimental effect of circadian disruption on overall survival in patients with distinct cancer types at an advanced or metastatic stage. Actual survival rates at 2 years according to circadian robustness (white boxes) or disruption (grey boxes) based on circadian patterns in cortisol (three studies on the left) or rest–activity (two studies on the right). For each study, survival outcomes differed with statistical significance independently of other prognostic factors. Redrawn with data from (Citation64,Citation65,Citation67,Citation68,Citation85,Citation118). CRC = colorectal cancer; MBC = metastatic breast cancer; NSCLC = non-small-cell lung cancer; RCC = renal cell carcinoma.
Disturbed sleep has also been found associated with poor survival in patients with cancer. Indeed, in 97 patients with advanced breast cancer, low objectively measured sleep efficiency independently predicted shorter overall survival (Citation115). Similarly, subjectively rated sleep troubles in 240 patients with metastatic colorectal cancer were independently associated with a 49% increased risk of earlier death (Citation119). Finally, misaligned bedtimes (going to bed earlier or later than preferred time) were associated with almost 3 years’ shorter disease-free interval in 85 women with breast cancer (Citation120). These results, observed while assessing sleep, a paramount component of circadian function, further support the clinical relevance of the regulation of circadian rhythms and sleep–wake cycles in cancer patients.
Circadian-based anticancer treatments
The CTS temporally controls whole-body and cellular pharmacokinetics and pharmacodynamics of anticancer drugs, as well as cell cycle and cell death mechanisms, with a phase regulated through circadian endogenous resetting signals () (Citation1,Citation2,Citation6,Citation9). Hence, optimally timed administration along the 24 hours span could improve the therapeutic index of anticancer agents. The adjustment of anticancer drug administration to circadian rhythms has led to their chronomodulated delivery through multidrug chronotherapy protocols. The development of programmable time pumps has enabled the safe and highly effective delivery of combination chronotherapy protocols involving up to four anticancer drugs in non-hospitalized patients with metastatic colorectal, pancreas, lung, breast, ovarian, kidney, or head-and-neck cancers (Citation1,Citation6,Citation121). Chronotherapy with oxaliplatin, 5-fluorouracil, and leucovorin has indeed achieved improved tumour response rate, progression-free survival, and overall survival as compared to conventional chemotherapy delivery in men with metastatic colorectal cancer, according to a recent meta-analysis (Citation122). However this was not the case in women with the same disease, which may result from sex-dependent optimal drug timing, as recently shown in mouse studies (Citation123,Citation124). Furthermore, women displayed increased treatment-related toxicities. Several chemotherapy-induced adverse events are associated with CTS disruption, thus ablate chronotherapy mechanisms (Citation125,Citation126). Several updated reviews describe in detail the rationale, achievements, limits, and future directions of cancer chronotherapy (Citation1,Citation6,Citation9,Citation31,Citation43). Circadian drug timing is further relevant for the treatment of rheumatoid, allergic, respiratory, psychiatric, cardiovascular, or endocrine diseases (Citation41,Citation42,Citation127,Citation128). We here summarize the recent results of circadian-based radiotherapy, for which literature data are scarce and have not yet been extensively reviewed. We then address the issue of personalized cancer chronotherapy that represents a current challenge for the field.
Circadian-based radiotherapy
Similarly to chemotherapy, radiotherapy damages cell cycle and DNA repair mechanisms, which are controlled by the molecular circadian clock (Citation129). Therefore, radiation therapy could follow a similar circadian-based optimization as chronomodulated chemotherapy, in order to improve therapeutic index. Both old and recent experimental data support this approach for optimizing both chronotolerance and chronoefficacy. For example, morning radiation caused more hair loss as compared with evening radiation in mice synchronized with 12-hour light and 12-hour dark cycles (LD 12:12), seemingly in relation with highest mitotic rate of hair follicle cells in the morning (Citation130). Radiation therapy combined with topotecan chemotherapy displayed better efficacy against xenografted human tumours during the activity rather than the rest span of the rest–activity cycle in mice kept in LD 12:12 conditions (Citation131). However, the few published reports in humans suggest that optimal timing could depend upon the irradiated region or volume, as well as patient sex and/or smoking habits. Thus both rate and grade of diarrhoea were higher in women receiving radiation therapy for cervical cancer in the morning as compared to the evening according to a randomized study (Citation132). Conversely, radiotherapy was associated with less oral mucositis and less body weight loss if it was applied in the morning rather than in the afternoon or in the evening, according to another randomized clinical trial involving a large majority of male patients with lifestyle-related head and neck cancer. Such a chronoradiotherapy effect was related to the higher rate of oral mucosa cells in the radiosensitive G2-M phase of the cell cycle in the late afternoon as compared to the early morning (Citation133,Citation134). This finding was confirmed in another trial involving patients with the same cancer type (Citation135). Similarly, improved skin tolerability was demonstrated in women undergoing adjuvant radiation therapy before 10.00 rather than after 15.00 after breast cancer surgery according to retrospective analysis of nearly 400 women (Citation136). While radiation timing modified healthy tissues tolerability in these four trials, no difference was found for efficacy.
In contrast, a retrospective analysis of the relevance of timing for gamma knife radiosurgery for brain metastasis of non-small cell lung cancer revealed improved outcomes in patients irradiated in the morning as compared to those treated in the afternoon, independent of other factors (Citation137). However, another retrospective study in a more than four times larger patient population treated with the same technique for the same medical indication, treatment time failed to influence local control and overall survival on multivariate analysis (Citation138). Prospective studies are needed to arbitrate between these contradictory findings.
Although not designed to be circadian-based, hyperfractionated radiotherapy, which delivers two or three fractions per day of radiation as the treatment of unresectable lung cancer, induced longer overall survival as compared to a single daily dose (Citation139,Citation140). Moreover, hyperfractionation in this setting was well tolerated, with the exception of increased acute oesophageal toxicity. Similar results have been obtained in the treatment of head and neck cancer with hyperfractionated radiotherapy (Citation141,Citation142). Therefore, it may be hypothesized that hyperfractionation gives radiotherapy the opportunity to act during an optimal period of the circadian clock to produce better clinical benefits with acceptable toxicity.
Precision oncology, personalized medicine, and individualized chronotherapeutics
Both experimental evidence and clinical findings demonstrate the disruptive effects of cytotoxic chemotherapy on the CTS (Citation1,Citation85,Citation143–149). Current anticancer treatments are being developed to halt tumour growth through targeting defects in cancer genetics (Citation150). Such precision oncology complements that of personalized medicine, in which drug doses are adapted to the metabolism phenotype of each individual patient. A pioneering example of personalized oncology is the pharmacokinetically based dose optimization of the antimetabolite 5-fluorouracil (5-FU). Indeed, the drug doses that achieved a clinically effective and safe blood concentration varied more than 3-fold among individual patients (Citation151). Moreover, similarly large differences in blood 5-FU concentrations were observed in most patients as a function of time of day or night, despite constant-rate infusion (Citation152). Thus, the extent of the 24-h changes in 5-FU pharmacokinetics is similar to that resulting from interindividual differences (Citation153) and warrant circadian-based chronotherapeutic delivery. However, the circadian phase of each patient, as estimated by the computed acrophase of physical activity, varies by up to 10 h among individual patients (). Thus the determination of the circadian phase in individual patients through the monitoring of relevant circadian biomarkers could drive the personalization of chronomodulated chemotherapy delivery schedules and improve treatment effects (Citation1,Citation31). Moreover, both anticancer drugs and supportive medications can modify CTS function in cancer patients (Citation31,Citation143,Citation153). This further supports the need for continuous monitoring of circadian rhythms and dynamic treatment adjustments ().
Optimization of cancer therapeutics with chronotherapy
The chronomodulated schedules used in clinical research trials and in daily practice have been derived from experiments performed on male mice. These studies revealed large differences in tolerability and efficacy according to dosing time or chronomodulated delivery schedule (Citation1,Citation31). Here, we focus on the newly described relationship between chronomodulated chemotherapy and toxicity with regard to patient outcomes.
Toxicity and efficacy
The current paradigm of conventional chemotherapy is to increase efficacy through dose increment until specific toxicities occur (Citation154). Thus, toxicity is regarded as a surrogate end-point for sufficient drug dosing for the individual patient. This approach has proven its relevance for both cytotoxic and targeted drugs (Citation155,Citation156). However, this positive association between toxicity and better outcomes does not apply to circadian-based chemotherapy (Citation1,Citation157,Citation158). Indeed, the patients who experienced better tolerability on chronotherapy displayed prolonged survival as compared to those who displayed toxicity. Thus, overall survival was influenced by significant interactions between chemotherapy schedule and treatment-induced severe neutropenia, clinically relevant fatigue, body weight loss, and subjective sleep complaints (). The patients who did not experience these toxicities displayed longer survival when treated with chronotherapy as compared to conventional chemotherapy () (Citation160–162). The therapeutic index of chronotherapy can therefore be increased through optimal dose and timing, so as to enhance tolerability and efficacy jointly (Citation1). This does not hold true for conventional chemotherapy, for which the occurrence of toxicity constitutes both a sought-after effect yet a limiting factor for dose increase and patient well-being. This observed difference is likely due to the disruptive effect of chemotherapy on the CTS, which we identified as a novel pharmacologic end-point of anticancer drugs (Citation143). Thus CTS disruption ablates the mechanistic rationale for chronotherapeutics and deteriorates outcomes both for healthy tissues (severe toxicity) and cancer cells (poor efficacy).
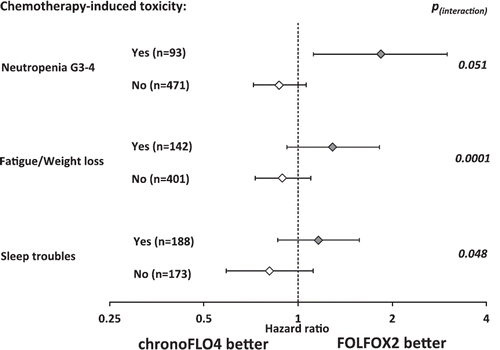
Figure 6. Schedule-specific relation between chemotherapy-induced toxicity and overall survival in patients with metastatic colorectal cancer. Divergent association between chemotherapy-induced toxicity and overall survival according to the delivery schedule of oxaliplatin, 5-fluorouracil/leucovorin, as first-line treatment (Citation159). Forest plot shows that the chronomodulated administration of this combination chemotherapy (chronoFLO4) is associated with longer survival in case of good tolerability (no toxicity, white diamonds), whereas the occurrence of toxicity (yes toxicity, grey diamonds) predicts better outcome on conventional chemotherapy delivery (FOLFOX2). Despite the fact that the 95% confidence limits for hazard ratios usually cross 1, the interactions, tested with univariate Cox proportional hazards model, were statistically significant for fatigue/weight loss and sleep troubles, or very close to statistical significance (P = 0.051 for severe neutropenia). Redrawn with data from (Citation157–158).
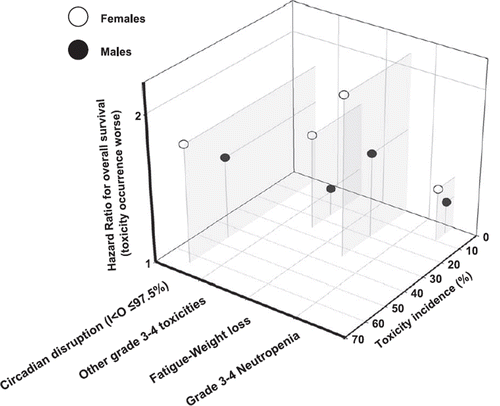
The negative association between chronotherapy-induced toxicity and overall survival could also partly explain the gender-related differences in chronotherapy efficacy against colorectal cancer (Citation122,Citation159). Indeed, whereas with conventional chemotherapy no obvious difference in outcomes was found according to gender, chronomodulated chemotherapy was associated with greater efficacy in men as compared to women (Citation6,Citation123). Thus, in a meta-analysis involving 842 patients with metastatic colorectal cancer, chronotherapy increased median overall survival by more than 3 months as compared to conventional delivery in men, while in women chronotherapy reduced survival by almost 2 months. This translated into a more than 4 month median survival difference according to gender in patients receiving chronomodulated chemotherapy (Citation122). Toxicity was more common in women as compared to men on either treatment modality. Moreover, the sex-related difference was more prominent for chronotherapy (85,157–159). Thus, the incidence of adverse events in women as compared to men was almost twice as high among the patients on chronotherapy. Moreover, the occurrence of clinically relevant toxicity on chronotherapy was even more detrimental for overall survival in women as compared to men, as evidenced by a larger hazard ratio for earlier death associated with toxicity occurrence in women as compared to men (). The excessive toxicity observed in women could be due to both excessive dosing and inadequate timing of administration. These hypotheses are supported by sex-related differences in the metabolism of drugs, such as 5-FU (Citation9,Citation160). Moreover, in a study testing lagged peak timings of chronomodulated infusion, an inconsistent pattern of toxicity was observed in women as compared to men, for whom the least toxic chemotherapy timing was that currently used in the chronotherapy protocols (Citation161).
Figure 7. Sex-related moderation of the relations between tolerance and efficacy of chronotherapy. Sex-related differences in the incidence (z-axis) and negative prognostic effect for overall survival (Cox's model; y-axis) of chemotherapy-induced toxicities in patients with metastatic colorectal cancer treated with chronoFLO4. Note that females (white circles) consistently display high incidence of toxicity as compared to males (black dots). Furthermore, the hazard ratio for an earlier death induced by toxicity is consistently higher in women as compared to men. Redrawn from (Citation85,Citation157,Citation158).
Circadian synchronization: therapeutic approaches
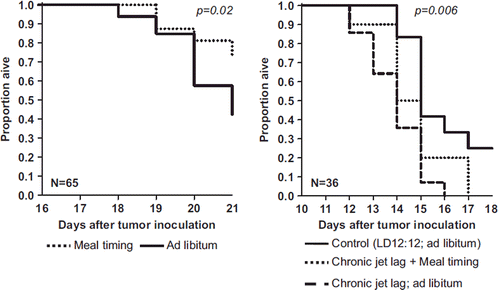
Circadian disruption involves both sleep inefficiency at night and fatigue/lack of physical activity during the day. Thus improving circadian synchronization would involve interventions aimed at improving sleep at night and physical activity during the day. Numerous factors are responsible for the development of sleep disruption among cancer patients, including stress associated with the diagnosis and its treatment, and side effects of cytotoxic chemotherapy. However, the mechanisms behind the development of sleep alterations in cancer patients are not well understood (Citation162,Citation163). Indeed, both sleep alterations and dysregulation of the HPA axis are common among cancer patients and survivors (Citation117,Citation164). In addition, women with metastatic breast cancer have flattened diurnal cortisol rhythm (Citation62,Citation164), which is predictive of shorter survival among those with metastatic breast (Citation64), ovarian (Citation62), and lung (Citation65) cancers. The flattening of diurnal cortisol rhythms is associated with the number of patient-reported awakenings during the night (Citation64). Consequently, many cancer patients with advanced disease spend a significant amount of time awake during the night and asleep during the day (Citation165). These behavioural abnormalities are also associated with immune dysregulation, including cytokine effects on brain function (Citation98). Poor sleep related to shift-work is associated with night-time light exposure and reduced melatonin levels. Melatonin has anti-oxidant and free radical scavenger properties, so its suppression with night-time light exposure may contribute to the increase in cancer incidence among shift-workers (Citation53). Disturbed sleep is also associated with decrements in the functioning of natural killer cells, which are known to be crucial to breast cancer surveillance and control (Citation166). In addition, disrupted sleep among cancer patients is associated with loss of vagal tone during the day, restricting the person's ability to self-soothe physiologically in the face of stress (Citation167). Disrupted sleep can cause or worsen fatigue, increase susceptibility to develop depressive and anxiety disorders, and may also reduce social support from spouses/partners (Citation168). Sleep problems are thus associated with poorer quality of life (Citation169). These data suggest that improving sleep efficiency and synchronization of circadian rhythms would improve quality of life and potentially survival time among cancer patients (Citation116,Citation170). While clinical trials have been conducted to improve sleep and enhance exercise among cancer patients, little attention has been paid to co-ordinated interventions designed to normalize circadian rhythms by both enhancing physical activity during the day and improving sleep at night. Even though there is no clinical proof of survival prolongation induced by circadian synchronization, experimental evidence shows that increasing the robustness of circadian function in tumour-bearing mice through scheduled meal timing slows cancer growth () (Citation16,Citation171).
Figure 8. Effect of circadian synchronization on tumour growth rate: experimental murine data. Kaplan–Meier survival curves showing the beneficial effect of meal timing, which reinforces the functioning of the CTS in mice with inoculated tumour cell lines. Left panel: mice, inoculated with P03 pancreatic adenocarcinoma, were synchronized with an alternation of 12 hours of light and 12 hours of darkness (LD 12:12), and fed either ad libitum (control, solid line) or only during the light span (usual rest phase, dotted line). Mice were sacrificed when tumour size reached 1.5 g. Right panel: mice inoculated with Glasgow osteosarcoma were either maintained in the same usual light–dark and ad libitum feeding conditions (control, solid line), or exposed to iterative light–dark schedule alterations that mimic chronic jet lag, thus producing circadian disruption, jointly with ad libitum feeding (dashed line) or meal timing (dotted line). Mice were sacrificed when tumour size reached 2 g. Results from log-rank test. Redrawn with data from (Citation16,Citation171).
Circadian synchronization through regulation of the sleep–wake cycle
Cognitive behavioural therapy for insomnia (CBT-I) is the gold standard for management of sleep disruption in the general population (Citation172,Citation173). CBT-I is a seven-session therapist-led intervention that includes stimulus control (instructions for behaviour modification), sleep education (sleep hygiene), sleep restriction (sleep scheduling), and management of maladaptive cognitions pertaining to sleep. Several randomized clinical trials conducted in cancer survivors showed that CBT-I can successfully improve sleep disruption (insomnia) in that population as well (Citation174,Citation175). Unlike medication for sleep, where the improvements are gone when the medication is no longer taken, the improvements associated with behavioural therapy are long-lasting. Specially modified versions of CBT-I have been used for patients diagnosed with cancer who are undergoing cancer treatments. The first trial, which utilized a non-specific sleep intervention (Citation176), showed no impact, but two other clinical trials (Citation177,Citation178) demonstrated that patients who participated in a brief behavioural sleep intervention modelled on CBT-I reported improvements in their sleep disturbance compared to patients who were in control conditions. While CBT-I and behavioural interventions based on the CBT-I model are effective for reducing sleep disruption in cancer, it is currently unknown whether management of sleep disturbance through behavioural therapy can indeed improve circadian rhythms, although one study showed that cancer patients who were in the behavioural intervention group for sleep had more robust circadian rhythms compared to patients who were in the healthy eating control condition (Citation179).
Circadian synchronization through physical activity
There is a growing body of research identifying psychosocial and physiological benefits that regular physical activity offers to patients diagnosed with cancer. For example, exercise helps to improve mood and decrease anxiety (Citation180) and more generally enhance quality of life (Citation181). While fatigue may discourage exercise, various studies have found that physical activity is associated with a decrease in cancer-related fatigue (Citation182). Exercise is also linked to improvement in body image, which is adversely affected by cancer and its treatment (Citation183). Several studies among cancer patients have found a positive association between physical activity, such as walking the equivalent of 3 to 5 hours per week at a moderate pace, and survival (Citation184,Citation185). Research suggests that physical activity can be beneficial for sleep and the circadian system (Citation190). However, the circadian system response to exercise is certainly mediated by exercise dosage, duration, intensity, mode, fitness level, and timing. In relation to timing, the data are conflicting—exercising during the morning has been shown to improve sleep, but exercising in the middle of the day or early evening can also provide sleep benefits (Citation186). Interestingly, exercise at high intensity and frequency (multiple times a day) can actually negatively impact sleep quality and duration (Citation186). For cancer patients and survivors, participating in various forms of physical activity is linked to improvements in sleep, quality of life, and side effects (Citation187,Citation188). For example, a recent randomized clinical trial of a hatha yoga intervention in 410 cancer survivors (YOCAS®) showed that a 4-week long (twice a week, 90-minute duration) yoga intervention can improve sleep quality, reduce sleep medication usage, and help regulate circadian rhythms (Citation188).
Circadian synchronization through light therapy
Light is a major synchronizer of the circadian timing system (Citation189). Light therapy has been used for management of SAD (seasonal affective disorder) and depression with success (Citation10,Citation190). More recently Ancoli-Israel and colleagues have been conducting clinical studies showing that bright light therapy is effective for prevention of circadian disruption and fatigue in women diagnosed with cancer undergoing chemotherapy (Citation146,Citation191).
Circadian synchronization through timed meals
The circadian timing system also helps regulate metabolic homeostasis. Circadian disruption leads to loss of rhythms in key clock peripheral genes and is associated with metabolic dysregulation, including obesity, diabetes, and high blood pressure (Citation60,Citation192). Studies show that delaying meal-time or having a meal during the night creates a misalignment between peripheral clocks and the SCN as eating only shifts the phase of the peripheral clocks (Citation193,Citation194). In animals, scheduled feeding can restore the peripheral rhythms that are missing and reverse the negative effects of circadian uncoupling (Citation8,Citation16,Citation171,Citation195,Citation196). Research is needed to understand whether scheduled meal-time can restore circadian synchronization in people as well. No studies to date have been conducted in cancer patients and survivors on circadian synchronization through timed meals.
Circadian synchronization through social rhythms
Social and family interactions constitute important circadian synchronizers in humans () (Citation11,Citation197). Social effects on sleep and activity patterns are known as social zeitgebers, and the social influence on regularity of sleep is associated with better sleep (Citation198,Citation199). Behavioural interventions aimed at reducing disruption in social rhythms have proven clinical effectiveness for the treatment of mood disorders (Citation200). This therapeutic approach should be tested in cancer patients, as no data exist in the oncology setting. Moreover, strengthening social interactions in cancer patients could have the potential of increasing overall survival, since poor social functioning has been shown to be an independent negative prognostic factor in metastatic colorectal cancer (Citation201,Citation202). A recent study involving 734,889 cancer patients from the SEER (Surveillance, Epidemiology and End Results) registry demonstrated that those who were married had significantly longer survival with 10 different cancers, independent of other risk factors (Citation203).
Circadian synchronization through pharmacological agents
Several drugs, available or in the development pipeline, have the property of affecting the circadian timing system () (Citation204–206). Such agents with chronobiotic effects could target the central pacemaker, the signalling pathways from the SCN to other brain or peripheral tissues, or directly the molecular circadian clock and/or clock-controlled pathways.
Table II. Summary of the potential pharmacological or behavioural therapeutic approaches for restoring robustness of the circadian timing system of cancer patients.
Melatonin and its agonists exert their effect on the SCN, whereas glucocorticoids exert their effect mostly upon peripheral tissues () (Citation207,Citation208). Recently, a novel, delayed-release formulation of prednisone has demonstrated an increase in its therapeutic index by better mimicking the physiological circadian rhythm of cortisol, with an early morning peak (Citation209). Conversely, higher nocturnal levels of melatonin can be maintained using a sustained-release formulation, with hypnotic properties as well (Citation210). However, fast-acting melatonin could also induce a phase shift the circadian clock (Citation73). For both hormones, scarce data exist on their use as synchronizers in the oncology setting (Citation6). A recent study in patients with advanced breast cancer showed an effect of bedtime melatonin on subjective and objective sleep, but not on circadian patterns.
Novel drugs directly modulating the function of the molecular clock, by altering its phase, period, or both (Citation204–206), have the potential for more fine-tuned targeting of the CTS, possibly selectively affecting tumour cells with specific molecular alterations. Moreover, given the molecular feedback between the circadian clock and several pharmacologically targetable cellular pathways, it is likely that various commonly used drugs could affect the CTS (Citation211).
Future directions
Going forward, more research is needed to develop novel multipronged approaches to the management of circadian disruption. Addressing day-time behaviour (physical activity) and night-time (sleep scheduling) through an integrated approach will potentially create a synergistic effect in managing sleep and circadian disruption in cancer patients and survivors. Moreover, a dynamic and continuous monitoring of circadian rhythms, made possible by technological progress, would benefit from real-time computer-assisted feedback, allowing constant improvement of CTS function in cancer patients. Thus, a dynamic and personalized approach including an integrated genetic and physiological evaluation of cancer patients should provide substantial improvement of the efficacy and safety of circadian-based chemotherapy. Initial development has both highlighted critically important achievements, and identified those obstacles yet to be surmounted for further optimization and routine implementation of chronotherapy (Citation1). The latter will clearly benefit from the use of information and communication technologies and mathematical modelling in order to link circadian biomarker assessments to personalized chronotherapy delivery, as already suggested in the ongoing European project InCASA and the national French project PiCADo. Expectedly, circadian-based drug delivery will shortly involve innovative telecommunicating perfusion devices for intravenous agents and novel modified- and chronomodulated-release formulations of oral drugs (Citation6). Taking circadian regulation into account as part of all aspects of cancer treatment, from chemo- and radiotherapies, to programmes of diet, sleep, and exercise, in a personalized and precise manner, has indeed the potential greatly to improve both quality and quantity of life for those with cancer.
Declaration of interest: The authors report no conflicts of interest.
References
- Levi F, Okyar A, Dulong S, Innominato PF, Clairambault J. Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol. 2010; 50:377–421.
- Dibner C, Schibler U, Albrecht U. The Mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49.
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62.
- Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet. 2010;44:419–44.
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–97.
- Innominato PF, Levi FA, Bjarnason GA. Chronotherapy and the molecular clock: clinical implications in oncology. Adv Drug Deliv Rev. 2010;62:979–1001.
- Reddy AB, O’Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44.
- Shibata S, Tahara Y, Hirao A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv Drug Deliv Rev. 2010;62:918–27.
- Lévi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628.
- Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. Handb Exp Pharmacol. 2013;(217):311–31.
- Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:293–9.
- Moore RY. The suprachiasmatic nucleus and the circadian timing system. Prog Mol Biol Transl Sci. 2013;119:1–28.
- Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–12.
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–22.
- Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–7.
- Filipski E, Innominato PF, Wu M, Li XM, Iacobelli S, Xian LJ, et al. Effects of light and food schedules on liver and tumor molecular clocks in mice. J Natl Cancer Inst. 2005;97:507–17.
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–85.
- Filipski E, Levi F. Circadian disruption in experimental cancer processes. Integr Cancer Ther. 2009;8:298–302.
- Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Handb Exp Pharmacol. 2013;(217):45–66.
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705.
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104:3342–7.
- Lévi F, Filipski E, Iurisci I, Li XM, Innominato P. Cross-talks between circadian timing system and cell division cycle determine cancer biology and therapeutics. Cold Spring Harb Symp Quant Biol. 2007; 72:465–75.
- Hunt T, Sassone-Corsi P. Riding tandem: circadian clocks and the cell cycle. Cell. 2007;129:461–4.
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–9.
- Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A. 2012;109:11758–63.
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22: 375–82.
- Gery S, Koeffler HP. The role of circadian regulation in cancer. Cold Spring Harb Symp Quant Biol. 2007;72:459–64.
- Fu L, Kettner NM. The circadian clock in cancer development and therapy. Prog Mol Biol Transl Sci. 2013;119:221–82.
- Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. Handb Exp Pharmacol. 2013;(217):3–27.
- O’Neill JS, Maywood ES, Hastings MH. Cellular mechanisms of circadian pacemaking: beyond transcriptional loops. Handb Exp Pharmacol. 2013;(217):67–103.
- Ortiz-Tudela E, Mteyrek A, Ballesta A, Innominato PF, Lévi F. Cancer chronotherapeutics: experimental, theoretical, and clinical aspects. Handb Exp Pharmacol. 2013;(217):261–88.
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–76.
- Kornmann B, Schaad O, Reinke H, Saini C, Schibler U. Regulation of circadian gene expression in liver by systemic signals and hepatocyte oscillators. Cold Spring Harb Symp Quant Biol. 2007;72:319–30.
- Minami Y, Ode KL, Ueda HR. Mammalian circadian clock: the roles of transcriptional repression and delay. Handb Exp Pharmacol. 2013;(217):359–77.
- Ukai H, Ueda HR. Systems biology of mammalian circadian clocks. Annu Rev Physiol. 2010;72:579–603.
- Ueda HR. Systems biology of mammalian circadian clocks. Cold Spring Harb Symp Quant Biol. 2007;72:365–80.
- Delaunay F, Laudet V. Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet. 2002;18:595–7.
- Mauvoisin D, Wang J, Jouffe C, Martin E, Atger F, Waridel P, et al. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc Natl Acad Sci U S A. 2014;111:167–72.
- Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–9.
- Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem. 2008;283:4535–42.
- Dallmann R, Brown SA, Gachon F. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol. 2014; 54:339–61.
- Paschos GK, Baggs JE, Hogenesch JB, FitzGerald GA. The role of clock genes in pharmacology. Annu Rev Pharmacol Toxicol. 2010; 50:187–214.
- Mormont MC, Levi F. Cancer chronotherapy: principles, applications, and perspectives. Cancer. 2003;97:155–69.
- Levi F. Circadian chronotherapy for human cancers. Lancet Oncol. 2001;2:307–15.
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24.
- Bertrand PP, Polglaz KE, Bertrand RL, Sandow SL, Pozo MJ. Detection of melatonin production from the intestinal epithelium using electrochemical methods. Curr Pharm Des. 2013 Nov 18. [Epub ahead of print]
- Li XM, Liu XH, Filipski E, Metzger G, Delagrange P, Jeanniot JP, et al. Relationship of atypical melatonin rhythm with two circadian clock outputs in B6D2F(1) mice. Am J Physiol Regul Integr Comp Physiol. 2000;278:R924–30.
- Chang A-M, Santhi N, St Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, et al. Human responses to bright light of different durations. J Physiol. 2012;590(Pt 13):3103–12.
- Sainz RM, Mayo JC, Tan D, León J, Manchester L, Reiter RJ. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005;63:29–43.
- Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014:64:207–18.
- Blask DE, Dauchy RT, Brainard GC, Hanifin JP. Circadian stage-dependent inhibition of human breast cancer metabolism and growth by the nocturnal melatonin signal: consequences of its disruption by light at night in rats and women. Integr Cancer Ther. 2009;8:347–53.
- Lahti T, Merikanto I, Partonen T. Circadian clock disruptions and the risk of cancer. Ann Med. 2012;44:847–53.
- Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17:273–84.
- Mormont MC, Langouet AM, Claustrat B, Bogdan A, Marion S, Waterhouse J, et al. Marker rhythms of circadian system function: a study of patients with metastatic colorectal cancer and good performance status. Chronobiol Int. 2002;19:141–55.
- Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010;21:277–86.
- Kudielka BM, Federenko IS, Hellhammer DH, Wüst S. Morningness and eveningness: the free cortisol rise after awakening in “early birds” and “night owls”. Biol Psychol. 2006;72:141–6.
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73.
- Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease?Brain Behav Immun. 2003;17:321–8.
- Eismann EA, Lush E, Sephton SE. Circadian effects in cancer-relevant psychoneuroendocrine and immune pathways. Psychoneuroendocrinology. 2010;35:963–76.
- Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119: 283–323.
- Kalsbeek A, Fliers E. Daily regulation of hormone profiles. Handb Exp Pharmacol. 2013;(217):185–226.
- Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, et al. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010; 116:4410–9.
- Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–7.
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000.
- Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl):S163–70.
- Mormon MC, Bogdan A, Cormont S, Touitou Y, Levi F. Cortisol diurnal variation in blood and saliva of patients with metastatic colorectal cancer: relevance for clinical outcome. Anticancer Res. 2002;22:1243–9.
- Spiegel D, Dugué P-A, Innominato PF, Karaboué A, Dispersyn G, Parganiha A, et al. Circadian rest-activity rhythm as a predictor of survival in metastatic colorectal cancer. 2012 ASCO Annual Meeting. J Clin Oncol. 2012;30:(suppl; abstr e14006).
- Levi F, Parganiha A, Karaboué A, Innominato PF, Dispersyn G, Giacchetti S, et al. Circadian robustness, an independent predictor of prolonged progression-free survival (PFS) and overall survival (OS) in 436 patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2012;30:(suppl 4; abstr 464).
- Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MA, Madrid JA. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput Biol. 2010;6:e1000996.
- Roche V, Mohamad-Djafari A, Innominato PF, Karaboue A, Gorbach AM, Lévi FA. Thoracic surface temperature rhythms as circadian biomarkers for cancer chronotherapy. Chronobiol Int. 2014;31:409–20.
- Waterhouse J, Fukuda Y, Morita T. Daily rhythms of the sleep-wake cycle. J Physiol Anthropol. 2012;31:5.
- Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. 2012;31:14.
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–102.
- Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–83.
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54.
- Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–8.
- Teunissen LPJ, de Haan A, de Koning JJ, Daanen HAM. Telemetry pill versus rectal and esophageal temperature during extreme rates of exercise-induced core temperature change. Physiol Meas. 2012;33:915–24.
- Scully CG, Karaboue A, Liu WM, Meyer J, Innominato PF, Chon KH, et al. Skin surface temperature rhythms as potential circadian biomarkers for personalized chronotherapeutics in cancer patients. Interface Focus. 2011;1:48–60.
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92.
- Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36:191–9.
- Hall JC, Chang DC, Dolezelova E. Principles and problems revolving around rhythm-related genetic variants. Cold Spring Harb Symp Quant Biol. 2007;72:215–32.
- Minors D, Akerstedt T, Atkinson G, Dahlitz M, Folkard S, Levi F, et al. The difference between activity when in bed and out of bed. I. Healthy subjects and selected patients. Chronobiol Int. 1996;13: 27–34.
- Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–45.
- Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, et al. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 2009;69:4700–7.
- Innominato PF, Giacchetti S, Bjarnason GA, Focan C, Garufi C, Coudert B, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer. 2012;131:2684–92.
- Martinez-Nicolas A, Ortiz-Tudela E, Madrid JA, Rol MA. Crosstalk between environmental light and internal time in humans. Chronobiol Int. 2011;28:617–29.
- Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6:407–14.
- Van den Heiligenberg S, Deprés-Brummer P, Barbason H, Claustrat B, Reynes M, Lévi F. The tumor promoting effect of constant light exposure on diethylnitrosamine-induced hepatocarcinogenesis in rats. Life Sci. 1999;64:2523–34.
- Filipski E, Subramanian P, Carriere J, Guettier C, Barbason H, Levi F. Circadian disruption accelerates liver carcinogenesis in mice. Mutat Res. 2009;680:95–105.
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50.
- Filipski E, Li XM, Levi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control. 2006;17:509–14.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100: 57–70.
- Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–32.
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, et al. Considerations of circadian impact for defining “shift work” in cancer studies: IARC Working Group Report. Occup Env Med. 2011;68:154–62.
- Stevens RG. Working against our endogenous circadian clock: breast cancer and electric lighting in the modern world. Mutat Res. 2009;679:6–8.
- Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(Suppl 1):35–42.
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–82.
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–77.
- Bower JE. Prevalence and causes of fatigue after cancer treatment: the next generation of research. J Clin Oncol. 2005;23:8280–2.
- Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19:313–23.
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100.
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–40.
- Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–64.
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–66.
- Cormie P, Newton RU, Taaffe DR, Spry N, Galvão DA. Exercise therapy for sexual dysfunction after prostate cancer. Nat Rev Urol. 2013;10:731–6.
- Van Gerpen RE, Becker BJ. Development of an evidence-based exercise and education cancer recovery program. Clin J Oncol Nurs. 2013;17:539–43.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20:17–25.
- Payne JK. Altered circadian rhythms and cancer-related fatigue outcomes. Integr Cancer Ther. 2011;10:221–33.
- Innominato PF, Mormont MC, Rich TA, Waterhouse J, Levi FA, Bjarnason GA. Circadian disruption, fatigue, and anorexia clustering in advanced cancer patients: implications for innovative therapeutic approaches. Integr Cancer Ther. 2009;8:361–70.
- Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, et al. Prevalence, putative mechanisms and management of sleep problems during chemotherapy. Nat Sci Sleep. 2012;4:151–62.
- Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–8.
- Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908.
- Palesh OG, Collie K, Batiuchok D, Tilston J, Koopman C, Perlis ML, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44.
- Palesh O, Aldridge Gerry A, Zeitzer JM, Koopman C, Jo B, Neri E, et al. Actigraphy measured sleep disruption as a predictor of survival in advanced breast cancer. 2013 ASCO Annual Meeting. J Clin Oncol. 2013;31:(suppl; abstr 9532).
- Spiegel D. Losing sleep over cancer. J Clin Oncol. 2008;26:2431–2.
- Innominato PF, Palesh O, Dhabhar FS, Levi F, Spiegel D. Regulation of circadian rhythms and hypothalamic-pituitary-adrenal axis: an overlooked interaction in cancer. Lancet Oncol. 2010;11:816–17.
- Cohen L, Cole SW, Sood AK, Prinsloo S, Kirschbaum C, Arevalo JM, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7:e42324.
- Palesh O, Bjarnason G, Deguzman C, Haitz K, Giacchetti S, Spiegel D, et al. Subjective sleep problems predict survival in 240 patients with metastatic colorectal cancer. 2013 MASCC Annual Meeting. Berlin, Germany; June 27–29, 2013.
- Hahm B, Jo B, Dhabhar F, Palesh O, Aldridge-Gerry A, Bajestan S, et al. Bedtime misalignment and progression of breast cancer. Chronobiol Int. 2014;31:214–21.
- Coudert B, Focan C, Donato di Paola E, Levi F. It's time for chronotherapy!Eur J Cancer. 2002;38(Suppl 4):S50–3.
- Giacchetti S, Dugue PA, Innominato PF, Bjarnason GA, Focan C, Garufi C, et al. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Ann Oncol. 2012;23:3110–6.
- Ahowesso C, Li XM, Zampera S, Peteri-Brunbäck B, Dulong S, Beau J, et al. Sex and dosing-time dependencies in irinotecan-induced circadian disruption. Chronobiol Int. 2011;28:458–70.
- Li X-M, Mohammad-Djafari A, Dumitru M, Dulong S, Filipski E, Siffroi-Fernandez S, et al. A circadian clock transcription model for the personalization of cancer chronotherapy. Cancer Res. 2013;73:7176–88.
- Lévi F, Zidani R, Misset JL. Randomized multicentre trial of chronotherapy with oxaliplatin, fluorouracil and folinic acid in metastatic colorectal cancer. Lancet. 1997;350:681–6.
- Levi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 1994;86:1608–17.
- Ohdo S. Chronopharmacology focused on biological clock. Drug Metab Pharmacokinet. 2007;22:3–14.
- Musiek ES, Fitzgerald GA. Molecular clocks in pharmacology. Handb Exp Pharmacol. 2013;(217):243–60.
- Sancar A, Lindsey-Boltz LA, Kang T-H, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010;584:2618–25.
- Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci U S A. 2013;110:E2106–15.
- Zhang Y, Chen X, Ren P, Su Z, Cao H, Zhou J, et al. Synergistic effect of combination topotecan and chronomodulated radiation therapy on xenografted human nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;87:356–62.
- Shukla P, Gupta D, Bisht SS, Pant MC, Bhatt ML, Gupta R, et al. Circadian variation in radiation-induced intestinal mucositis in patients with cervical carcinoma. Cancer. 2010;116:2031–5.
- Bjarnason GA, Mackenzie RG, Nabid A, Hodson ID, El-Sayed S, Grimard L, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). Int J Radiat Oncol Biol Phys. 2009;73:166–72.
- Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol. 1999;154:613–22.
- Goyal M, Shukla P, Gupta D, Bisht SS, Dhawan A, Gupta S, et al. Oral mucositis in morning vs. evening irradiated patients: a randomised prospective study. Int J Radiat Biol. 2009;85:504–9.
- Noh JM, Choi DH, Park H, Huh SJ, Park W, Seol SW, et al. Comparison of acute skin reaction following morning versus late afternoon radiotherapy in patients with breast cancer who have undergone curative surgical resection. J Radiat Res. 2014;55:553–8.
- Rahn DA, Ray DK, Schlesinger DJ, Steiner L, Sheehan JP, O’Quigley JM, et al. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment?Cancer. 2011;117:414–20.
- Badiyan SN, Ferraro DJ, Yaddanapudi S, Drzymala RE, Lee AY, Silver SA, et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer. 2013;119:3563–9.
- Belani CP, Wang W, Johnson DH, Wagner H, Schiller J, Veeder M, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lu. J Clin Oncol. 2005;23:3760–7.
- Mauguen A, Le Péchoux C, Saunders MI, Schild SE, Turrisi AT, Baumann M, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol. 2012;30:2788–97.
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–54.
- Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16.
- Ortiz-Tudela E, Iurisci I, Beau J, Karaboue A, Moreau T, Rol de Lama MA, et al. The circadian rest-activity rhythm, a potential safety pharmacology endpoint of cancer chemotherapy. Int J Cancer. 2014; 134:2717–25.
- Savard J, Liu L, Natarajan L, Rissling MB, Neikrug AB, He F, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32:1155–60.
- Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs Forum. 2010;37:E359–69.
- Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer. 2012;20: 1211–19.
- Li XM, Vincenti M, Levi F. Pharmacological effects of vinorelbine on body temperature and locomotor activity circadian rhythms in mice. Chronobiol Int. 2002;19:43–55.
- Li XM, Kanekal S, Crepin D, Guettier C, Carriere J, Elliott G, et al. Circadian pharmacology of L-alanosine (SDX-102) in mice. Mol Cancer Ther. 2006;5:337–46.
- Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nat Med. 2001;7:356–60.
- Morere JF. Oncology in2012: from personalized medicine to precision medicine. Target Oncol. 2012;7:211–12.
- Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–105.
- Petit E, Milano G, Levi F, Thyss A, Bailleul F, Schneider M. Circadian rhythm-varying plasma concentration of 5-fluorouracil during a five-day continuous venous infusion at a constant rate in cancer patients. Cancer Res. 1988;48:1676–9.
- Innominato P, Lévi F, Bjarnason G. Chronotherapy and the molecular clock: clinical implications in oncology. Adv Drug Deliv Rev. 2010; 62:979–1001.
- Felici A, Verweij J, Sparreboom A. Dosing strategies for anticancer drugs: the good, the bad and body-surface area. Eur J Cancer. 2002;38:1677–84.
- Shitara K, Matsuo K, Oze I, Mizota A, Kondo C, Nomura M, et al. Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol. 2011;68:301–7.
- Rini BI, Melichar B, Ueda T, Grünwald V, Fishman MN, Arranz JA, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol. 2013;14:1233–42.
- Innominato PF, Giacchetti S, Moreau T, Smaaland R, Focan C, Bjarnason GA, et al. Prediction of survival by neutropenia according to delivery schedule of oxaliplatin-5-Fluorouracil-leucovorin for metastatic colorectal cancer in a randomized international trial (EORTC 05963). Chronobiol Int. 2011;28:586–600.
- Innominato PF, Giacchetti S, Moreau T, Bjarnason GA, Smaaland R, Focan C, et al. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer. 2013;119:2564–73.
- Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Can. J Clin Oncol. 2006;24:3562–9.
- Chansky K, Benedetti J, Macdonald JS. Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer. 2005;103:1165–71.
- Levi F, Focan C, Karaboue A, de la Valette V, Focan-Henrard D, Baron B, et al. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv Drug Deliv Rev. 2007;59:1015–35.
- Costanzo ES, Stawski RS, Ryff CD, Coe CL, Almeida DM. Cancer survivors’ responses to daily stressors: implications for quality of life. Health Psychol. 2012;31:360–70.
- Palesh OG, Mustian KM, Peppone LJ, Janelsins M, Sprod LK, Kesler S, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med. 2012;13:1184–90.
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–92.
- Parker KP, Bliwise DL, Ribeiro M, Jain SR, Vena CI, Kohles-Baker MK, et al. Sleep/wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26: 2464–72.
- Levy S, Herberman R, Lippman M, d’Angelo T. Correlation of stress factors with sustained depression of natural killer cell activity and predicted prognosis in patients with breast cancer. J Clin Oncol. 1987;5:348–53.
- Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–9.
- Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18:163–76.
- Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat. 1996;39:261–73.
- Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864–6.
- Li XM, Delaunay F, Dulong S, Claustrat B, Zampera S, Fujii Y, et al. Cancer inhibition through circadian reprogramming of tumor transcriptome with meal timing. Cancer Res. 2010;70:3351–60.
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15.
- Harvey AG, Tang NKY. Cognitive behaviour therapy for primary insomnia: can we rest yet?Sleep Med Rev. 2003;7:237–62.
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–8.
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083–96.
- Barsevick A, Beck SL, Dudley WN, Wong B, Berger AM, Whitmer K, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manag. 2010;40:200–16.
- Berger AM, Kuhn BR, Farr LA, Von Essen SG, Chamberlain J, Lynch JC, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol. 2009;27:6033–40.
- Palesh O, Innominato PF, Mustian K, Janelsins M, Neri E, Koopman C, et al. Brief behavioral therapy for insomnia (BBT-I) in breat cancer during chemotherapy: a randomized pilot study. 2013 MASCC Annual Meeting. Berlin, Germany; 2013.
- Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18:105–14.
- Blacklock R, Rhodes R, Blanchard C, Gaul C. Effects of exercise intensity and self-efficacy on state anxiety with breast cancer survivors. Oncol Nurs Forum. 2010;37:206–12.
- Kendall AR, Mahue-Giangreco M, Carpenter CL, Ganz PA, Bernstein L. Influence of exercise activity on quality of life in long-term breast cancer survivors. Qual Life Res. 2005;14:361–71.
- Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–7.
- Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney-Tedder S, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 1994;21:899–907; discussion 908.
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86.
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41.
- Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4: 387–402.
- Mustian KM, Griggs JJ, Morrow GR, McTiernan A, Roscoe JA, Bole CW, et al. Exercise and side effects among 749 patients during and after treatment for cancer: a University of Rochester Cancer Center Community Clinical Oncology Program Study. Support Care Cancer. 2006;14:732–41.
- Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Palesh OG, Chandwani K, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233–41.
- Gronfier C, Wright KP, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004; 287:E174–81.
- Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11:509–22.
- Neikrug AB, Rissling M, Trofimenko V, Liu L, Natarajan L, Lawton S, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10:202–16.
- Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013;(217):127–55.
- Challet E. Circadian clocks, food intake, and metabolism. Prog Mol Biol Transl Sci. 2013;119:105–35.
- Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Togo F, et al. Influence of dietary behavior on the circadian rhythm of the autonomic nervous system as assessed by heart rate variability. Physiol Behav. 2013;118:122–8.
- Wu MW, Li XM, Xian LJ, Levi F. Effects of meal timing on tumor progression in mice. Life Sci. 2004;75:1181–93.
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3.
- Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc. 2004;79:533–56.
- Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry. 2000;48:593–604.
- Monk TH, Reynolds CF, Buysse DJ, DeGrazia JM, Kupfer DJ. The relationship between lifestyle regularity and subjective sleep quality. Chronobiol Int. 2003;20:97–107.
- Swartz HA, Frank E, O’Toole K, Newman N, Kiderman H, Carlson S, et al. Implementing interpersonal and social rhythm therapy for mood disorders across a continuum of care. Psychiatr Serv. 2011; 62:1377–80.
- Efficace F, Innominato PF, Bjarnason G, Coens C, Humblet Y, Tumolo S, et al. Validation of patient's self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and. J Clin Oncol. 2008;26:2020–6.
- Efficace F, Bottomley A, Coens C, Van Steen K, Conroy T, Schoffski P, et al. Does a patient's self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer?Eur J Cancer. 2006;42:42–9.
- Aizer AA, Chen M-H, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76.
- Schroeder AM, Colwell CS. How to fix a broken clock. Trends Pharmacol Sci. 2013;34:605–19
- Antoch MP, Kondratov RV. Pharmacological modulators of the circadian clock as potential therapeutic drugs: focus on genotoxic/anticancer therapy. Handb Exp Pharmacol. 2013;(217):289–309.
- Hirota T, Kay SA. High-throughput screening and chemical biology: new approaches for understanding circadian clock mechanisms. Chem Biol. 2009;16:921–7.
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–7.
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–7.
- Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet. 2008;371:205–14.
- Morris CJ, Aeschbach D, Scheer FAJL. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104.
- Zhang EE, Liu AC, Hirota T, Miraglia LJ, Welch G, Pongsawakul PY, et al. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell. 2009;139:199–210.
- Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14(3):201–9.
- Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manag. 2007;33: 398–409.
- Berger AM, Kuhn BR, Farr LA, Lynch JC, Agrawal S, Chamberlain J, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18:634–46.
- Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62.
- Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26: 1663–71.
- Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum. 2000;27:1443–8.
- Kuo HH, Chiu MJ, Liao WC, Hwang SL. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2006;105:64–9.
- Liu L, Rissling M, Natarajan L, Fiorentino L, Mills PJ, Dimsdale JE, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35:237–45.
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–13.
- Liu L, Rissling M, Neikrug A, Fiorentino L, Natarajan L, Faierman M, et al. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: a controlled study. Fatigue. 2013;1:12–26.
- Payne J, Piper B, Rabinowitz I, Zimmerman B. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: a pilot study. Oncol Nurs Forum. 2006;33:775–83.
- Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–36.
- Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manag. 1999;17:320–32.
- Sarna L, Conde F. Physical activity and fatigue during radiation therapy: a pilot study using actigraph monitors. Oncol Nurs Forum. 2001;28:1043–6.
- Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005;93:1202–8.
- Hinds PS, Hockenberry MJ, Gattuso JS, Srivastava DK, Tong X, Jones H, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110:2321–30.
- Vallance K, Liu W, Mandrell BN, Panetta JC, Gattuso JS, Hockenberry M, et al. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in paediatric patients receiving treatment for ALL. Eur J Cancer. 2010;46:1848–55.
- Coleman EA, Goodwin JA, Coon SK, Richards K, Enderlin C, Kennedy R, et al. Fatigue, sleep, pain, mood, and performance status in patients with multiple myeloma. Cancer Nurs. 2011;34:219–27.
- Gholam D, Giacchetti S, Brezault-Bonnet C, Bouchahda M, Hauteville D, Adam R, et al. Chronomodulated irinotecan, oxaliplatin, and leucovorin-modulated 5-Fluorouracil as ambulatory salvage therapy in patients with irinotecan- and oxaliplatin-resistant metastatic colorectal cancer. Oncologist. 2006;11:1072–80.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.

