Abstract
Several lines of evidence demonstrate that the immune system plays a pivotal role in development and progression of ischemic heart disease (IHD). More recently, a series of biological and clinical investigations has generated new interest about the existence of a relationship between a specific class of immunoglobulin, that is immunoglobulin E (IgE), and IHD. Data obtained in several epidemiological studies have convincingly demonstrated that the concentration of total serum IgEs is significantly increased in patients with IHD and often correlates with the prognosis. The putative mechanisms are essentially mediated by a physiological interaction between IgEs and mast cells, which triggers the direct or indirect release of a variety of substances that are actively involved in the pathogenesis of myocardial ischemia and thrombosis. Regardless of these important evidences, a causality dilemma remains, since it is still unclear whether increased IgE levels are a consequence of IHD or, rather, IHD is an underlying cause of increased IgE levels. The answer would allow us to recognize whether total IgEs may be considered simple biomarkers or risk factors of IHD, thus paving the way to investigations focused on immunotherapy or avoidance of allergenic foods for reducing serum IgEs in patients at risk of IHD.
Key messages
The immune system plays a pivotal role in development and progression of ischemic heart disease.
Data obtained in epidemiological studies demonstrate that serum IgEs are increased in patients with ischemic heart disease.
Serum IgE often correlates with the prognosis of ischemic heart disease.
IgEs interplay with mast cells and trigger direct or indirect release of a variety of substances that are actively involved in the pathogenesis of myocardial ischemia and thrombosis.
Introduction
The pathogenesis of ischemic heart disease (IHD), which typically encompasses stable angina, unstable angina, and acute myocardial infarction (AMI)—either ST-elevation MI (STEMI) or non-ST-elevation MI (NSTEMI)—is complex and multifactorial (Citation1). Among the various mechanisms implicated in development, growth, and rupture of an atherosclerotic plaque (i.e. the leading mechanisms supporting the pathogenesis of AMI) (Citation2,Citation3), several lines of evidence support a pivotal role of the immune system. It has been clearly shown that atherosclerotic lesions contain a broad array of inflammatory cells, thus including monocytes, macrophages, mast cells, basophils, lymphocytes, and dendritic cells, along with cholesterol that infiltrates from the bloodstream (Citation4). The progression of disease also involves a kaleidoscope of inflammatory and autoimmune reactions, which may become potential targets for future therapies (Citation5). In particular, the immune response against specific antigens contained in the atherosclerotic plaque, which include oxidized low-density lipoproteins (LDLs), micro-organisms, phospholipid proteins such as beta2-glycoprotein I (beta2GPI) and cardiolipin (Citation6), has now been recognized as an active part of development, evolution, and complications associated with atherosclerosis. More recently, a series of biological and clinical investigations has generated new interest in the existence of a potential relationship between a specific class of immunoglobulin, that is immunoglobulin E (IgE), and IHD. Therefore, the aim of this narrative review is to provide an updated overview about epidemiological and biological evidence linking IgE with IHD and its complications.
Immunoglobulin E (IgE)
Immunoglobulins E (IgEs) are a class of antibodies that exist in the form of monomers, consisting of two heavy chains (ϵ chain) and two light chains. These immunoglobulins are synthesized and released by B lymphocytes as a consequence of a complex interaction among genes, cytokines, and environment (Citation7). At variance with other types of immunoglobulins, the serum concentration of IgEs is very modest, usually 0.05% of total serum immunoglobulin levels, and typically lower than 100 U/mL (i.e. < 240 ng/mL) in adults (Citation8).
The IgEs are key components of a network of proteins involved in signalling response to an allergen/antigen, thus participating in the so-called ‘type I hypersensitivity’. Their biological activity is mainly dependent upon binding to specific Fc receptors present at the surface of mast cells, basophils, and eosinophils (Citation7). Two leading IgE cellular receptors have been identified so far, the Fcϵ receptor 1 (FcϵR1), that is a high-affinity IgE receptor, and the Fcϵ receptor 2 (FcϵR2), a low-affinity IgE receptor also known as CD23. The binding of IgE to FcϵR1 activates an intracellular signalling that culminates in degranulation of mast cells and release of a vast array of mediators including proteases, lipid mediators, cytokines, chemokines, and growth factors, which are ultimately responsible for the typical signs and symptoms associated with elevated IgE levels (Citation7). Atopy is indeed the leading clinical correlate of increased IgE levels. This disorder may typically manifest in the form of eczema (atopic dermatitis), allergic rhinitis (hay fever), allergic conjunctivitis, or allergic asthma. Interestingly, atopy and allergy are not synonyms, in that atopic individuals may not necessarily develop symptoms of allergy, but are predisposed to develop one or more allergic diseases for the presence of detectable serum IgE antibodies against specific allergens (Citation9). Another important function of IgE is the antibody-dependent, cell-mediated cytotoxic response against helminthic parasites. In brief, the IgE binds through the antigen binding site to a specific parasite antigen, then also binds to the eosinophil Fcϵ receptor with its Fc domain, and finally promotes eosinophil degranulation toward the parasite, with release of a variety of substances that damage, destroy, or dislodge the micro-organism (Citation7,Citation8).
Clinical evidence
The first ever study that has investigated the relationship between total IgEs and cardiovascular disease was published by Criqui et al. in 1987 (Citation10). The authors carried out a cross-sectional, population-based study including 577 subjects (262 men and 315 women, aged 38 to 82 y), and found that the mean total serum IgE levels were increased 1.2-fold (P < 0.05) in men with a previous history of AMI as compared with those who did not experience an AMI. In a fully adjusted model including age, cigarette smoking, fasting plasma glucose, diastolic blood pressure, and LDL level, the concentration of total serum IgEs remained positively and independently associated with cardiovascular disease (P = 0.03). Similar analysis in women did not reveal any statistically significant association between total serum IgE levels and AMI.
Szczeklik et al. assessed the concentration of total serum IgEs in 100 patients with recent AMI (Citation11). The mean total IgEs value steadily increased after the ischemic episode, achieving a statistically significant variation on day 3, and the peak on day 7. The concentration of IgEs declined on day 14 and returned to the initial level after 3 weeks. The pattern of increase was similar to that of the eosinophil count, and was more accentuated in patients with higher initial values (i.e. total IgE concentration > 200 U/mL).
Korkmaz et al. also assessed the serum IgE values in 156 patients with IHD (61 with AMI, 52 with unstable angina, and 43 with stable angina) and in 53 healthy controls (Citation12), reporting that total serum IgE levels were significantly higher in the subsets of patients with AMI and unstable angina than in those with stable angina and in controls.
In a following study, Szczeklik et al. investigated the concentration of total serum IgEs in 386 AMI patients at the time of coronary care unit admission (Citation13). After subdivision of patients according to the presence (n = 55) or absence (n = 331) of sudden cardiac arrest upon admission, the authors found that the serum levels of total IgEs were significantly increased in the former group.
Edston and van Hage-Hamsten studied 29 cases of sudden death due to coronary artery thrombosis and 27 controls (Citation14), reporting that the former group of patients had a nearly double concentration of serum total IgEs (200 ± 392 versus 101 ± 204 U/mL) and a significantly greater frequency of increased total serum IgE levels (i.e. > 200 U/mL) when compared with the control group (20% versus 8%).
Langer et al. also measured IgE levels in 621 subjects, who were prospectively followed for a mean period of 8.9 years (Citation15). The levels of total serum IgEs were found to be significantly higher in men who experienced IHD on follow-up than in those who did not (33.9 ± 5.6 versus 21.4 ± 4.2 U/mL; P < 0.05), and also in those who experienced non-fatal AMI on follow-up than in those who did not (61.7 ± 7.2 versus 21.9 ± 4.2 U/mL; P < 0.01). This trend was not confirmed in women, however, in whom the levels of total serum IgEs were found to be non-significantly different in those who experienced IHD on follow-up and in those who did not (17.0 ± 4.2 versus 17.8 ± 4.1 U/mL; P = ns), as well as in those who experienced non-fatal AMI on follow-up and in those who did not (19.1 ± 3.6 versus 17.0 ± 4.1 U/mL; P = ns). These results were at least partially explained by the fewer ischemic events in women (i.e. 23 versus 41) and probably by the lack of statistical power due to the small sample size. In Cox proportional hazards models adjusted for cigarette smoking, diastolic blood pressure, LDL, and fasting plasma glucose, men with a concentration of total IgEs > 200 U/mL had a significantly increased risk of developing both IHD (relative risk (RR) 3.4; 95% CI 1.4–8.4; P = 0.01) and non-fatal AMI (RR 7.3; 95% CI 2.2–23.9; P < 0.01).
Kovanen et al. assessed the potential association between total serum IgEs levels and IHD in dyslipidemic men (i.e. non-high-density lipoprotein (HDL) cholesterol > 201 mg/dL) participating in the Helsinki Heart Study, and who were followed up for 5 years (Citation16). The cases consisted in 135 patients who developed fatal or non-fatal AMI, who were compared with 135 controls who did not suffer coronary end-points during follow-up. It was hence found that total serum IgE levels at baseline were significantly higher in cases than in controls (5.0 versus 3.8 U/mL; P < 0.002). After stratification of the population in quartiles of IgE concentration, patients in the fourth quartile displayed a significant higher risk of fatal or non-fatal AMI than those in the first quartile (odds ratio (OR) 2.8; 95% CI 1.3–5.9; P < 0.01). Interestingly, this association was greater in non-smokers (OR 1.7) than in smokers (OR 1.3).
In a small prospective clinical study, Erdogan et al. measured the concentration of total serum IgEs in patients with AMI (n = 16), unstable angina (n = 14), stable angina (n = 15), and in 14 healthy controls (Citation17). The total serum IgE levels on admission were significantly lower in healthy controls (38.4 ± 19.0 U/mL) compared to patients with stable angina (87.5 ± 75.6 U/mL; P = 0.023), unstable angina (146.3 ± 112.8 U/mL; P = 0.001), and AMI (106.6 ± 140.8 U/mL; P = 0.04). In patients with AMI, the serum level of total IgEs increased gradually from baseline (106.6 ± 140.8 U/mL), reaching the peak on day 7 (113.0 ± 151.3; P = 0.024), and then gradually declined in the following 3 weeks. At 3 months after the ischemic episode, the concentration of total IgEs was less than half of that recorded upon patient admission (i.e. 48.6 ± 44.1 U/mL).
In a further investigation, Jong et al. studied the concentration of IgEs in 120 patients with different stages of IHD (i.e. 40 patients with AMI, 40 with unstable angina, and 40 with stable angina), and in 40 healthy controls (Citation18), and found that the total serum IgE values of all classes of patients with IHD (AMI, 106.6 ± 19.0 U/mL; unstable angina, 149.9 ± 51.6 U/mL; stable angina, 117.2 ± 38.2 U/mL) were significantly higher than those of healthy controls (31.7 ± 4.4 U/mL; all P < 0.01).
Sinkiewicz et al. investigated 195 patients with IHD (80 patients with AMI, 58 with unstable angina, and 57 patients with stable angina), along with 39 healthy controls (Citation19). The concentration of total serum IgEs was found to be nearly double in IHD patients (i.e. 40.0 U/mL in patients with AMI, 35.9 U/mL in those with unstable and stable angina) as compared with the control population (24.9 U/mL; P < 0.05). In patients with AMI, IgEs values modestly and non-significantly declined in the first 40 days after the ischemic episode, from 40.0 U/mL on admission to 39.9 U/mL on the 7th day, 37.4 U/mL on the 14th day, and 33.8 U/mL on the 40th day after AMI, respectively.
Wang et al. studied 982 subjects consecutively admitted to several hospitals in Central China with suspected chronic heart disease (CHD). Approximately 72% of these patients were diagnosed as having CHD (i.e. 207 with AMI, 255 with unstable angina, and 247 with stable angina), whereas the remaining 273 were considered as controls, since they had no or less than 50% luminal narrowing of the coronary arteries (Citation20). The concentration of total serum IgEs was found to be nearly 60% higher in patients diagnosed with CHD than in those without (90.6 ± 2.9 versus 57.1 ± 5.3 U/mL; P < 0.001). Even more interestingly, the concentration of total serum IgEs was significantly associated with severity of CHD, in that patients with AMI exhibited significantly higher values (126.1 ± 6.4 U/mL) than those with unstable angina (120.8 ± 1.5 U/mL; P < 0.01) and those with stable angina (114.7 ± 1.6 U/mL; P < 0.05).
Baalash and co-workers evaluated the total IgE levels before and after coronary artery bypass grafting (CABG) surgery (Citation21). The total serum IgE levels were found to be significantly higher (P < 0.05) in the preoperative samples (99.8 ± 8.6 U/mL) when compared with both the postoperative samples (37.4 ± 5.7 U/mL) and those of a control population (31.6 ± 3.4 U/mL), whereas the postoperative samples did not show any significant difference when compared with controls.
Jaramillo et al. carried out a retrospective study, analysing data of allergen-specific and total IgEs obtained in 4002 participants aged > 20 y (Citation22).The personal history of AMI was defined by means of a health questionnaire, as a positive answer to the question ‘Has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction)?’. In the 159 subjects who responded ‘yes’, the concentration of total serum IgEs was marginally higher than in those who responded ‘no’ to the survey (55.1 ± 7.3 versus 40.5 ± 1.6 U/mL; P = 0.05). In fully adjusted regression analysis, the concentration of total serum IgEs was significantly associated with previous AMI (OR 1.41; 95% CI 1.01–1.98). Surprisingly, however, the levels of allergen-specific IgEs were marginally lower in the subjects who responded ‘yes’ than in those who responded ‘no’ to the questionnaire (5.5 ± 0.1 versus 7.7 ± 0.2 U/mL; P < 0.01). Accordingly, a positive allergen-specific IgE test was inversely associated with AMI (OR 0.91; 95% CI 0.85–0.97) after adjustment for age, gender, ethnicity, diabetes mellitus, hypertension, family history of AMI, cigarette smoking, blood lipids, body mass index, and C-reactive protein. Among the various allergens tested, mites (OR 0.36; 95% CI 0.20–0.64) but not foods (e.g. eggs, milk), moulds (e.g. Alternaria, Aspergillus), plants, roaches (cockroach, shrimp), pets (e.g. dog, cat), and rodents (mouse, rat) were inversely associated with AMI in fully adjusted regression analysis.
Shiue examined the relationship between serum total or seafood-specific IgE levels and cardiovascular risk in a national and population-based setting, including 4979 subjects (Citation23). The serum levels of total IgEs were found to be significantly higher in patients with AMI than in those without (240.9 ± 553.6 versus 160.1 ± 475.7 U/mL; P = 0.02). In a fully adjusted regression model, total IgE serum values were also modestly but significantly associated with the presence of IHD (OR 2.02; 95% CI 1.001–4.08; P = 0.05). Even more interestingly, only seafood-specific IgE antibodies appeared to be significantly associated with IHD (OR 2.96; 95% CI 1.06–8.28; P = 0.04) among all allergen-specific IgE antibodies.
Finally, Kovcic et al. have found that the levels of IgEs were higher in patients with coronary artery disease with a significant coronary artery stenosis (> 70%) when compared with patients with a coronary artery stenosis < 70% and with patients without verified stenosis (Citation24).
It is also noteworthy that in a large and prospective investigation, Linneberg et al. followed up for 10 years 18,841 persons receiving subcutaneous allergen-specific immunotherapy (SCIT) along with 428,484 subjects receiving conventional allergy treatment (i.e. nasal steroids or oral antihistamines) (Citation25). As compared with conventional allergy treatment, patients receiving SCIT had an overall lower risk of mortality (hazard ratio (HR) 0.71; 95% CI 0.62–0.81), IHD (HR 0.88; 95% CI 0.73–1.05), and AMI (HR 0.70; 95% CI 0.52–0.93).
Coronary artery abnormalities in hyper-IgE syndrome (HIES)
Hyper-IgE syndrome (HIES), also known as Job's syndrome, is a rare (i.e. < 1/1,000,000) immunodeficiency disorder due to mutations in the STAT3 (signal transducer and activator of transcription 3) and—less frequently—TYK2 (tyrosine kinase 2 deficiency) and DOCK8 (dedicator of cytokinesis 8) genes (Citation26). The syndrome is typically characterized by eczema, recurrent skin and lung infections, extremely elevated serum IgEs (typically > 2000 U/mL, occasionally > 10,000 U/mL), along with connective tissue and skeletal abnormalities. The leading vascular abnormalities that are commonly found in HIES patients include middle-sized artery tortuosity and aneurysms, which may be occasionally associated with the onset of IHD (Citation26). Although the cardiac involvement in HIES patients is probably independent of the elevated serum IgE levels, it may be relevant to this article briefly to describe the abnormalities of coronary arteries that are more frequently encountered in these patients.
In 2004, Hilfiker-Kleiner et al. first reported that STAT3-knockout mice develop low myocardial capillary density and high interstitial fibrosis early after birth, with following onset of dilated cardiomyopathy, abnormal cardiac function, and premature death (Citation27). It is also noteworthy that knockout of STAT3 in mice was associated with greater vulnerability to myocardial ischemia/reperfusion injury and infarction, followed by apoptosis of cardiomyocytes, greater infarct sizes, cardiac dysfunction, and reduced survival.
In 2007, Ling et al. originally described two cases of aneurysmal coronary artery disease in two men, aged 43 and 48 y (Citation28). In the same year, Young et al. described the case of a 30-year-old woman with HIES syndrome, who was diagnosed as having coronary artery aneurysms by coronary angiogram (Citation29). Gharib et al. studied nine patients with HIES syndrome (Citation30), and reported that six of them displayed some forms of coronary arterial abnormality, including the presence of tortuous and ectatic right coronary artery along with the presence of smooth ectatic short segments or focal aneurysms in the left anterior descending coronary artery. Interestingly, no signs of coronary calcification or a significant degree of atherosclerosis could be detected in the coronary arteries by multi-detector computed tomography angiography. Freeman et al. studied 33 healthy controls and 38 patients with HIES by computed tomography or magnetic resonance imaging, to assess tortuosity, dilation, and aneurysm of coronary arteries (Citation31), and found that tortuosity (70% versus 21%) and dilation (37% versus 3%) were present in a greater percentage of HIES cases than in controls, thus concluding that STAT3 may be a crucial determinant of vascular remodelling.
Biological background
The proatherogenic and prothrombotic activity of total IgEs is probably complex and multi-faceted, thus involving a variety of putative pathogenetic pathways. One of the leading mechanisms linking total IgEs with IHD is probably attributable to the well-established capacity of these immunoglobulins to promote vascular injury through activities on cellular elements that display the receptor FcϵR, and thus principally including mast cells, macrophages and platelets.
As regards the specific cellular subtypes, the role of mast cells in the pathogenesis of coronary heart disease is now clearly acknowledged (Citation32), since these are also the leading actors of the so-called ‘Kounis syndrome’ (Citation33). This paradigmatic condition has been originally described in 1991 as the concurrent occurrence of chest pain and allergic reactions in association with clinical and laboratory data of angina, and has been associated with release of inflammatory mediators during an allergic episode () (Citation34). The syndrome may hence be classified as an episode of IHD due to coronary artery spasm with (i.e. Kounis syndrome type II) or without (i.e. Kounis syndrome type I) the presence of coronary atherosclerosis (Citation35).
Figure 1. Potential mechanisms linking immunoglobulin E (IgE) with the pathogenesis of ischemic heart disease. bFGF = basic fibroblast growth factor; IL-8 = interleukin-8; LDL = low-density lipoprotein; MCP-1 = monocyte chemoattractant protein-1; PAF = platelet activating factor; PAI-1 = plasminogen activator inhibitor 1; TF = tissue factor; VEGF = vascular endothelial growth factor.
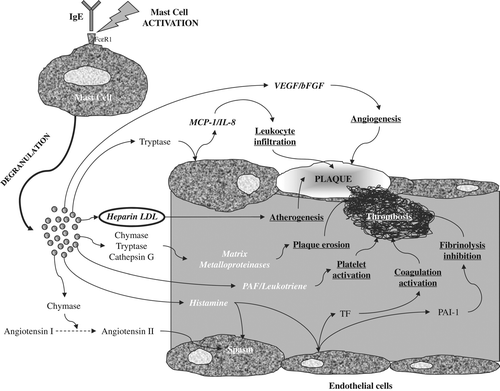
In Kounis syndrome type I, the leading pathogenetic mechanism is indeed represented by coronary artery spasm, which may progress to persistent ischemia up to myocardiocyte necrosis. In brief, the IgE binds to FcϵR1, an event that leads to activation and degranulation of mast cells. Immediately upon activation, mast cells release the intracellular contents of their granules by two separate mechanisms, i.e. rapid (anaphylactic) and (slow) piecemeal degranulation. The leading substances that are released by mast cells include vasoconstricting agents (e.g. histamine), collagen-degrading enzymes (e.g. tryptase, chymase, and cathepsin G), heparin, proangiogenic mediators (e.g. vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF)), as well as other important cytokines that modulate platelet function (e.g. platelet-activating factor (PAF), and arachidonic acid metabolites such as leukotrienes and thromboxane) (Citation32,Citation35,Citation36). Histamine is indeed the leading mediator, since it is capable of directly triggering the spasm of coronary arteries through an H1 receptor mechanism and amplifying angiotensin II-vasoconstriction (the enzymes chymase tryptase and cathepsin G released by mast cells convert angiotensin I to angiotensin II) () (Citation37).
Besides the vasoconstriction mechanism that typically characterizes Kounis syndrome type I, the activation of mast cells in Kounis syndrome type II exacerbates a pre-existing atheromatous disease by amplifying atherogenesis and promoting plaque erosion, ulceration, rupture, and occurrence of subsequent thrombotic complications. More specifically, histamine is capable of amplifying the inflammatory response of neutrophils, monocytes, and eosinophils, directly activating platelets, acting in synergy with other platelet-activating factors such as thrombin and adrenaline for enhancing platelet aggregation, and promoting expression and release into the circulation of both tissue factor (TF) and plasminogen activator inhibitor 1 (PAI-1) (Citation35,Citation36). The release of tryptase from activated mast cells may then trigger interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) expression in endothelial cells, which both promote leukocyte recruitment and infiltration into the atherosclerotic plaque (Citation38). Another important pathogenetic mechanisms of IgE-dependent activation of mast cells involves the release of heparin, the binding of this glycosaminoglycan with LDL particles (Citation39), the subsequent association with macrophages, and the enhanced formation of foam cells () (Citation40,Citation41). The IgE-induced release of a number of collagen-degrading enzymes such as tryptase, chymase, and cathepsin G also triggers the activation of metalloproteinases, collagenase, gelatinase, and stromelysin, thus powerfully promoting the instability of atherosclerotic plaques (Citation32,Citation35,Citation36). The further complication of plaque rupture entails activation of primary and secondary hemostasis, which ultimately leads to coronary thrombosis. The evidence of a direct role of IgEs on blood coagulation has been provided in 1975 by Pinckard et al., who showed that these immunoglobulins may trigger, either directly or indirectly, the activation of intrinsic blood coagulation in vivo (Citation42). Further evidence of indirect prothrombotic activity of IgEs has been provided by Steffel et al., who showed that histamine released by activated mast cells may trigger the release of TF by human aortic endothelial and vascular smooth muscle cells, in a concentration-dependent manner (Citation43). IgE-mediated mast cell activation may also activate secondary hemostasis, by release of PAF and thromboxane, which both promote platelet activation and aggregation (Citation36). After development of coronary thrombosis, increased values of IgEs may then impair the fibrinolytic system, as reflected by the positive association observed between serum levels of total IgEs and delayed thrombin generation in survivors of AMI (Citation13). It seems hence reasonable to conclude that IgEs may be effective to destabilize atheromatous plaques and further promote intraluminal thrombosis in patients with significant burden of coronary atherosclerosis ().
As regards other cell types targeted by IgEs, Wang et al. found that these immunoglobulins and their high-affinity receptor FcϵR1 were co-localized in human atherosclerotic plaques, particularly in macrophage-rich areas (Citation20). It was also observed that mice lacking FcϵR1α had reduced inflammation and apoptosis in atherosclerotic lesions, along with a lower burden of atherosclerotic disease. The IgEs were also found to exert an FcϵR1- and Toll-like receptor 4 (TLR4)-dependent activity, thus triggering macrophage signal transduction, expression of inflammatory molecules (i.e. interleukin-6 and MCP-1), and apoptosis (Citation20).
It is also noteworthy that the concentration of total IgEs in patients with an AMI was found to be significantly associated with that of some well-established biomarkers of IHD, thus including increased LDL (r = 0.40; P < 0.05), increased lipoprotein(a) (r = 0.51; P = 0.02), and reduced HDL (r = 0.40; P < 0.05) (Citation44).
As regards coronary aneurysms, several lines of evidence now suggest that mast cells are actively involved in the pathogenesis of these conditions. More specifically, the release of proteolytic enzymes, the activation of both matrix metalloproteinases and the renin–angiotensin system, along with their contribution to smooth muscle cell apoptosis, would represent major events that support neovascularization, inflammation, and atherosclerosis, thus promoting development and growth of aneurysms (Citation45).
Conclusions
Although the clinical evidence available so far seems to confirm the existence of a strong epidemiological association between increased serum levels of total IgE and IHD (), some doubts remain about the pathophysiology of this association.
Table I. Synthesis of studies that have explored the epidemiological association between serum levels of Immunoglobulin E (IgE) and ischemic heart disease.
Firstly, we are facing a typical causality dilemma, i.e. which came first, the chicken or the egg? More specifically, are increased IgE levels a consequence of IHD, or, rather, is IHD an underlying cause of increased IgE levels? This question is far from ancillary, since the answer would allow identification of whether IgE may be considered a risk factor or a simple biomarker of IHD. In the former case, specific interventions should be planned to decrease the levels of IgEs, especially in the part of the population more vulnerable to IHD. In the latter circumstance, the combination of total serum IgEs along with other biomarkers of IHD may be tested to verify whether their diagnostic efficiency may be increased.
The current scientific evidence seems to support the existence of a convincing causal relationship between total IgE levels and IHD, wherein these immunoglobulins were proven to exert important proatherogenic, prothrombotic, and antifibrinolytic activities. Interestingly, the finding of high IgE levels already observed at hospital admission would suggest that the concentration of these proteins must have been elevated before the event, and this aspect further supports the hypothesis that an increased IgE level is perhaps a risk factor for IHD. This is also consistent with the evidence that the serum half-life of IgEs is between 1 and 5 days, but the catabolism is enormously delayed when these proteins are bound to mast cells or eosinophils (i.e. from weeks to months) (Citation46). In apparent contradiction with this hypothesis is the evidence that IgE levels steadily decline after an acute episode of myocardial ischemia and infarction, which is consistent with their kinetics (i.e. degradation occurs between 5 and 12 hours) (Citation46), and with the development of a temporary and self-limited immunological event, characterized by transitory activation of immune system and production of IgE.
Another important issue that has not been adequately clarified in the current scientific literature is the specific nature of the IgEs. According to recent evidence, it seems more plausible that the association with IHD is limited to polyclonal (i.e. total) IgEs, wherein studies that have measured allergen-specific IgEs failed to find significant associations with IHD (Citation47). An alternative explanation emerges from the current diagnostic panels of allergen-specific IgEs, which entail common human allergens, but virtually exclude oxidation-specific epitopes and other potentially immunoreactive substances that may be contained in atherosclerotic plaques and which may be potential targets of type I hypersensitivity.
The current statistics attest that 20% of the general population may suffer from some forms of allergic disease (Citation48). Moreover, recent epidemiological data describe that atopy has remarkably increased during the last quarter of the twentieth century, by nearly 4.5% per decade, with a steady trend that is not expected to reverse soon (Citation49). This is not really unexpected, since the remarkably decreased frequency of parasitic diseases that has been attributed to better sanitation and hygiene has virtually left the immune system ‘unoccupied’, thus fostering immune responses to benign antigens (e.g. allergens) that are associated with type I hypersensitivity reactions (Citation8). Provided the chicken-and-egg dilemma is solved and IgE turns out to be a risk factor, specific investigations may be planned to establish the effectiveness of immunotherapy or avoidance of allergenic foods for reducing total serum IgEs in patients at higher risk of IHD. As specifically regards the latter issue, it is undeniable that the avoidance of certain aliments such as seafood may be effective to prevent mast cell activation and the following allergic reactions, but this should be weighed against the evidence that elimination of heart-healthy seafood from the diet may paradoxically increase the risk of IHD (Citation50).
Declaration of interest: The authors report no conflicts of interest.
References
- Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8:502–12.
- Silvestre-Roig C, de Winther MP, Weber C, Daemen MJ, Lutgens E, Soehnlein O. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res. 2014;114:214–26.
- Chávez-Sánchez L, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Montoya-Díaz E, Blanco-Favela F. Innate immune system cells in atherosclerosis. Arch Med Res. 2014;45:1–14.
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12.
- Bjorkbacka H, Fredrikson GN, Nilsson J. Emerging biomarkers and intervention targets for immune-modulation of atherosclerosis - a review of the experimental evidence. Atherosclerosis. 2013;227:9–17.
- Nicolo D, Varadhachary AS, Monestier M. Atherosclerosis, antiphospholipid syndrome, and antiphospholipid antibodies. Front Biosci. 2007;12:2171–82.
- Pate MB, Smith JK, Chi DS, Krishnaswamy G. Regulation and dysregulation of immunoglobulin E: a molecular and clinical perspective. Clin Mol Allergy. 2010;8:3.
- Winter WE, Hardt NS, Fuhrman S. Immunoglobulin E: importance in parasitic infections and hypersensitivity responses. Arch Pathol Lab Med. 2000;124:1382–5.
- Linneberg A. Changes in atopy over 25 years: allergy epidemic has spread to old age. BMJ. 2005;331:352.
- Criqui MH, Lee ER, Hamburger RN, Klauber MR, Coughlin SS. IgE and cardiovascular disease. Results from a population-based study. Am J Med. 1987;82:964–8.
- Szczeklik A, Sladek K, Szczerba A, Dropinski J. Serum immunoglobulin E response to myocardial infarction. Circulation. 1988;77:1245–9.
- Korkmaz ME, Oto A, Saraclar Y, Oram E, Oram A, Ugurlu S, et al. Levels of IgE in the serum of patients with coronary arterial disease. Int J Cardiol. 1991;31:199–204.
- Szczeklik A, Dropinski J, Gora PF. Serum immunoglobulin E and sudden cardiac arrest during myocardial infarction. Coron Artery Dis. 1993;4:1029–32.
- Edston E, van Hage-Hamsten M. Immunoglobulin E, mast cell-specific tryptase and the complement system in sudden death from coronary artery thrombosis. Int J Cardiol. 1995;52:77–81.
- Langer RD, Criqui MH, Feigelson HS, McCann TJ, Hamburger RN. IgE predicts future nonfatal myocardial infarction in men. J Clin Epidemiol. 1996;49:203–9.
- Kovanen PT, Manttari M, Palosuo T, Manninen V, Aho K. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med. 1998; 158:1434–9.
- Erdogan O, Gul C, Altun A, Ozbay G. Increased immunoglobulin E response in acute coronary syndromes. Angiology. 2003;54:73–9.
- Jong GP, Wang YF, Tsai FJ, Tsai CH, Wu CL, Liu RH, et al. Immunoglobulin E and matrix metalloproteinase-9 in patients with different stages of coronary artery diseases. Chin J Physiol. 2007;50: 277–82.
- Sinkiewicz W, Błazejewski J, Bujak R, Kubica J, Dudziak J. Immunoglobulin E in patients with ischemic heart disease. Cardiol J. 2008; 15:122–8.
- Wang J, Cheng X, Xiang MX, Alanne-Kinnunen M, Wang JA, Chen H, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J Clin Invest. 2011;121:3564–77.
- Baalash AA, Hamouda HE, Ismail GM, Yassein IK, Ibrahim BM. The role of heat shock protein 70, IgE and MMP-9 in detecting early minor myocardial damage and evaluating the efficacy of coronary artery bypass grafting (CABG). J Life Sci. 2012;6:260–7.
- Jaramillo R, Cohn RD, Crockett PW, Gowdy KM, Zeldin DC, Fessler MB. Relation between objective measures of atopy and myocardial infarction in the United States. J Allergy Clin Immunol. 2013;131:405–11.
- Shiue I. Are higher serum IgE concentrations associated with adult cardiovascular disease?Int J Cardiol. 2013;168:1580–1.
- Kovcic J, Nurkic J, Nurkic M. C-reactive protein, immunoglobuline E and natural killer cells in patients with coronary artery disease. J Am Coll Cardiol. 2013;62:C203.
- Linneberg A, Jacobsen RK, Jespersen L, Abildstrom SZ. Association of subcutaneous allergen-specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol. 2012;129:413–19.
- Yong PF, Freeman AF, Engelhardt KR, Holland S, Puck JM, Grimbacher B. An update on the hyper-IgE syndromes. Arthritis Res Ther. 2012;14:228.
- Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–95.
- Ling JC, Freeman AF, Gharib AM, Arai AE, Lederman RJ, Rosing DR, et al. Coronary artery aneurysms in patients with hyper IgE recurrent infection syndrome. Clin Immunol. 2007;122:255–8.
- Young TY, Jerome D, Gupta S. Hyperimmunoglobulinemia E syndrome associated with coronary artery aneurysms: deficiency of central memory CD4 + T cells and expansion of effector memory CD4 + T cells. Ann Allergy Asthma Immunol. 2007;98:389–92.
- Gharib AM, Pettigrew RI, Elagha A, Hsu A, Welch P, Holland SM, et al. Coronary abnormalities in hyper-IgE recurrent infection syndrome: depiction at coronary MDCT angiography. AJR Am J Roentgenol. 2009;193:W478–81.
- Freeman AF, Avila EM, Shaw PA, Davis J, Hsu AP, Welch P, et al. Coronary artery abnormalities in Hyper-IgE syndrome. J Clin Immunol. 2011;31:338–45.
- Bot I, Biessen EA. Mast cells in atherosclerosis. Thromb Haemost. 2011;106:820–6.
- Kounis NG, Giannopoulos S, Goudevenos J. Beware of, not only the dogs, but the passionate kissing and the Kounis syndrome. J Cardiovasc Med (Hagerstown). 2011;12:149–50.
- Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45:121–8.
- Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm?Int J Cardiol. 2006;110: 7–14.
- Potaczek DP. Links between allergy and cardiovascular or hemostatic system. Int J Cardiol. 2014;170:278–85.
- Kovanen PT. Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev. 2007;217:105–22.
- Kinoshita M, Okada M, Hara M, Furukawa Y, Matsumori A. Mast cell tryptase in mast cell granules enhances MCP-1 and interleukin-8 production in human endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:1858–63.
- Kokkonen JO, Kovanen PT. Low-density-lipoprotein binding by mast-cell granules. Demonstration of binding of apolipoprotein B to heparin proteoglycan of exocytosed granules. Biochem J. 1987; 241:583–9.
- Ma H, Kovanen PT. IgE-dependent generation of foam cells: an immune mechanism involving degranulation of sensitized mast cells with resultant uptake of LDL by macrophages. Arterioscler Thromb Vasc Biol. 1995;15:811–19.
- Ma H, Kovanen PT. Inhibition of mast cell-dependent conversion of cultured macrophages into foam cells with antiallergic drugs. Arterioscler Thromb Vasc Biol. 2000;20:E134–42.
- Pinckard RN, Tanigawa C, Halonen M. IgE-induced blood coagulation alterations in the rabbit: consumption of coagulation factors XII, XI, and IX in vivo. J Immunol. 1975;115:525–32.
- Steffel J, Akhmedov A, Greutert H, Luscher TF, Tanner FC. Histamine induces tissue factor expression: implications for acute coronary syndromes. Circulation. 2005;112:341–9.
- Sinkiewicz W, Blazejewski J, Bujak R, Zekanowska E, Sobański P, Kubica J, et al. Immunoglobulin E as a marker of the atherothrombotic process in patients with acute myocardial infarction. Cardiol J. 2007;14:266–73.
- Swedenborg J, Mäyränpää MI, Kovanen PT. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:734–40.
- Achatz G, Achatz-Straussberger G, Feichtner S, Koenigsberger S, Lenz S, Peckl-Schmid D, et al. The biology of IgE: molecular mechanism restraining potentially dangerous high serum IgE titres in vivo. In: Penichet ML, Jensen-Jarolim E, editors. Cancer and IgE: introducing the concept of allergooncology. New York, NY: Humana Press, Inc.; 2010; pp. 13–36.
- Binder CJ, Witztum JL. Is atherosclerosis an allergic disease?Circ Res. 2011;109:1103–4.
- Ebert CS Jr, Pillsbury HC 3rd.Epidemiology of allergy. Otolaryngol Clin North Am. 2011;44:537–548, vii.
- Law M, Morris JK, Wald N, Luczynska C, Burney P. Changes in atopy over a quarter of a century, based on cross sectional data at three time periods. BMJ. 2005;330:1187–8.
- Sirot V, Leblanc JC, Margaritis I. A risk-benefit analysis approach to seafood intake to determine optimal consumption. Br J Nutr. 2012;107:1812–22.

