Abstract
Aim. To characterize blood monocyte subsets in patients with different degrees of carotid atherosclerosis and pathological carotid plaque neovascularization.
Methods. Assessment of carotid plaque neovascularization using contrast ultrasonography and flow cytometric quantification of monocyte subsets and their receptors involved in inflammation, angiogenesis, and tissue repair was done in 40 patients with carotid stenosis ≥ 50% and CAD (CS > 50), 40 patients with carotid stenosis < 50% and documented CAD (CS < 50), 40 hypercholesterolaemic controls (HC group), and 40 normocholesterolaemic controls (NC).
Results. CS > 50 and CS < 50 groups had increased counts of Mon1 (‘classical’ CD14++ CD16-CCR2 + cells) compared to HCs (P = 0.03, and P = 0.009). Mon3 (‘non-classical’ CD14 + CD16++ CCR2- cells) were only increased in CS < 50 compared with HCs (P < 0.01). Both CS>50 and CS < 50 groups showed increased expression of proinflammatory interleukin-6 receptor on Mon1 and Mon2 (‘intermediate’ CD14++ CD16 + CCR2+ cells); TLR4, proangiogenic Tie2 on all subsets (P < 0.01 for all). In multivariate regression analysis only high Mon1 count was a significant predictor of carotid stenosis (P = 0.04) and intima-media thickness (P = 0.02). In multivariate regression analysis only the Mon1 subset was significantly associated with severe, grade 2 neovascularization (P = 0.034).
Conclusion. In this pilot study classical monocytes (Mon1) represent the only monocyte subset predictive of the severity of carotid and systemic atherosclerosis, such as carotid intima-media thickness, degree of carotid stenosis, and presence of carotid intraplaque neovascularization.
Key messages
Classical CD14++ CD16-CCR2 monocytes (Mon1) represent the only monocyte subset predictive of the severity of carotid and systemic atherosclerosis, such as carotid intima-media thickness and degree of carotid stenosis.
Classical CD14++ CD16-CCR2 monocytes (Mon1) is also the only monocyte subset associated with presence of pathologic carotid intraplaque neovascularization.
All monocyte subsets show varying degrees of changes in expression of receptors mediating inflammatory, angiogenic, and remodelling processes in patients with carotid atherosclerosis.
Introduction
Approximately 60% of recently symptomatic carotid artery disease is caused by disruption of an atheromatous plaque (Citation1,Citation2). Cerebrovascular events related to carotid atherosclerosis are common, with many patients requiring surgical intervention. Pathologic intimal thickening constitutes the earliest atherosclerotic change and is characterized by surface smooth muscle cells overlying relatively acellular lipid-rich pools. Measurement of carotid intima-media thickness (IMT) has developed as a widely used marker of carotid and systemic atherosclerosis (Citation3,Citation4). Although the underlying mechanism(s) for conversion of an asymptomatic fibroatheromatous area to a lesion vulnerable to rupture are not precisely established, intraplaque neovascularization has been proposed as an important contributor to this process (Citation5–7).
As a plaque enlarges, the ensuing local tissue hypoxia and inflammatory cell infiltration promotes local neovascularization (Citation8). Whilst intimal thickening (e.g. carotid IMT) is largely conceived as an early marker of atherosclerosis, pathological neovascularization is implicated in both early and late atherosclerosis (Citation9–12). In the process of plaque progression, adventitial vasa vasorum give rise to intraplaque vessels (Citation13), whose presence correlates with risk of its rupture (Citation14). In a study of 269 patients with sudden coronary death, the number of vasa vasorum in coronary plaques was increased 2-fold in vulnerable plaques and up to 4-fold in ruptured plaques, when compared to stable plaques (Citation15).
The changes in arterial wall vascularization are uniformly present in different arterial beds (in contrast to often selected location of the plaques themselves) and support the concept of symptomatic atherosclerosis as a panarterial disease (Citation16). However, the factors/mechanisms triggering the switch from relatively benign systemic atherosclerosis (i.e. intima-media thickening, increased density of adventitial vasa vasorum) to rapidly progressive stenotic lesions or associated with intraplaque neovascularization are poorly understood.
A potential link between monocytes and abnormal plaque angiogenesis has a strong biological justification. Monocyte activity is directly associated with the inflammatory status. Activated monocytes promote the synthesis of proinflammatory molecules, such as interleukin-6 (IL6) and tumour necrosis factor-α, mediated by stimulation of Toll-like receptors (TLR4) (Citation17,Citation18). Monocyte activation enhances the affinity of monocyte ligands to adhesion molecules, thus promoting monocyte–endothelium adhesion (Citation19). In support of this argument, microvessels within lipid-rich plaques strongly express adhesion molecules, such as vascular cell adhesion molecule (VCAM), with monocytes expressing receptors to the molecule (Citation20,Citation21). Their interaction may facilitate transendothelial migration of inflammatory cells (i.e. monocytes) into the plaque microenvironment. Also, monocytes per se have proangiogenic properties. A large proportion of so-called endothelial progenitor cells resident in vessels have a monocytic origin (Citation22). Additionally, monocytes have been found to constitute the dominant population among circulating cells expressing type 2 receptor for vascular endothelial growth factor (VEGF), Tie2, CXCR4—which are receptors implicated in angiogenesis and tissue remodelling (Citation23). Whilst a role for macrophages in the development and progression of atherosclerosis has long been recognized, the role of their blood-borne ancestors—monocytes, represented by different functional subsets—has scarcely been addressed (Citation24).
In this pilot study we firstly aimed to assess different monocyte subsets and their expression of receptors involved in inflammation, angiogenesis/tissue remodelling in patients with different degrees of carotid atherosclerosis with evidence of a systemic atherosclerotic process (i.e. concomitant coronary artery disease (CAD)), who were compared to hypercholesterolaemic and normocholesterolamic controls. Second, we aimed to establish a relation between the monocyte subsets and carotid plaque neovascularization, by using a novel method of contrast carotid ultrasonography. Different types of contrast have been used for over a decade for detection of carotid stenosis, but only more recently have the contrast agents been utilized for assessment of intraplaque neovascularizaion (Citation25).
Methods
Study population
We recruited 160 patients divided into four groups: 1) 40 patients with carotid stenosis ≥ 50% and documented CAD (CS > 50 group); 2) 40 patients with carotid stenosis < 50% and documented CAD (CS < 50 group); 3) 40 asymptomatic control subjects with hypercholesterolaemia (HC group); and 4) 40 asymptomatic control subjects without hypercholesterolaemia (NC group). The participants were recruited from Sandwell and West Birmingham Hospital NHS Trust during the period May 2010 to May 2011. The study was approved by the local research ethics committee, and all participants gave written informed consent.
Carotid stenosis and intraplaque neovascularization were assessed using carotid ultrasonography (see below). Presence of stable CAD was confirmed by history and/or previous elective coronary angiography, with no acute hospital admission for ≥ 3 months. HCs were recruited from our outpatient lipid clinic and had total cholesterol level above 5.0 mmol/L but without clinical evidence of symptomatic atherosclerosis. NCs were identified and recruited from hospital staff, relatives of patients, and local general practices and were healthy with no clinical evidence of symptomatic arthrosclerosis and cholesterol levels below 5.0 mmol/L. Exclusion criteria for all groups included any neoplastic, inflammatory diseases and significant kidney disease.
All subjects were invited to attend our research clinic in the morning, after abstaining from smoking from midnight of the preceding day. Following a 20-minute supine rest, a 20 mL blood sample was taken for flow cytometric studies and ELISA (below), and carotid ultrasound imaging was performed (below).
Flow cytometry
Flow cytometric analysis was performed using the BD FACS Calibur flow cytometer (Becton Dickinson (BD), Oxford, UK) as published previously (Citation26). The technique is robust and highly reproducible.
Absolute count of monocyte subsets
Mouse anti-human monoclonal fluorochrome-conjugated antibodies anti-CD16-Alexa Fluor 488 (clone DJ130c, AbD Serotec, Oxford, UK), anti-CD14-PE (clone MфP9, BD), anti-CD42a-PerCP (clone Beb1, BD), and anti-CCR2-APC (clone 48607, R&D Systems (R&D), Abingdon, UK) were mixed with 50 μL of fresh EDTA anticoagulated whole blood in BD TruCount tubes (BD) containing a strictly defined number of fluorescent count beads (Citation26–29). After incubation for 15 minutes red blood cells were lysed by 450 μL of BD lysing solution® (BD) for 15 minutes followed by dilution in 1.5 mL of PBS and immediate flow cytometric analysis. Monocytes were selected by gating strategies based on forward and side scatter properties to select monocytes, side scatter properties versus CD14 expression to exclude granulocytes, and ungated CD14 versus CD16 expression to exclude natural killer lymphocytes. Monocyte subsets were defined as CD14++ CD16-CCR2 + cells (Mon1), CD14++ CD16 + CCR2+ cells (Mon2), and CD14 + CD16++ CCR2- cells (Mon3). Monocyte-platelet aggregates (MPAs) were defined as events positive to both monocyte markers (as above) and the platelet marker CD42a (glycoprotein IX) (). Absolute counts of monocyte subsets (cells/μL) were calculated using cout beads according to the manufacturer recommendations.
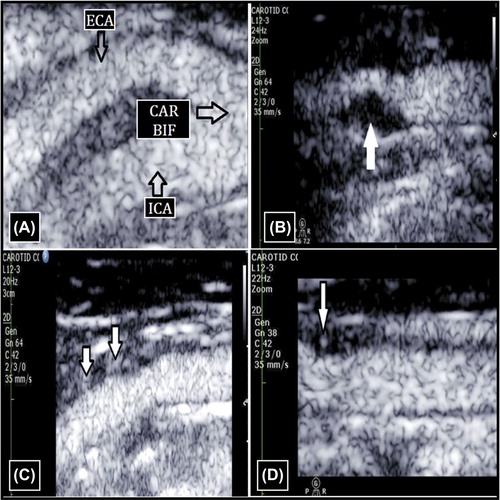
Figure 1. Contrast-enhanced carotid ultrasound. (A) Carotid bifurcation with contrast agent. (B) Grade 0 ICA lesion (no contrast within the plaque). (C) Grade 1 ICA lesion (bubbles confined to the plaque adventitial side). (D) Grade 2 ICA lesion (bubbles reaching the plaque core and/or contrast agent enhancement throughout the plaque). CAR BIF = carotid bifurcation; ECA = external carotid artery; ICA = internal carotid artery.
Expression of surface antigens on monocyte subsets
For analysis of surface antigens, 100 μL of whole blood was incubated with mouse anti-human monoclonal fluorochrome-conjugated antibodies for 15 minutes in the dark. Red blood cells were lysed with 2 mL of BD lysing solution® for 10 min, washed in phosphate-buffered saline followed by immediate flow cytometric analysis. Anti-CD16-Alexa Fluor 488 (clone DJ130c, AbD Serotec, Oxford, UK) and anti-CD14-PerCP-Cy5.5 (clone M5E2, BD) were used for definition of monocyte subsets into CD14++ CD16- monocytes (Mon1), CD14++ CD16 + monocytes (Mon2), and CD14 + CD16++ monocytes (Mon3). PE-conjugated antibodies were used against Toll-like receptor (TLR4, clone 285219, R&D) and CXCR4 (clone 12G5, R&D). APC-conjugated antibodies were used against interleukin-6 (IL6) receptor (clone 17506, R&D), integrin α4/CD49d (vascular cells adhesion molecule-1 (VCAM-1) receptor, clone 7.2R, R&D), vascular endothelial growth factor (VEGF) receptor 2 (clone 89106, R&D), and Tie2 (clone 83715, R&D).
Carotid ultrasonography
Carotid ultrasonography was performed with CX50 (Phillips Medical Systems, Guildford, Surrey, UK) with a L12-3 linear array transducer. Scans of the common carotid arteries (CCA) and internal carotid arteries (ICA) were obtained bilaterally in three different longitudinal planes (anterior oblique, lateral, and posterior oblique) and in the transverse projection. IMT and the degree of carotid stenosis (≥ 50% or < 50%) were determined according to the current guidelines (Citation30). The inter- and intra-observer coefficient of variation in our laboratory was < 5%.
Contrast carotid ultrasonography
The contrast ultrasound investigation was performed using Sonovue (Bracco, Milan, Italy) echocontrast using a previously validated protocol (Citation31). The contrast was infused in an antecubital vein (20 gauge canula) at a rate of 1.3 mL/s. The linear array probe with a mechanical index 0.08 to 0.10 and contrast pulse sequencing was used to achieve best visualization of the plaque morphology and vascularization. The studies were digitally stored for off-line analysis. Plaque neovascularization were classified as grade 0 (no contrast within plaque), grade 1 (bubbles confined to plaque adventitial side), or grade 2 (bubbles reaching plaque core and contrast agent enhancement throughout the plaque (). The inter- and intra-observer coefficient of variation in our laboratory was < 5% (Citation31).
ELISAs
Following centrifugation of peripheral venous blood, the plasma samples obtained were stored at –70°C for batched analysis. Tie2, angiopoietin, and VEGF were measured in citrated plasma by commercial ELISAs (R&D). Intra- and inter-assay coefficients of variation were < 5% and < 10%, respectively. Lower limits of detection were 0.16 ng/mL for Tie2 and angiopoietin and 31.2 pg/mL for VEGF.
Power calculation
Based on our previous work (Citation26), the calculated minimum number of participants required to achieve 80% power to detect a difference of 0.5 standard deviations in mean monocyte count between the study groups was n = 35 for the cross-sectional study.
Statistical analysis
Normal data are expressed as mean (standard deviation, SD), non-normal data are reported as median (interquartile range, IQR). The between-group comparisons were done using ANOVA with Tukey's post hoc test (normal data) or Kruskal–Wallis test with Dunn's post hoc test (non-normal data). Predictive values of the study parameters for carotid stenosis (assessed as maximal stenosis percentage detected from both sides) and from IMT (assessed as average IMT from the right and left sides) were established using linear regression analysis. These analyses were done across the whole study population (n = 160).
Predictive values for intraplaque neovascularization were done using multinomial regression analysis among participants with detectable atherosclerotic plaques and good-quality contrast study (n = 128). The choice of variables for multivariate regression analyses was based on their significance on univariate analysis and relevance. Two-tailed P values were used. Correlation analyses were performed using the Spearman test. The differences/effects were considered significant with a P value of less 0.05, except for comparisons of surface monocyte markers where a P value of less 0.01 was considered significant to account for multiple comparisons. Statistical analysis was performed with IBM SPSS20 software with GraphPad Prism 4.0 software (La Jolla, CA, USA) to produce the graphs.
Results
Monocyte subsets and carotid atherosclerosis
The study groups were matched for age and gender, but, as expected, there were significant differences between the groups in clinical characteristics (). A proportion of control subjects had non-significant asymptomatic carotid atherosclerotic lesions (i.e. < 50%) reflecting the natural pattern of high prevalence of subclinical stable atherosclerotic plaques in older populations (). The CS > 50 and CS < 50 groups were well matched for the majority of demographic and clinical characteristics. There was no significant difference in IMT between the study groups (). All patients from CS > 50 and CS < 50 groups received statins, whilst control groups were free of statins.
Table I. Demographic, clinical, and carotid artery characteristics in the study groups.
The CS > 50 group had increased counts of Mon1 compared to HCs (P = 0.03) and MPAs lower than in the CS < 50 group (P < 0.001) (). This group also had increased expression of CD14 on Mon2 (P = 0.001 versus HC); IL6R on Mon1 and Mon2 (P < 0.001 versus NC); TLR4 on Mon1 and Mon3 versus CS < 50 (P < 0.01) and TLR4 on all subsets versus HC and NC (P < 0.001); VEGF receptor 2 on Mon2 (P < 0.001 versus NC) and on Mon3 (P < 0.01 versus HC and P < 0.001 versus NC); CXCR4 on all subsets (P < 0.001 versus NC for all three subsets); Tie2 on Mon1 (P < 0.001 versus NC and HC), Mon2 (P < 0.001 versus NC), and Mon3 (P < 0.01 versus NC). The CS>50 group had decreased CD16 expression on all subsets (P ≤ 0.001 versus all other groups).
Table II. Monocyte subsets in the study groups.
Similarly to CS > 50, the CS < 50 group had increased counts of Mon1 compared to HCs (P = 0.009) (). But Mon3 were also increased in CS < 50 compared with HCs (P < 0.01). MPAs were higher in the CS < 50 group than in both the CS > 50 group (P < 0.001) and NCs (P < 0.001). The CS < 50 group showed increased expression of CCR2 on Mon3 (P = 0.002 versus NC); IL6R on Mon1 and Mon2 (P < 0.001 versus NC); TLR4 on Mon1 (P < 0.001 versus HC), Mon2 (P < 0.001 versus HCs, P < 0.01 versus NCs), and Mon3 (P < 0.001 versus NC); VEGF receptor 2 on Mon3 (P < 0.01 versus HC); CXCR4 on Mon3 (P < 0.01 versus HC); Tie2 on all subsets versus NC (P < 0.001 for Mon1 and Mon2, P < 0.01 for Mon3). Patients from the CS < 50 groups had the highest expression of CD49d, of all the groups, on all monocyte subsets (P < 0.01 for all). In contrast to the CS>50 group, monocyte CD16 expression was not changed in CS < 50 patients.
In univariate regression analysis, higher Mon1 counts were predictive of degree of maximal carotid stenosis (P < 0.001), whilst there was modest predictive value for Mon3 (P = 0.03) and a non-significant trend for Mon2 (P = 0.06) (). In multivariate regression analysis only high Mon1 counts remained a significant predictor of carotid stenosis (P = 0.04). Mon1 was a significant predictor of IMT on multivariate analysis (P = 0.02) ().
Table III. Linear regression analysis for predictors of carotid stenosis and intima-media thickness.
Monocyte subsets and plaque neovascularization
From the whole study population, 128 subjects had atherosclerotic plaques suitable for assessment of intraplaque neovascularization (). The grade 0 group had slightly higher body mass index (BMI) and estimated glomerular filtration rate (eGFR) than the grade 2 group (P = 0.03 for both). Patients with grade 2 neovascularization had higher Mon1 counts compared to those with grade 0 (P = 0.048 for ANOVA and P = 0.06 for post hoc analysis) (). There were no differences in levels of other monocyte subsets and MPA, nor in expression of the studied monocyte surface markers at the P < 0.01 significance threshold. No differences between groups were seen in levels of plasma markers of angiogenesis, with no correlation found between their levels and density of the corresponding receptors on monocytes (full data not shown).
Table IV. Demographic and clinical characteristics of patients with different grades of plaque neovascularization.
Table V. Monocyte subsets and plasma markers of angiogenesis in patients with different grades of plaque neovascularization.
In univariate regression analysis, predictors of grade 1 neovascularization were low BMI (P = 0.02), smoking (P = 0.02), degree of carotid stenosis (P = 0.02), and lower eGFR (P = 0.05 for a trend) (). Predictors of grade 2 neovascularization were degree of carotid stenosis (P = 0.03), reduced eGFR (P = 0.02), with a trend seen for smoking (P = 0.06). High Mon1 count was significantly associated with both grade 1 (P = 0.046) and grade 2 (P = 0.023) neovascularization. On multivariate analysis, Mon1 remained significantly associated with grade 2 neovascularization after adjustment for age, BMI, eGFR, carotid artery stenosis, and aspirin and statin use (P = 0.034).
Table VI. Multinomial regression analysis for predictors of plaque angiogenesis.
Discussion
This is the first study that has assessed the relation of different monocyte subsets to the severity of carotid atherosclerosis and carotid plaque neovascularization. The study demonstrates for the first time that ‘classical’ monocytes (Mon1) are the only monocyte subset associated with severity of carotid atherosclerosis independently of other risk factors. This subset was also the only monocyte subset whose counts were predictive of IMT, a marker of carotid and generalized atherosclerosis (Citation32,Citation33).
Despite some controversy in respect to the link between the total monocyte count and carotid atherogenesis, overall, the published data support the role of monocytosis in carotid atherogenesis (Citation34,Citation35). In a prospective study of subjects free from carotid disease during a 10-year follow-up, total monocyte count was significantly predictive of future plaque development (Citation34).Until now it has been unclear which of the monocyte subsets were principally implicated in the process.
Of interest, Mon3 monocytes (i.e. ‘non-classical’ monocytes) which were previously shown to be increased in stable CAD were only significantly increased in patients with moderate, but not with severe, carotid stenosis in the present study (Citation36). The exact reasons for this difference cannot be fully explained based on design of the present study, but it could be due to the predominant role of the subset in the initial development and growth of atherosclerotic plaques. We also observed a similar phenomenon in relation to expression of CD49d by all monocyte subsets (i.e. a receptor that mediates recruitment of monocytes to areas of vascular inflammation and atherogenesis) (Citation20) and levels of MPAs (another phenomenon contributing to monocyte migration to the vascular tissues) (Citation37).
Not only numbers of monocytes but also their functional status could play a role in atherosclerotic plaque formation. The present study supports this hypothesis by demonstrating significant up-regulation of different monocyte receptors mediating inflammatory and angiogenic processes in the patients with carotid atherosclerosis. These changes were not confined to a single monocyte subset but were present variably across all three monocyte subpopulations, possibly reflecting complex implication and functions of different subsets in atherogenesis. Of note, some features of excessive proinflammatory activation, such as increased expression of IL6R on Mon2, was only seen in CS > 50 patients, suggesting a higher degree of monocyte proatherogenic changes in subjects prone to more severe coronary atherosclerosis. Interestingly, patients with severe carotid stenosis had a highly significant reduction in CD16 expression by all monocytes. The presence of this phenomenon does not have a straightforward explanation, and further investigation of related changes in monocyte functionality may be of interest.
The present study is the first to evaluate the role of monocytes and their subsets in carotid plaque neovascularization and to show a significant and independent association of the Mon1 subset in the development of plaque neovascularization. Two major factors influencing intravascular neovascularization are local ischaemia and inflammatory burden (either local or systemic). Diffusion of oxygen and other nutrients is limited to 100 μm from the lumen of the blood vessel, which in normal arteries is adequate to nourish the inner media and intimal layers. As vessel wall thickness increases in the setting of vascular disease, proliferation of the vasa vasorum and intimal neovascularization is observed, and the degree of adventitial neovascularization is associated with carotid IMT (Citation38). The role of local ischemia in angiogenesis is supported by evidence from a rabbit model of high local levels of hypoxia inducible factor-1 (Citation39), which is a recognized promoter of production of vascular endothelial growth factor (VEGF) (Citation40). Consequently, VEGF (a potent stimulator of angiogenesis) is able to orchestrate a local proangiogenic environment and to mobilize endothelial progenitors. Indeed, VEGF is abundantly expressed within atherosclerotic lesions (Citation41). Lack of significant systemic plasma VEGF level changes could be due to the relatively small area of localized carotid plaque angiogenesis.
Hypoxia-independent pathways of angiogenesis within the vessel wall have been identified and appear to depend on an inflammatory stimulus (Citation42). The density of intraplaque vessels corresponds to the focal accumulation of inflammatory cells (such as monocytes/macrophages) forming a vicious circle: enhanced angiogenesis—enhanced mobilization of inflammatory cells—enhanced angiogenesis (Citation43). The present study indicates that pathological carotid intraplaque angiogenesis determined by carotid contrast ultrasonography is related to the ‘classical’ Mon1 subset with potent proinflammatory and phagocytic properties (Citation26,Citation44). However, the clinical significance of findings of this pilot study needs to be addressed in larger studies with appropriate follow-up and adequate statistical power, as well as studies with direct assessment of plaque histology, which was beyond the scope of the present study.
Limitations
This pilot study is limited by its observational nature, and the clinical significance of the finding for adverse outcome is still to be established. Characteristics and counts of blood monocytes were used as surrogates of monocyte function, which may differ for their distribution within atherosclerotic plaques. These pilot data are obtained from a relatively small study population and require further confirmation from larger prospective analyses of plaque progression, outcome assessment, and analysis of the plaque histology. Variability in statin agents used, dosages, and duration of treatment could bias the study findings. The study design cannot rule out asymptomatic myocardial and cerebral ischaemia in the study participants, and this needs to be considered in any interpretation of the study results.
Conclusion
Classical monocytes (Mon1) represent the only monocyte subset predictive of the severity of carotid and systemic atherosclerosis, such as carotid IMT, degree of carotid stenosis, and presence of carotid intraplaque neovascularization. Changes in monocyte phenotype featured by increased expression of receptors on inflammatory molecules, factors of angiogenesis, and CXCR4 are related to more severe carotid stenosis but not to plaque angiogenesis. These observations may have important implications for the pathogenesis of carotid plaque and intraplaque angiogenesis.
Declaration of interest: The authors report no conflicts of interest.
References
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–82.
- Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113:2320–8.
- de Groot E, van Leuven SI, Duivenvoorden R, Meuwese MC, Akdim F, Bots ML, et al. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:280–8.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67.
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25.
- Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–75.
- Milei J, Parodi JC, Alonso GF, Barone A, Grana D, Matturri L. Carotid rupture and intraplaque hemorrhage: immunophenotype and role of cells involved. Am Heart J. 1998;136:1096–105.
- Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR Jr, Schwartz RS, et al. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–6.
- de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–8.
- McCloskey K, Vuillermin P, Ponsonby AL, Cheung M, Skilton MR, Burgner D. Aortic intima-media thickness measured by trans-abdominal ultrasound as an early life marker of subclinical atherosclerosis. Acta Paediatr. 2014;103:124–30.
- Zureik M, Ducimetiere P, Touboul PJ, Courbon D, Bonithon-Kopp C, Berr C, et al. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: longitudinal results from the Aging Vascular Study (EVA) study. Arterioscler Thromb Vasc Biol. 2000;20:1622–9.
- Del Rincon I, Williams K, Stern MP, Freeman GL, O’Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–40.
- Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26:450–6.
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–8.
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61.
- Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–50.
- Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. 2006;8:810–15.
- Shantsila E, Lip GY. Monocytes in acute coronary syndromes. Arterioscler Thromb Vasc Biol. 2009;29:1433–8.
- Lauener RP, Geha RS, Vercelli D. Engagement of the monocyte surface antigen CD14 induces lymphocyte function-associated antigen-1/intercellular adhesion molecule-1-dependent homotypic adhesion. J Immunol. 1990;145:1390–4.
- O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–82.
- van der Wal AC, Das PK, Tigges AJ, Becker AE. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992;141:1427–33.
- Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–52.
- Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, et al. CD14 + CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–22.
- Schmitz G, Grandl M. Lipid homeostasis in macrophages - implications for atherosclerosis. Rev Physiol Biochem Pharmacol. 2008;160:93–125.
- Huang PT, Huang FG, Zou CP, Sun HY, Tian XQ, Yang Y, et al. Contrast-enhanced sonographic characteristics of neovascularization in carotid atherosclerotic plaques. J Clin Ultrasound. 2008;36:346–51.
- Shantsila E, Wrigley B, Tapp L, Apostolakis S, Montoro-Garcia S, Drayson MT, et al. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost. 2011;9:1056–66.
- Shantsila E, Tapp LD, Wrigley BJ, Montoro-Garcia S, Ghattas A, Jaipersad A, et al. The effects of exercise and diurnal variation on monocyte subsets and monocyte-platelet aggregates. Eur J Clin Invest. 2012;42:832–9.
- Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GY. The CD14++ CD16 + monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost. 2012;10:1231–41.
- Wrigley BJ, Shantsila E, Tapp LD, Lip GY. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ Heart Fail. 2013;6:127–35.
- Wardlaw JM, Lewis S. Carotid stenosis measurement on colour Doppler ultrasound: agreement of ECST, NASCET and CCA methods applied to ultrasound with intra-arterial angiographic stenosis measurement. Eur J Radiol. 2005;56:205–11.
- Jaipersad AS, Shantsila A, Silverman S, Lip GY, Shantsila E. Evaluation of carotid plaque neovascularization using contrast ultrasound. Angiology. 2013;64:447–50.
- Bots ML, Grobbee DE. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther. 2002; 16:341–51.
- Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, et al. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33:183–90.
- Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njølstad I, et al. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36:715–19.
- Huang ZS, Wang CH, Yip PK, Yang CY, Lee TK. In hypercholesterolemia, lower peripheral monocyte count is unique among the major predictors of atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:256–61.
- Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, et al. CD14 + CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–24.
- van Gils JM, da Costa Martins PA, Mol A, Hordijk PL, Zwaginga JJ. Transendothelial migration drives dissociation of plateletmonocyte complexes. Thromb Haemost. 2008;100:271–9.
- Magnoni M, Coli S, Marrocco-Trischitta MM, Melisurgo G, De Dominicis D, Cianflone D, et al. Contrast-enhanced ultrasound imaging of periadventitial vasa vasorum in human carotid arteries. Eur J Echocardiogr. 2009;10:260–4.
- Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870–6.
- Kuwahara F, Kai H, Tokuda K, Shibata R, Kusaba K, Tahara N, et al. Hypoxia-inducible factor-1alpha/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension. 2002;39:46–50.
- Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–16.
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9: 653–60.
- Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–41.
- Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80.

