Abstract
Short sleep duration has been shown to be associated with elevated body mass index (BMI) in many epidemiological studies. Several pathways could link sleep deprivation to weight gain and obesity, including increased food intake, decreased energy expenditure, and changes in levels of appetite-regulating hormones, such as leptin and ghrelin. A relatively new factor that is contributing to sleep deprivation is the use of multimedia (e.g. television viewing, computer, and internet), which may aggravate sedentary behavior and increase caloric intake. In addition, shift-work, long working hours, and increased time commuting to and from work have also been hypothesized to favor weight gain and obesity-related metabolic disorders, because of their strong link to shorter sleep times. This article reviews the epidemiological, biological, and behavioral evidence linking sleep debt and obesity.
Key messages
Epidemiological data have identified an association between short sleep duration and overweight and/or obesity. This association is more consistent in children than in adults.
Sleep restriction results in metabolic and endocrine alterations (e.g. increased levels of ghrelin, decreased levels of leptin, increased hunger and appetite, decreased glucose tolerance, decreased insulin sensitivity, increased evening concentrations of cortisol).
Insufficient sleep is associated with increased food intake, snacking, and poor diet quality.
Introduction
Sleep is a restorative process that plays an important role in the balance of psychological, emotional, and physical health. Increasing evidence suggests that not having enough sleep may be associated with adverse health effects, such as obesity, type 2 diabetes, hypertension, and cardiovascular disease (Citation1–3).
Reduced sleep duration and sleep quality are increasingly frequent in modern society and are likely linked to changes in the socio-economic environment and lifestyle (Citation4). The percentage of adults who reported sleeping 6 hours or less increased by 5%–6% between 1985 and 2004 (Citation5). Most studies in college students indicate that they are chronically sleep-deprived with self-reported average sleep duration of around 7 hours per night (Citation6) versus the 9–10 hours usually recommended for adolescents.
Simultaneously, obesity has become a major public health epidemic worldwide. In the past three decades, obesity rates for adults have doubled, and rates for children have tripled (Citation7).The increasing prevalence of obesity affects all industrialized countries. In 2008, 1.46 billion adults worldwide had a body mass index (BMI) of 25 kg/m² or greater, and, of these, 205 million men and 297 million women were obese (BMI > 30 kg/m²) (Citation8). The prevalence of obesity is not distributed equally across nations and socio-economic groups; for example, in France, national epidemiological data in 2012 showed that 32% of adults were overweight and 15% obese (Citation9). Asian countries, in comparison with others countries such as USA, have lower but increasing rates of overweight and obesity (Citation10). Economic development, industrialization, urbanization, decreased physical activity, and disturbed food habits may probably partly explain this increasing prevalence of obesity observed.
Obesity is a multifactorial disease occurring as the result of complex interactions between genetic and environmental factors (Citation11). The dramatic increase in obesity prevalence observed in the last decade seems to be largely attributable to environmental changes promoting the intake of energy-dense foods and/or the reduction in physical activity associated with the high number of sedentary jobs, available transportation systems, and increasing urbanization (Citation11). Although poor diet and reduced physical activity both play a major role in the risk of weight gain and the obesity epidemic, other factors may also be involved.
Reduced sleep duration was proposed as a possible contributing factor 15 years ago (Citation12). In adults, short sleep is usually defined as reported sleep duration of ‘less than 6 hours per regular (working or active) day’ including night sleep, napping, and resting (Citation13). Epidemiological studies suggest that sleeping for less than 6 h is associated with increased morbidity in terms of development of obesity, type 2 diabetes, cardiovascular disease, and risk of accidents (Citation14).
Based on the literature, no consensus definition exists for the term ‘sleep debt’, which may reflect a voluntary (due to work conditions, transport, or leisure) or involuntary (due to insomnia or noisy environment) shortening of sleep duration (Citation13). Similarly, there is no agreed definition of inadequate sleep, which can refer to short sleep duration (generally < 6 hours per night) or to poor sleep quality with or without sleep disorder (Citation15).
In this review, we focus only on reduced sleep duration with no associated sleep disorder (e.g. insomnia, sleep apnea). We first review the epidemiological data that support an association between short sleep duration and obesity. We then examine the different physiological and behavioral pathways that may explain the association between sleep reduction and weight gain. Finally, we wonder whether improving sleep duration could be a preventive tool against weight gain and obesity.
Sleep debt and obesity: the epidemiological evidence
The reduction in sleep quantity and quality has been associated with obesity in numerous epidemiological surveys.
Review and meta-analyses
One of the first observations published on the association between sleep duration and weight was a case–control study in which 327 obese children and 704 controls aged 5 years were observed (Citation16). Several environmental factors contributing to child obesity (snacks, television viewing) were highlighted, but the most important factor identified was the reduction in sleep duration; this was the only risk factor that remained significant after adjustment (adjusted OR = 1.4). Since this study, numerous cross-sectional and longitudinal epidemiological studies have examined the association between sleep duration and weight gain in children, adolescents, and adults, and we will discuss the results of some recent meta-analyses on this issue.
Cappuccio et al. provided quantitative estimates of the cross-sectional association between sleep duration and obesity in children and in adults (Citation17). Thirty-six studies were included in the meta-analysis, with a total of 30,002 children and 604,509 adults. In children, the pooled OR for short duration of sleep (< 10 hours) and obesity (BMI > 95th percentile) was 1.89 (1.43–1.68; P < 0.0001). In adults, the pooled OR was 1.55 (1.43–1.68; P < 0.0001). The authors also demonstrated that adults reporting five or fewer hours of sleep per night were at a higher risk of being obese and that every additional hour of sleep was associated with a 0.35 kg/m² decrease in BMI.
The same year, another meta-analysis of cohort and cross-sectional studies in the general pediatric population identified 17 studies (Citation18). The pooled OR predicting obesity from short sleep duration was 1.58 (1.26–1.98). This meta-analysis supported a sex difference in the association, with boys having a stronger inverse association than girls (OR = 2.50 (1.88–3.84) versus OR = 1.24 (1.07–1.45)). The results also showed a significant linear dose–response relationship between decreased sleep duration and increased BMI in young children (< 10 years old).
Two key aspects can be drawn from these meta-analyses: First, the association is more robust in children than in adults; second, a gender difference may exist.
Are children more vulnerable than adults?
Several systematic reviews concluded that short sleep duration was consistently associated with a higher risk of obesity in children, but not in adults () (Citation19–22), with an effect of sleep duration on weight gain declining when age increases in the adults population. The association is also more difficult to assess in adults due to the higher percentage of associated sleep disorders (insomnia, sleep apnea) which may disturb total sleep time (Citation22).
Table I. Summary of principal systematic reviews examining the association between sleep duration and obesity in child and in adult populations. This table shows that almost all the studies performed in children found an association between short sleep duration and obesity, but not in adults, especially for prospective (1/7) or longitudinal (8/13) studies.
A recent systematic review of 20 longitudinal studies produced similar results (Citation23). Among the 13 studies in adult populations, 4 found an association between short sleep duration and weight gain, 4 found an association between short and long sleep durations and weight gain, and 5 studies found no significant association. All the 7 longitudinal studies involving children reported an association between short sleep duration and increased weight.
Data from the National Health and Nutrition Examination Survey demonstrated that, among participants without depression or a diagnosed sleep disorder, sleep duration was associated with BMI and waist circumference and the association was stronger among young adults (age between 20 and 39 years) (Citation24).
However, a recent large cross-sectional study also showed an association between short sleep duration and obesity in adults aged 45 years or older (Citation25). This result was confirmed by data from the NIH-AARP Diet and Health Study Cohort. Among participants aged 51–72 years and followed during 7.5 years, who were not obese at baseline, those who reported less than 5 hours of sleep per night had an approximately 40% higher risk of developing obesity than did those who reported 7–8 hours of sleep (Citation26).
In summary, the impact of short sleep duration on obesity risk appears greater in children than in adults and probably greater in young adults than in mid-life or late life, although the relationship is still present at older ages. This finding suggests that children and adolescents may be more vulnerable to the effects of insufficient sleep. Sleep is important for brain development. Sleep loss at young age may alter the hypothalamic mechanisms that regulate appetite and energy expenditure. Another possibility to explain the effect of age is that the impact of sleep duration on weight gain may alter over time such that short-duration sleepers may not continue to gain weight linearly.
However, we did not find any longitudinal survey which allows us to determine which age group may be determinant in the association. Another important limitation in studies on children is that different methods were used to determine obesity.
Is there a gender effect?
Gender differences are consistently found in some sleep disorders: for example, insomnia is more prevalent in women, and sleep apnea in men. However, the gender difference in the association between sleep duration and obesity remains unclear.
In children, the majority of studies have not reported a gender difference. For example, a recent cross-sectional study on 1589 children demonstrated that short sleep duration was associated with obesity in girls (RR 1.2; 95% CI 1.0–1.4) and boys (PR 1.3; 95% CI 1.1–1.5) after adjustment for behavioral problems and social risk factors (Citation27). Researchers who did identify a gender difference suggested that girls may be more resilient to environmental stressors than boys and would probably need greater sleep deprivation to affect their metabolism than boys (Citation18,Citation28,Citation29).
Similarly, in adults, it is unclear whether sleep duration is differentially related to obesity according to gender. Some studies observed no differences by gender or ethnicity (Citation24), whereas others reported a positive association only in men (Citation30,Citation31). In a prospective study, Watanabe et al. found an association between short sleep duration and obesity in men but not in women. In men, the adjusted odds ratios for development of obesity were 1.91 (95% CI 1.36–2.67) and 1.50 (95% CI 1.24–1.80), respectively, in those who slept less than 5 hours and in those who slept between 5 and 6 hours (Citation31). In this study, the sex difference could have been explained by the low prevalence of obesity and newly developed obesity in the women.
Still others report that the link is stronger or only present in women (Citation32,Citation33). Mezick et al. have investigated associations between sleep and anthropometric variables using both subjective and objective measures of sleep (Citation32). Participants (n = 1248, age ranged from 34 to 84 years, 43% men) reported their habitual sleep duration, and 441 of them (40% men) underwent 7 nights of wrist actigraphy. Among all participants, increased BMI was associated with shorter and less efficient sleep. But further examinations by gender have shown that these associations were only present in women. One possible explanation for this finding is that ‘women often report needing more sleep and feeling less rested than men’; it is therefore possible that the physiological consequences of sleep loss may be more pronounced in women (Citation32). Moreover, menopausal state has an influence on sleep, and menopause is often related to weight gain, which may, thus, explain the greater difference observed in women in some studies.
Limitations of epidemiological studies: objective measures of sleep
An important issue in epidemiological studies is that reliable and valid measures of both sleep duration and obesity need to be used. Most of the literature examining the relationship between sleep and obesity is based on self-reported sleep assessments, and the correlation between self-reported sleep and objective measures of sleep is moderate (Citation34). In population-based samples, self-reports of habitual sleep duration are biased by systematic over-reporting compared to objective measures of sleep duration.
Another issue is that the causes of short sleep duration are generally not reported, and it is particularly difficult to know whether short sleep duration was the result of voluntary chronic restriction in a healthy sleeper or of the inability to sleep longer in an individual suffering from a sleep disorder (Citation35).
A few studies have provided data on objective duration of sleep and BMI. In 2004, Taheri et al. used polysomnography records (PSG, the gold standard of sleep evaluation) in a large longitudinal survey and demonstrated a U-shaped curvilinear association between sleep duration and BMI. In subjects sleeping less than 8 hours, increased BMI was proportional to decreased sleep (Citation36).
Another recent study also used PSG and found that short sleep duration was associated with central obesity (waist circumference and abdominal diameter), a risk factor for cardiometabolic disorders, in a population of middle-aged women (Citation33). The results of this study indicated that the loss of slow-wave sleep and REM sleep (two qualitatively essential stages of sleep) may play an important role in the association between short sleep and obesity (Citation33). A relationship between slow-wave sleep and waist circumference was also previously reported in elderly men in a cross-sectional study (Citation30).
In a pediatric sleep clinic population, short sleep duration (assessed by PSG and defined as 1 hour of sleep less than the duration recommended per age) was correlated to obesity independently of the presence of obstructive sleep apnea (Citation37).
Several other PSG studies did not, however, identify a strict association between sleep duration and weight gain (Citation34,Citation36,Citation38,Citation39).
In a longitudinal population-based study using PSG, Vgontzas et al. found that the incidence of obesity was higher in women and in individuals who reported poor sleep, but also in subjects with subjective short sleep duration and emotional stress (Citation40). The associations were stronger in young and middle-aged adults. However, objective short sleep duration was not significantly associated with obesity (Citation40). The authors suggested that an individual's perception of sleep duration may more clearly correlate with obesity than does objective sleep loss. One explanation is that self-reported short sleep duration does not reflect real short sleep duration, but may be a marker of sleep complaints, such as insomnia, poor sleep, and chronic psycho-social stress (Citation41).
In 2008, in a large population study (Penn State Cohort), the same authors showed that obese individuals reporting short sleep duration had a higher incidence of subjective sleep disturbances and a higher score for chronic and emotional stress than did non-obese subjects (Citation40). Data from the National Health Interview Survey indicated that adults who usually slept less than 6 hours were more likely to engage in certain health risk behaviors (cigarette smoking, alcohol drinking, physical inactivity) than adults who slept 7–8 hours (Citation42). Furthermore, these behaviors used to reduce stress and to improve sleep were associated with increased food intake and significant weight gain.
Reducing emotional stress may become a target of preventive strategies against obesity. Stress and emotional disturbance have also been associated with sleep duration and obesity and may represent a potential link between these two factors.
Limitations of epidemiological studies: objective measures of adiposity or visceral fat
Self-report of BMI (based on weight and height) is often used to determine overweight and obesity status. However, BMI does not necessarily provide accurate estimates of percentage body fat or of body composition.
Few epidemiological studies have evaluated the association between sleep duration, body weight, and adiposity. Among 330 young adult women (20.2 ± 1.5 years), inconsistent sleep patterns and poor sleep efficiency were related to adiposity (Citation43). In this study, sleep was monitored by actigraphy and physical activity, and body composition was also objectively measured. Interestingly, other sleep variables, such as sleep quality and sleep efficiency, had a stronger association with BMI than did sleep duration. These findings support the importance of considering consistent sleep, which can be defined as sleep with no interruption, and wake schedules in future studies.
A study conducted in two minority groups in the US—African American and Hispanic adults—focused not only on BMI but on visceral and subcutaneous fat. These investigators found that younger subjects (18–39 years old) who slept less than 5 hours per night experienced a larger increase in BMI, visceral fat, and subcutaneous fat over 5 years than those who slept 6–7 hours per night (Citation44). No association between sleep and BMI or fat was observed in older subjects (> 40 years old).
Recently, the association between sleep time (assessed by actigraphy) and BMI was reported in women but not in men (Citation32).
Perspectives
It is important to mention that epidemiological evidence alone cannot establish a cause–effect relationship between sleep duration and obesity. Inadequate sleep may lead to obesity, but obese individuals are more likely to have inadequate sleep. A bidirectional effect may thus exist: short sleep duration leading to weight gain and weight gain leading to short sleep duration in a vicious cycle. Epidemiological studies have also included many confounders, which vary across the studies, and some important confounders are not always taken into account in the analyses, such as socio-economic status, physical activity, alcohol, caffeine consumption, mood, and psychological disorders. For example, despite the inverse relationship observed between sleep duration and weight gain, a recent publication demonstrated that parental rules may confound the association, particularly in 3-year-old children (Citation45). Three-year-old children and their parents were recruited in nursery schools in socio-economically deprived and non-deprived areas. Parents were interviewed to assess their use of sleep, television-viewing, and dietary rules. Children were measured for height, weight, waist circumference, and triceps and sub-scapular skin fold thicknesses. Parental rules were associated with longer night-time sleep and were more prevalent in the non-deprived area. Television-viewing and dietary rules were associated with leaner body composition. As rules cluster across behavioral domains and are associated with sleep duration and body composition, the authors conclude that this could play a role and confound the association observed between sleep duration and obesity in young adults. However, this preliminary study needs to be replicated (Citation45).
It is also important to note that the majority of research has been conducted in Western countries, with the exception of Japan (Citation31,Citation46). However, the findings from Japan and Western countries did not differ. In Japan's studies, short sleep remains associated with weight gain and development of obesity. Comparative research on sleep and its association with obesity is lacking (Citation15). Only the Behavioral Risk Factor Surveillance System (BRFSS) showed, in a multi-ethnic sample of US adults, that perceived insufficient rest or sleep was independently associated with cardiovascular disease, stroke, diabetes mellitus, and obesity (Citation47).
To clarify the effects of sleep duration on the risk of obesity, randomized prospective interventional trials are needed. Although depriving subjects of sleep for extended periods of time may be unethical, extending sleep in individuals with short sleep duration and obesity could help to clarify the relationships (Citation48).
Sleep debt and obesity: the biological hypothesis
In addition to epidemiological data, several hormonal and metabolic pathways may be involved in the association between short sleep duration, overweight, and obesity ().
Figure 1. Schematic representation of the possible biological and behavioral pathways linking sleep debt and obesity.
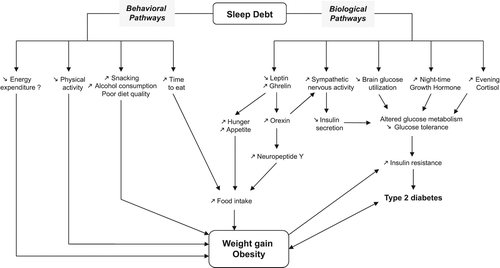
It is well known that during sleep the secretion of various hormones varies, contributing to the metabolism and energy balance of our body (Citation49). Sleep reduction or deprivation may disturb this balance. Overweight or obesity is usually associated with an energy intake that is greater than energy expenditure (Citation15). Energy intake is dependent on appetite regulation, which determines the quantity and quality of food intake (Citation15). Two peripheral hormones, leptin (a 16-kDa polypeptide cytokine) and ghrelin, act on the hypothalamic nuclei to regulate energy balance and food intake (Citation50). Leptin is produced mainly by the adipocytes and inhibits appetite, whereas ghrelin is released by the stomach and stimulates appetite (Citation35).
Sleep duration and BMI
The Wisconsin Sleep Cohort Study, a population-based longitudinal study of sleep disorders, included 1024 volunteers with stable sleep duration, who underwent questionnaires about their sleep habits, completed sleep diaries, had nocturnal polysomnography (PSG), and had fasted blood samples taken the morning following the PSG. Serum leptin, ghrelin, glucose, insulin, and lipid profiles were measured. After controlling for confounding factors, a U-shaped curvilinear association was found between sleep duration and BMI. Subjects sleeping less than 8 h had an increased BMI proportional to the decreased length of sleep. Short sleep duration was associated with low leptin (predicted 15.5% lower leptin for sleep of 5 h versus 8 h) and high ghrelin (predicted 14.9% higher ghrelin for sleep of 5 h versus 8 h), independent of BMI, age, sex, and other confounding factors (Citation36). No significant correlation was found between sleep duration, insulin, glucose, and lipid profiles.
Sleep restriction and leptin and ghrelin
As early as 2003, Mullington et al. showed that prolonged sleep loss (88 consecutive hours of sleeplessness) in 10 healthy men decreased the circadian amplitude of leptin (Citation51). Experimental studies of partial sleep restriction in humans were conducted in Van Cauter's laboratory in Chicago. The first studies were conducted in 11 healthy young, non-obese men (4 hours in bed for 6 nights followed by 6 nights with 12 hours in bed) and in 8 healthy men (4 hours in bed for 2 nights followed by 10 hours of sleep extension) with a constant glucose infusion as the only source of calories. Caloric intake and activity level were carefully controlled in the studies. The first study showed a 30% reduction of the leptin maximum amplitude during sleep restriction compared with rested conditions (Citation12). In the other study, sleep restriction was associated with an 18% reduction in mean leptin level, a 28% increase in ghrelin level, and increased hunger and appetite for every kind of food but especially for carbohydrates. A strong association was found between the leptin/ghrelin ratio increase and hunger (Citation50). In another experiment, seven days of sleep restriction (4 hours per 24 h) in eight young men was also associated with a significant decrease in the leptin rhythm amplitude (Citation52). Interestingly, one study observed 15 healthy women with sleep restricted to 3 h for a single night, and found a very small increase in leptin level after sleep restriction with no change in appetite (Citation53).
Leptin has not always been found to be altered by sleep restriction. In 14 healthy young men who underwent 24 h total sleep deprivation, Benedict et al. found no change in the leptin circadian rhythm (Citation54). Similar findings were reported in a prospective study in 21 lean teenage boys (15–19 years) after three consecutive nights of 4 h sleep duration (Citation55). Finally, no change in leptin levels was observed in young men (20–40 years old) submitted to a single night of 4.5 h sleep duration compared to 1 night of 7.5 h sleep, despite increased ghrelin levels and hunger (Citation56).
Sleep restriction and other nutrition hormones
Blood levels of other hormones have also been assessed during chronic partial sleep loss studies (Citation12). Thyrotropin concentrations were depressed and growth hormone (GH) secretion patterns biphasic with a first pre-sleep circadian pulse (during wakefulness in sleep restriction conditions with delayed sleep onset) and a post-sleep pulse (during sleep).The pre-sleep pulse, which was the larger one, occurred at the habitual time of the first cycle of sleep. The post-sleep onset pulse was negatively related to the pre-sleep onset GH secretion but tended to be positively correlated with the amount of concomitant slow-wave activity (SWA). The amount of GH secreted during the first 3 hours of sleep was less during sleep restriction (34% of 24 h secretion) than during sleep extension (53% of 24 h secretion). During sleep extension, the subjects recovered their normal GH secretion profile (Citation57).
Sleep restriction and glucose
It has been shown that glucose regulation is markedly affected by the sleep–wake cycle. Glucose levels decline during sustained wakefulness and remain quite stable during overnight sleep. Whole-brain metabolism declines from wakefulness to NREM sleep, resulting in reduced glucose utilization. As brain glucose is not mediated by insulin, the decline in brain glucose utilization results in reduced glucose effectiveness. Glucose tolerance is optimal in the morning and decreases during the day and the night (Citation58).
Some experimental studies of sleep restriction showed a link between sleep debt and impairments in glucose metabolism leading to a risk of type 2 diabetes. In 11 young men, Spiegel et al. compared glucose tolerance, cortisol, and activity of the sympathetic nervous system after sleep restriction of 4 hours in bed for 6 nights and 12 hours sleep recovery for 7 nights (Citation12). During the sleep restriction period, insulin sensitivity was lower than during sleep recovery, but this difference did not reach statistical significance; glucose tolerance (ability of the tissues to absorb glucose from blood and return blood glucose level to baseline), measured using the intravenous glucose tolerance test, was lower than in recovery conditions (P < 0.02) as observed in older adults with impaired glucose tolerance; and glucose effectiveness (ability of glucose to mediate its own disposal independently of insulin) was 30% lower, as was the acute insulin response (P = 0.05), which is an early marker of diabetes. Glucose tolerance returned to the normal range after sleep extension. Evening cortisol was higher during sleep restriction (P = 0.0001), and activity of the sympathetic nervous system was increased (P < 0.02). Glucose metabolism was also studied during a frequently sampled intravenous glucose tolerance test (IVGTT) after 5 days of sleep restriction and after 6 days of sleep recovery. Glucose tolerance was 40% lower at the end of the sleep restriction period than after sleep extension. The insulin profile showed a biphasic pattern with the first phase (corresponding to the rapid release of insulin from the β cells) reduced by 30%, as was the level of C-peptide by 25%. Glucose effectiveness, a measure of non-insulin-dependent glucose uptake, was also reduced by 30%–40%. The disposition index (product of acute insulin response to glucose and insulin sensitivity index) considered as a predictor of diabetes risk was decreased by 37% in sleep debt compared with the rested state (Citation59). More extended periods of less severe sleep curtailment gave similar results, as did a study with a randomized cross-over design—2 nights with 4 hours in bed and 2 nights with 10 hours in bed—to eliminate an order effect (Citation49). The suppression of slow-wave sleep (SWS) without any reduction in total sleep time resulted in decreased insulin sensitivity, reduced glucose tolerance, and increased risk of type 2 diabetes (Citation60). Nedeltcheva et al. noted that 5.5 hours in bed for 14 nights provoked an 18% reduction in insulin sensitivity and 10% decrease in glucose tolerance compared with 2 weeks of 8.5 hours in bed per night (Citation61); in these two conditions, weight gain was similar. In another experimental study, 20 young (20–35 years) men spent 10 h in bed for 8 nights or more followed by 5 hours in bed for 7 nights (Citation62). After the sleep restriction period, insulin sensitivity was reduced by approximately 11% (11% to 20% according to measurement method), and glucose tolerance was reduced (Citation62). Even a single night of 4 hours was able to decrease peripheral insulin sensitivity by 20%–25% and increase hepatic insulin resistance (Citation63).
Interestingly, a recent study evaluated the effect of sleep restriction on insulin signaling in human adipocytes in a randomized, two-period, two-condition, cross-over protocol (4 days of reduced sleep of 4.5 h versus 8.5 h) in seven young healthy volunteers, under controlled conditions of caloric intake and physical activity (Citation64). Four nights of reduced sleep led to increased insulin resistance and a 30% decrease in insulin sensitivity in fat cells. The impaired cellular insulin sensitivity paralleled a 16% reduction in total body insulin sensitivity. Thus, reducing sleep may have deleterious effects even at the cell level.
Sleep restriction and obesity: a biological hypothesis?
In summary, sleep restriction has a deleterious impact on BMI, which may involve multiple biological pathways. Sleep deprivation was associated with decreased leptin levels in some studies, but not in all, and increased ghrelin level in numerous works. The imbalance between these hormones, which are part of the orexin system that integrates control of feeding, wakefulness, and energy expenditure, may explain the change in hunger, with increased appetite for snacks, fat, and carbohydrates, ultimately leading to weight gain (Citation65). Sleep debt also decreases brain glucose utilization, which may therefore be one of the mechanisms for the decrease in glucose tolerance (Citation59). Altered sympathetic–vagal balance with an increase in sympathetic activity as shown by RR variability and increased catecholamine levels may be a pathway by which sleep loss exerts systemic effects, explaining the decreased insulin response to intravenous glucose perfusion (Citation57,Citation59). This altered balance may also be implicated in the reduction in leptin levels during sleep debt conditions. Experimental studies also demonstrated an increase in cortisol level in the evening, which may impact on insulin sensitivity the following morning and promote a delayed night-time of GH secretion (when sleep time is delayed and sleep restricted). This effect may adversely influence glucose regulation, leading to transient insulin resistance in muscle cells, a decrease in glucose uptake, an elevated blood glucose level, and an increase in insulin resistance in other tissues. GH secretion is indeed known to facilitate a regular glucose level during the night, despite fasting conditions (Citation57–59). Increased sympathetic nervous activity can also decrease insulin secretion from pancreatic β cells. Moreover, we know that sleep restriction is associated with increased levels of pro-inflammatory cytokines predisposing to insulin resistance and diabetes (Citation58).
Sleep debt and obesity: the behavioral pathways around energy balance
As there is accumulating evidence showing that inadequate sleep is associated with poor health outcomes including obesity, two recent reviews have examined the link between sleep duration and energy balance (Citation66,Citation67). St-Onge evaluated studies that had assessed food intake, energy expenditure, and leptin and ghrelin levels after periods of normal and restricted sleep varying from total sleep deprivation (no sleep at all during ≥ 24-h period) to partial sleep restriction (sleep prescription < 7 h per 24-h period) (Citation66). The objective of the Chaput review was to discuss the evidence linking sleep patterns (especially insufficient sleep and sleep timing) to appetite control, feeding behavior, and energy balance (Citation67). We will not discuss the biological results of these studies, discussed previously, but just focus on the behavioral hypotheses.
Sleep deprivation and energy intake
Acute or short-term sleep restriction is consistently reported in association with an increase in food intake, calorie consumption and poor dietary quality, and also alcohol consumption. After partial sleep deprivation, the increased meal and calorie intake was attributed to snacks with a higher carbohydrate or fat content. For example, 14 normal-weight men received a standard breakfast and were told to purchase as much as they could (for a given fixed budget, from 20 high-caloric and 20 low-caloric foods) after a normal night of sleep or after total sleep deprivation (Citation68). After sleep deprivation, ghrelin concentrations were higher and men purchased significantly more calories and grams of food than after 1 night of sleep. Altered food purchasing and increased food intake may represent two behavioral mechanisms that explain weight gain in sleep-deprived men. In a study in which 12 lean men were observed after 2 nights of 4 hours in bed and after 2 nights of 8 hours in bed, the authors reported a 22% increase in energy intake and a 98% increase in fat consumption in the sleep-deprived condition during an ad libitum dinner (Citation69). A significant increase in calorie intake, especially fat, without change in energy expenditure was also found after 6 nights of 4 hours in bed in non-overweight adults (Citation70).
The mechanism that was first proposed was an alteration in the key appetite hormones (e.g. leptin and ghrelin), such that the sensation of hunger is enhanced under restriction of sleep, so that levels of leptin are suppressed and those of ghrelin increased (Citation50,Citation71). However, this finding has not been universally observed, and Chaput suggested in his review that food intake can be overridden by hedonic rather than homeostatic factors (Citation67). This suggestion was based on the recent observation that overeating and weight gain occurred after sleep restriction (5 days) in healthy adults despite increases in leptin and peptide YY (PYY) and decreases in ghrelin that signaled food intake was in excess (Citation72). Moreover, Nedeltcheva et al. showed that recurrent bedtime restriction under free-living conditions did not alter leptin and ghrelin levels but increased snacking (Citation61). In another 8-night partial sleep-restriction survey, caloric intake in the sleep-restricted group increased by + 559 kcal/day and decreased in the control group for a net change of + 667 kcal/day (P = 0.014), but there was no change in leptin or ghrelin levels (Citation73).
These hypotheses were confirmed by fMRI studies showing, in normal-weight adults, that inadequate sleep enhances hedonic stimulus-processing in the brain underlying the drive to consume foods and is consistent with the notion that reduced sleep may lead to a greater propensity to overeat. For example, it has been shown that sleep restriction (4 h/night during 6 days) enhanced regional brain activity during food stimuli in regions involved in reward (Citation74) and enhanced also the neuronal circuitry related to unhealthy food types (Citation66). Recently, Hogenkamp et al. showed that young men had increased feelings of hunger in the morning after total sleep deprivation, which was accompanied by overeating with a preference for snacks specifically after breakfast and not in the fasted state. For these authors, the observed food behavior was driven by both homeostatic and hedonic factors (Citation75).
Chaput, therefore, postulated that in an environment where energy-dense foods are highly palatable and readily available, caloric intake may be directly proportional to the time spent awake, especially if most of the time spent awake is spent participating in screen-based sedentary activities where snacking is common (Citation67). Increased television viewing and computer and internet use during adolescence have been shown to be associated with higher odds of consumption of sweetened beverages, especially at the upper tail of the BMI distribution between ages 14 and 18 years (Citation76).
Another possible behavioral link between sleep deficit and obesity is the time at which people go to sleep. Recent studies have shown that sleep timing can predict weight loss effectiveness in humans (Citation77). For these authors, eating late compared with early (e.g. lunch-time after versus before 3.00 p.m.) impaired the success of a 20-week weight loss therapy in overweight/obese patients. Because energy intake, dietary composition, estimated energy expenditure, appetite hormones, and sleep duration were similar in early- and late-eaters, the authors suggested that changes in the chronotype, genetic background, and/or circadian system function may be implicated in this outcome. In healthy non-obese adults, Spaeth et al. showed that increased daily caloric intake in sleep-restricted participants (5 nights of 4 h in bed) was due to the consumption of 553 additional calories between the hours of 22.00 and 03.59, with a greater percentage of these calories derived from fat during late-night hours compared to day-time and evening hours (Citation78). The importance of sleep timing on energy intake has also been shown in obese adolescents in the sense that later bedtime (mid-point of sleep > 3.30 a.m.) was associated with greater caloric intake and screen time independent of total sleep duration (Citation79).
Sleep deprivation and energy expenditure (EE)
Despite considerable evidence on the association between sleep deficit and food intake, results from experimental studies on energy expenditure and sleep debt are at the moment more limited and contradictory. Some protocols tried to study both mechanisms. For example, a careful cross-over protocol analyzed energy intake and energy expenditure in two groups of eight adults sleeping 5 nights of 5 hours, followed by 5 nights of 9 hours (Citation72). Restricted sleep increased daily energy expenditure by 5% but also increased energy intake with a 0.82 ± 0.47 kg weight gain, despite an increase in 24 h leptin and peptide YY and a significant decrease in 24 h ghrelin levels. Curiously, women, but not men, maintained their weight during normal sleep duration, although food availability led to an increase in food intake. During sleep deprivation both men and women gained weight. The authors’ hypothesis was that increased food intake during sleep deprivation was a physiological adaptation to provide energy, needed to sustain longer wakefulness. When food was accessible, intake exceeded what was needed.
In a study by Schmid et al., sleep restriction (4.25 hours) was significantly associated with a decrease in global physical activity and in the percentage of intense physical activity, and thus a decrease in energy expenditure (Citation80). After acute total sleep deprivation, postprandial (after breakfast) estimated energy expenditure (i.e. indirect calorimetry) was also reduced on the following morning (Citation81).
In contrast, no change in energy expenditure was described in other sleep restriction studies (Citation69,Citation73), and energy expenditure was increased when measured in a calorimetric chamber (Citation82). For these authors, the weight gain seemed to be related more to an up-regulation of appetite, with a subsequent increase in energy intake and a net positive balance, than to a decrease in energy expenditure due to lack of exercise.
In conclusion, short sleep duration does not seem substantially to affect total energy expenditure, nor is there sufficient evidence in support of any meaningful effect of restricted sleep on the different components of energy expenditure (i.e. sleeping metabolic rate, thermal effect of food, physical activity energy expenditure, and non-exercise activity thermogenesis). Each of these components requires different measurement tools, and each can be differentially affected by sleep.
Perspectives
Can sleeping more prevent overweight and obesity?
Beyond the evidence showing an association between sleep deficit and weight gain lies a crucial question: Is ‘sleeping more’ a solution for overweight and obesity? There is no obvious answer to this debate, but several perspectives can be considered to suggest avenues for research.
First, ‘sleeping more’ is not an easy solution. Multiple epidemiological studies have shown an increase in the percentage of adults and adolescents around the globe reporting short sleep durations (Citation13,Citation83). The determinants of short sleep duration are linked to occupational duties (shift-work, increased commuting time between home and work) but also to leisure activities and the use of internet, mobile phone, and video games (Citation84–86). In addition to the behavioral components of short sleep duration, there are also multiple clinical causes of poor sleep—insomnia, sleep apnea, restless leg syndrome—which affect 20% to 30% of adults (Citation87,Citation88).
There is currently no evidence that improving sleep may help people to reduce their weight. Prevention of obesity is a complex problem, and we have discussed how sleep interacts with various biological and behavioral pathways. Further research is needed to determine whether interventions aimed at increasing sleep duration may be useful in combating obesity. However, this research may be difficult to conduct. To observe a measurable difference in weight, a long time will be needed, and maintaining good compliance in such trials will not be easy. Physical activity, environmental help, and even napping may be proposed to improve sleep quality and quantity (Citation89).
Declaration of interest: Professor Leger has received funding or has been main investigator in studies sponsored by Sanofi-Aventis, Merck, Vanda, Actelion, Bioprojet, Philips, ResMed, Vitalaire in the last 5 years. He declares no conflict of interest for this manuscript. Doctor Vecchierini has received funding in studies sponsored by Sanofi-Aventis, Merck, Vanda, Bioprojet, ResMed, in the last 5 years. She declares no conflict of interest for this manuscript. Other authors declare no conflict of interest.
References
- Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-report and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15:42–50.
- Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14:324–32.
- Altman NG, Schopfer E, Jackson N, Izci-Balzerak B, Rattanaumpawan P, Gehrman PR, et al. Sleep duration versus sleep insufficiency as predictors of the cardiometabolic health outcomes. Sleep Med. 2012; 13:1261–70.
- Bixler E. Sleep and society: an epidemiological perspective. Sleep Med. 2009;10:S3–9.
- National Sleep Foundation. American poll. Washinghton, DC. 2005 http://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2005-adult-sleep-habits-and-styles accessed on 24 June 2014.
- Taylor DJ, Bramoweth AD. Patterns and consequences of inadequate sleep in college students: substance use and motor vehicle accidents. J Adolesc Health. 2010;46:610–12.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;131:1–8.
- Finucade MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al.; Global Burden of Metabolic Risk Factors of Chronic Disease Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67.
- National Survey on obesity and Overweight 2012. http://www.roche.fr/home/recherche/domaines_therapeutiques/cardio_metabolisme/enquete_nationale_obepi_2012.html (accessed on 24 June 2014).
- Ramachandran A, Chamukuttan S, Shetty SA, Arun N, Susairaj P. Obesity in Asia - is it different from the rest of the world. Diabetes Metab Res Ref. 2012;28:47–51.
- Chaput JP, Perusse L, Despres JP, Tremblay A, Bouchard C. Findings from the Quebec Family Study on the etiology of obesity: genetics and environmental highlights. Curr Obes Rep. 2014;3:54–66.
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9.
- Leger D, du Roscoat E, Bayon V, Guignard R, Paquereau J, Beck F. Short sleep in young adults: prevalence and clinical description of short sleep in a representative sample of 1004 young adults from France. Sleep Med. 2011;12:454–62.
- Knutson KL, Van Cauter E, Rathouz PJ, Deleire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33:37–45.
- Knutson KL. Does inadequate sleep play a role in vulnerability to obesity?Am J Hum Biol. 2012;24:361–71.
- Locard E, Mamelle N, Miginiac M, Munoz F, Rey S. Risk factors in a five year old population. Parental versus environmental factors.Int J Obes Relat Metab Disord. 1992;16:721–9.
- Cappuccio FP, Taggart FM, Kandala NB, Curie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26.
- Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity?A systematic review and meta-analysis. Obesity. 2008;16:265–74.
- Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16:643–53.
- Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity?A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98.
- Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J of Endocrinol. 2008;159:S59–66.
- Nielsen LS, Danielsen KV, Sorensen IA. Short sleep duration as possible cause of obesity: critical analysis of epidemiological evidence. Obes Rev. 2011;12:78–92.
- Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev. 2012;16:231–41.
- Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity (Silver Spring). 2014;22:598–607.
- Liu Y, Wheaton AG, Chapman DP, Croft JB. Sleep duration and chronic disease among US adults age 45 years and older: evidence from the 2010 Behavioral Risk Factor Surveillance System. Sleep. 2013;36:1421–7.
- Xiao Q, Arem H, Moore SC, Hollenbeck AR, Matthews CE. A large prospective investigation of sleep duration, weight change, and obesity in the NIH-AARP Diet and Health Study Cohort. Am J Epidemiol. 2013;178:1600–10.
- Suglia SF, Duarte CS, Chambers EC, Boynton-Jarrett R. Social and behavioral risk factors for obesity in early childhood. J Dev Behav Pediatr. 2013;34:549–56.
- Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr. 2005;147:830–4.
- Eisenmann JC, Ekkekakis P, Holmeq M. Sleep duration and overweight among Australian children and adolescents. Acta Pediatr. 2006;95: 956–63.
- Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–90.
- Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–7.
- Mezick EJ, Wing RR, McCaffery JM. Associations of self-reported and actigraphy-assessed sleep characteristics with body mass index and waist circumference in adults: moderation by gender. Sleep Med. 2014;15:64–70.
- Theorell-Haglow J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33:593–8.
- Lauderdale DS, Knutsson KL, Yan LL, Liu K, Rathouz PJ. Sleep duration: how well do self-report reflect objective measures?The CARDIA Sleep Study. Epidemiology. 2008;19:838–45.
- Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21.
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62.
- Moraleda-Cibrian M, O’Brien LM. Sleep duration and body mass index in children and adolescents with and without obstructive sleep apnea. Sleep Breath. 2013 Nov 28. [Epub ahead of print]
- Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, et al. Short sleep duration and obesity: the role of the emotional stress and sleep disturbances. Int J Obes (Lond). 2008; 32:801–9.
- Vgontzas AN, Bixler EO. Short sleep and obesity: are poor sleep, chronic stress, and unhealthy behaviors the link?Sleep. 2008;31:1203.
- Vgontzas AN, Fernadez-Mendoza J, Miksiewicz T, Krittikou I, Shaffer ML, Liao D, et al. Unveiling the longitudinal association between short sleep duration and the incidence of obesity: the Penn State Cohort. Int J Obes (Lond). 2014;38:825–32.
- Vzontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54.
- Schoenborn CA, Adams PF. Sleep duration as a correlate of smoking, alcohol use, leisure-time physical inactivity, and obesity among adults: United States, 2004–2006. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Health Statistics, May 2008. Available at: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/sleep04-06/sleep04-06.htm (accessed on 24 June 2014).
- Bailey BW, Allen MD, LeCheminant JD, Tucker LA, Errico WK, Christensen WF, et al. Objectively measured sleep patterns in young adult women and the relationship to adiposity. Am J Health Promot. 2013 Nov 7. [Epub ahead of print]
- Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33:289–95.
- Jones CH, Pollard TM, Summerbell CD, Ball H. Could parental rules play a role in the association between short sleep and obesity in young children?J Biosoc Sci. 2013;11:1–14.
- Kawada T, Inagaki H, Suzuki S. Sleep duration and body mass index. Sleep Med. 2008;9:808.
- Shankar A, Syamala S, Kalidindi S. Insufficient rest or sleep and its relation to cardiovascular disease, diabetes and obesity in a national, multiethnic sample. PLoS One. 2010;5:e14189.
- Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10:37–45.
- Knutson KK, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–77.
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50.
- Mullington JM, Chan JL, Van Dongen PA, Szuba MP, Samaras J, Price NJ, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4.
- Guilleminault C, Powell NB, Martinez S, Kushida C, Raffray T, Palombini L, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4:177–84.
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010; 99:651–6.
- Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36.
- Klingenberg L, Chaput JT, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescents boys. Am J Clin Nutr. 2012;96:240–8.
- Schmid SM, Hallschmid M, Jaud-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal weight healthy men. J Sleep Res. 2008; 17:331–4.
- Spiegel K, Leproult R, Colecchia EF, L'hermite-Balériaux M, Nie Z, Copinschi G, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R874–83.
- Broussard J, Knutsson KL. Sleep and metabolic disease. In: Cappucio FP, Miller MA, Lockey SW, editors. Sleep, health and society from aetiology to public health. Oxford University Press, Oxford, UK); 2010. pp. 111–40.
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19.
- Tasali E, Leproult R, Ehrman DR, van Cauter E. Slow wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9.
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33.
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33.
- Donga E, von Djik M, van Djik JG, Biermasz NR, Lammers GJ, van Kralingen KW, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8.
- Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction. Ann Intern Med. 2012;157:549–57.
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci. 2008; 1129:287–304.
- St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9:73–80.
- Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav. 2013 Sep 17. [Epub ahead of print]
- Chapman CD, Nilsson EK, Nilson VC, Cedernaess J, Rangtell FH, Vogel H, et al. Acute sleep deprivation increases food purchasing in men. Obesity (Silver Spring). 2013;21:E555–60.
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;9:1550–9.
- St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, Roychoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;9:410–16.
- Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129–43.
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700.
- Calvin AD, Carter RE, Adachi A, Macedo PG, Alburquerque FN, van der Walt P, et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144:79–86.
- St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24.
- Hogenkamp PS, Nilsson E, Nilsson VC, Chapman CD, Vogel H, Lundberg LS, et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinol. 2013;38:1668–74.
- Mitchell JA, Rodriguez D, Schmitz KH, Audrain-McGovern J. Greater screen time is associated with adolescent obesity: a longitudinal study of the BMI distribution from Ages 14 to 18. Obesity. 2013; 21:572–5.
- Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37:604–11.
- Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36:981–90.
- Adamo KB, Wilson S, Belanger K, Chaput JP. Later bedtime is associated with greater daily energy intake and screen time in obese adolescents independent of sleep duration. J Sleep Disorders Ther. 2013;2:1–5.
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82.
- Benedict C, Shostak A, Lange T, Brooks SJ, Schioth HB, Schultes B, et al. Diurnal rhythm of circulating nicotinamide phosphoribosyltransferase (nampt/visfatin/PBEF): impact of sleep loss and relation to glucose metabolism. J Clin Endocrinol Metab 2012;97: E218–22.
- Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011; 589:235–44.
- Leger D, Beck F, Richard JB, Godeau E. Total sleep time severely drops during adolescence. PLoS One. 2012;7:e45204.
- Pilcher JJ, Lambert BJ, Huffcutt AI. Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep. 2000;23:155–63.
- Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gilman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–11.
- Ortega FB, Chillon P, Ruiz JR, Delado M, Albers U, Alvarez-Granda JL, et al. Sleep patterns in Spanish adolescents: associations with TV watching and leisure-time physical activity. Eur J Appl Physiol. 2010;110:563–73.
- Vgontzas AN, Fernandez-Mendoza J. Insomnia with short sleep duration: nosological, diagnostic, and treatment implications. Sleep Med Clin. 2013;8:309–22.
- Hargens TA, Kaleth AS, Edwards ES, Butner KL. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep. 2013; 5:27–35.
- Sayon-Orea C, Bes-Rastrollo M, Carlos S, Beunza JJ, Basterra-Gorttari FJ, Martinez-Gonzalez MA. Association between sleeping hours and siesta and the risk of obesity: the SUN Mediterranean Cohort. Obesity Facts. 2013;6:337–47.

