Abstract
Glucocorticoids (GC) are steroid hormones with important implications in the treatment of various inflammatory and autoimmune diseases. At the same time GC are known to have numerous side-effects. Endogenous GC are predominantly produced by the adrenal glands, and adrenal-derived GC serve important functions in the regulation of development, metabolism, and immune regulation. The last two decades of research have led to the identification of numerous alternative sources of extra-adrenal GC synthesis. Among other tissues the intestine and lung are capable of locally producing considerable amounts of immunoregulatory GC. This local steroidogenesis in these mucosal tissues appears to be regulated by transcription factors and mediators different from those in the adrenals, likely reflecting an adaptation to the local requirements and conditions. Here we summarize the current knowledge about the extra-adrenal GC synthesis in the mucosal tissues, with special emphasis on the intestinal epithelium, and its implication on the regulation of immune homeostasis and inflammatory processes.
Key messages
In addition to the adrenals, various other tissues produce immunoregulatory glucocorticoids.
In the intestine glucocorticoids are produced in the intestinal crypts in response to immune cell activation.
Locally produced glucocorticoids contribute to the maintenance of intestinal immune homeostasis and the regulation of inflammatory processes, as seen in inflammatory bowel disease.
Introduction
Glucocorticoids as adrenal hormones
Uncountable studies have been performed since the initial description of the stress reaction and the role of glucocorticoids (GC) in this process by Hans Selye in 1936 (Citation1) and the subsequent isolation and first medical use of these adrenal hormones by Edward Kendall, Phillip Hench, and Tadeus Reichstein, who shared the Nobel Prize in 1950 (Citation2). In the early 1950s different though still expensive approaches were developed to produce fully synthetic cortisone (Citation3), which enabled its use first in rheumatology, then further extended to the treatment of various inflammatory and autoimmune diseases. Nowadays cheap and refined synthetic corticoid derivatives are available from numerous pharmaceutical companies and for different purposes. The success of these GC-containing drugs is based on their potent activity on the synthesis and action of pro-inflammatory cytokines, and the activation of immune cells. Nevertheless, none of them is deprived of the severe side-effects of long-term systemic GC therapy, and even the topical administration cannot completely avoid them (Citation4–6).
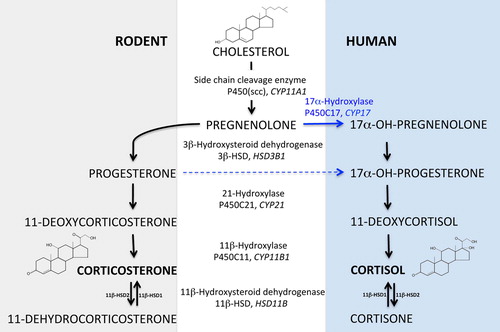
While synthetic GC as active components of various drugs have an important use in the treatment of a large number of inflammatory conditions and disorders, its ‘original’ function is clearly as an endogenous hormone regulating a variety of different processes, including development, metabolism, and inflammation. In particular, with regard to its role in the regulation of metabolism and inflammation, GC are classical stress hormones that help the body to cope with exceptional physical and emotional stress events. Thus far, the best-characterized source, both in terms of biosynthesis and enzymatic cascades, as well as regulatory processes, is the adrenal glands. Though the adrenal glands are relatively tiny organs, their capacity to produce steroid hormones and GC in particular is exceptional. GC synthesis occurs in the zonae fasciculata et reticularis in the adrenal cortex, involving the enzymatic conversion of cholesterol to bioactive GC via a series of enzymatic steps (). While in rodents the most abundant bioactive GC is corticosterone, cortisol (17-hydroxycorticosterone) predominates in humans due to the expression and activity of 17-hydroxylase in the cortex of human adrenal glands, which is absent in rats and mice.
Figure 1. The GC synthesis pathways in rodents and humans. The different metabolites in the synthesis of GC (corticosterone or cortisol) from cholesterol and the enzymes involved in specific steps are indicated.
Various signals regulate the synthesis and release of GC from the adrenals. The best-characterized is the regulation via the hypothalamic–pituitary–adrenal (HPA) axis. Stimulus-induced release of corticotropin-releasing hormone (CRH) from the hypothalamus promotes the release of adrenocorticotropic hormone (ACTH), which triggers the synthesis and release of GC in the adrenal cortex. CRH and ACTH are not only released upon stimulation of the nervous system, but also in response to inflammatory cytokines, such as IL-6 and TNFα (Citation7,Citation8) Thus, this hormonal network system is ideally suited to cope with the immediate demand of the body during emotional, physical, and inflammatory stress.
Extra-adrenal GC synthesis—an overview
The enormous capacity of the adrenal glands to produce large amounts of GC in response to stress signals has obscured our search for alternative sources of these important regulatory hormones until the early 1990s. This search was particularly complicated by the fact that systemic stress leads to the rapid release of adrenal GC, which have critical functions in coping with this stress response. For example, while mice can tolerate strong systemic immune cell activation, e.g. as triggered by lipopolysaccharide (LPS) injection, the simultaneous removal of the adrenal glands results in rapid death due to cytokine storm and shock (Citation9). This important function of systemic, adrenal-derived GC has hampered the study of the synthesis and function of GC in other tissues. Various gene expression studies have, however, characterized the expression of steroidogenic enzymes and factors involved in the GC synthesis pathway in organs and tissues previously not being recognized as hormone-producing tissues. For example, it was found that the steroidogenic enzymes Cyp11a1 and Cyp11b1 are expressed in the developing lung and intestine during specific stages of the embryonic development (Citation10,Citation11).
While these findings suggested that tissues may indeed be capable of producing steroid hormones, formal proof was lacking. Important work by Jonathan Ashwell and colleagues in the thymus in the early 1990s has paved the way for subsequent studies in other tissues. These scientists identified the thymic epithelial cells as a constitutive source of GC in the thymus and proposed that thymic GC are critically involved in the selection process of developing thymocytes (reviewed in (Citation12)). While the role of thymic GC in thymic selection has been subject of intensive and controversial scientific discussion (Citation13,Citation14), the work by Ashwell and colleagues impressively showed that adrenals are not the sole source of GC. These studies were later on confirmed by numerous studies in the thymus (Citation15,Citation16), the skin (Citation17,Citation18), the brain (Citation18), intestine (Citation19–21), and lung (Citation22), and it can be foreseen that a growing number of tissues will be further identified as producing GC. Along these lines it is of interest to note that also thymocytes themselves have been identified as a source of GC (Citation23), and more recently the expression of steroidogenic enzymes has been shown in mature T cells (Citation24), suggesting that also hematopoietic cells may be able to produce GC.
Of interest is the fact that while all these tissues produce from minor to large amounts of GC (), the regulation of GC synthesis seems to be rather diverse and often distinct. For example, while adrenal GC synthesis is largely regulated by releasing hormones derived from the brain, the skin appears to have its own autonomous regulatory network. The skin and hair follicles, for example, have been shown to produce all the components of the HPA axis, and their synthesis is completely independent of central stimuli (Citation17,Citation25). The nuclear receptor and transcription factor steroidogenic factor-1 (SF-1; NR5a1) is critical for the transcriptional regulation of adrenal GC synthesis (Citation26), yet is not expressed in the intestine and seems to be functionally replaced by a close homolog, liver receptor homolog-1 (LRH-1; NR5a2) (Citation27,Citation28). Similarly, intestinal GC synthesis does not respond to the action of ACTH (Citation29). Furthermore, while adrenals, thymus, skin, and intestine appear to produce bioactive GC via de novo synthesis from cholesterol or at least cholesterol metabolites, the lung seems to use another strategy, also known for the liver. GC can be inactivated to dehydrocorticosterone, respectively cortisone. Inactive serum-distributed metabolites can, however, be reactivated in the lung by 11β-hydroxysteroid dehydrogenase B1 (HSD11B1) to active GC (Citation22). This enzymatic pathway is also actively explored by GC derivatives in drugs, which require a first-pass metabolism and activation in the liver.
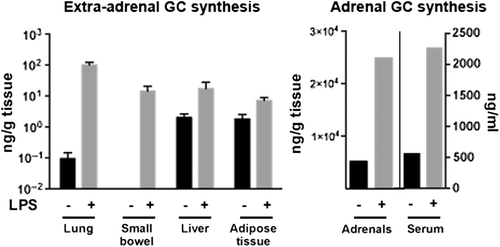
Figure 2. Ex vivo GC synthesis (ng/g tissue) under steady-state conditions and after inflammation triggering through LPS injection. C57B/6 mice were either treated i.p. with 100 μg LPS or left untreated to avoid stress. Three hours later the mice were sacrificed, organs were taken for ex vivo culture, and blood was taken through cardiac puncture to measure serum corticosterone levels. Organs were chopped into small pieces and rinsed with ice-cold PBS, containing 2% horse serum, then cultured for 4 h in medium supplemented with charcoal-stripped 5% horse serum. The adrenals were incubated for 3 h only. Each organ was cultured in duplicates with and without the steroidogenesis inhibitor metyrapone. Corticosterone concentration in the supernatant was measured by radioimmunoassay. Data are presented as the metyrapone blockable corticosterone fraction, i.e. locally synthesized GC. n = 3 mice for all organs, except adrenals.
Local GC production
The extra-adrenal GC synthesis has likely no great input on the systemic levels of these hormones, as adrenalectomy leads to almost undetectable GC levels in the serum (Citation19), but rather regulates various local processes, especially under inflammatory conditions. In a comparative experiment we examined the capacity of the adrenals and some other organs to synthesize GC. Not surprisingly, the adrenals demonstrated an enormous GC-producing capacity compared to the extra-adrenal sources, especially when considering their tiny size (). Nonetheless, based on the GC concentrations measured in ex vivo organ cultures, interesting calculations regarding the total amounts of immunoregulatory GC produced by extra-adrenal sources can be made.
The lung of a 25 g mouse weighs around 120 mg. In our experiments we measured corticosterone production of approximately 200 ng/g tissue over 4 h ex vivo culture, which reflects approximately 6 ng corticosterone per hour, produced under inflammatory conditions in the lung only. Similarly, the small intestine produces around 15 ng corticosterone per gram tissue after triggering by immune cell activation. The weight of the small intestine is around 600 mg, which results in 2.5 ng corticosterone per hour produced by the entire intestine. The adrenals (with an approximate weight of around 5 mg each), on the other hand, show under these experimental conditions a production of approximately 17 ng per hour in control treated mice and an increase of up to 100 ng per hour in response to immunological stress. The latter is in concordance with the in vivo measurements showing that adrenals in rodents produce corticosterone under steady-state conditions at the rate of around 4.3 μg/g body weight/h (Citation30). Thus, ex vivo cultures of different mouse organs and measurement of GC synthesis under these experimental conditions can give an idea about the relative contribution of these tissues to the GC synthesis in the organism (). While such comparative studies have not been done yet with human tissue, analysis of cortisol produced in ex vivo cultures of human colonic tissue (Citation31) suggests that GC-producing capacities may be comparable, when correcting for size.
In addition to adrenals, lung, and intestine, two other tissues were assessed for their capacity to produce GC. The liver and the adipose tissue are known with their capability to boost the GC effects via rehydrating the inactive GC metabolites cortisone, respectively 11-dehydrocorticosterone, and thereby generating active GC. It is believed that in doing so the liver as well as adipose tissue can regulate their own as well as the systemic metabolism. Of interest, their GC-reactivating capacity was only slightly enhanced under inflammatory conditions compared to the mucosal tissues, likely reflecting only the extreme increase of the serum GC or metabolites rather than representing an inflammation-regulated process, at least under the experimental conditions examined. In summary, in particular the mucosal tissues lung and intestine can respond to immunological stress by rapidly producing considerable amounts of immunoregulatory GC.
It should, however, be kept in mind that also extra-adrenal GC synthesis may be dependent on adrenal GC synthesis, as reactivation of dehydrocorticosterone to corticosterone, respectively cortisone to cortisol via an HSD11B1-dependent mechanism requires initial synthesis of bioactive GC, for example in the adrenal glands. In this respect, it is of interest to note that adrenalectomy leads to a massive reduction of serum GC (Citation19). Similarly, also lung GC synthesis is drastically reduced in adrenalectomized animals, indicating that adrenal steroidogenesis provides critical substrates for GC synthesis in the lung (Citation22).
Intestinal GC synthesis
What is the function of these locally produced GC? Given the potent immunoregulatory and immunosuppressive activity of GC in general, it is suggested that locally mucosal GC serve similar functions. Indeed, several lines of evidence from experimental mouse data but also human patients support the idea that GC are produced locally in these mucosal tissues in response to immune cell activation, and in turn contribute to balancing these immune responses to avoid tissue damage. In this respect, locally produced GC represent a negative feed-back loop to regulate and maintain immune homeostasis. This feed-back function appears to be particularly useful and important in mucosal surfaces such as the intestine and the lung, given the close and extensive interactions between local immune cells and micro-organisms.
In the gut, the key steroidogenic enzymes P450ssc (encoded by Cyp11a1) and 11β-hydroxylase (encoded by Cyp11b1) are expressed only at very low levels under basal conditions, but induced in response to immunological stress (Citation19). However, if intestinal epithelial cells are fractioned and the crypt cell are examined separately, or novel and sensitive methods such as laser capture microdissection microscopy (LCM) are used, expression of these two enzymes can be reproducibly demonstrated also under steady-state conditions. This might suggest that GC, though at very low amounts, are produced constitutively by the intestine, possibly fulfilling important functions in the regulation of immune homeostasis and epithelial layer integrity. In vitro data show the importance of GC for the maturation and differentiation of the intestinal epithelial cells (Citation32,Citation33). GC are also implicated in the regulation of the expression of tight junction proteins and maintaining the intestinal epithelial barrier, especially antagonizing the tight junction-destructing effect of TNFα during inflammation. Myosin light chain kinase has been shown to be responsible for the elevated tight junction permeability, and an important antagonizing effect of GC is the transsuppression of its TNFα-induced expression in the gut (Citation33). Indeed, mice deficient in intestinal GC synthesis demonstrate higher intestinal epithelial layer permeability compared to controls (F. Kostadinova, T. Brunner, unpublished). These observations indicate that intestinal GC might have also autocrine functions by regulating the intestinal epithelial layer permeability.
Though the expression of steroidogenic enzymes in the intestinal mucosa has been known for a considerable time (Citation10), formal proof that the intestinal epithelium produces considerable amounts of immunoregulatory GC was only provided in 2004 (Citation19). Of interest was the finding that the intestinal expression of steroidogenic enzymes and the synthesis of GC could be triggered in response to strong immune cell activation, suggesting a direct relationship between the activation of immune cells, the release of inflammatory mediators, and the induction of steroidogenesis in the intestinal epithelium. At present, in particular the role of certain cytokines and transcription factors in these processes has been established.
Regulatory mechanisms and immune mediators: role of LRH-1 and TNFα
Steroidogenesis in different tissues is tightly controlled by the transcriptional control of steroidogenic enzymes. In particular, members of the nuclear receptor family of transcription factors play critical roles in these processes. Steroidogenic factor-1 (SF-1/NR5a1) is essential in the regulation of adrenal steroidogenesis, and SF-1-deficient mice not only lack adrenal GC synthesis, but even have no adrenal glands (Citation26). SF-1 binds to the promoter of many genes involved in steroidogenesis and thereby promotes the expression of steroidogenic enzymes. Of interest is the fact that SF-1 expression is basically absent in the intestinal mucosa, thus cannot account for the regulation of intestinal GC synthesis (Citation20,Citation27). Instead, it was found that the close homolog liver receptor homolog-1 (LRH-1/NR5a2) functionally replaces SF-1 in the intestine. LRH-1 binds to the identical response elements in the promoter of steroidogenic genes (Citation34), and its expression in the intestinal epithelium closely correlates with that of steroidogenic enzymes. Thus, LRH-1 as well as steroidogenic enzymes are predominantly expressed in the lower parts of the intestinal crypts (Citation19,Citation35) and gradually fade in the more mature epithelium. This restricted localization of LRH-1 is of interest regarding its dual role in intestinal epithelial cell biology. LRH-1 has a critical role in the regulation of intestinal GC synthesis. LRH-1 haploinsufficiency as well as targeted deletion of LRH-1 in the intestinal epithelium strongly compromises immune cell-induced intestinal GC synthesis (Citation20,Citation28). The importance of LRH-1-regulated local GC synthesis was illustrated by the fact that mice lacking LRH-1 expression in the intestinal epithelium, and therefore lacking intestinal GC synthesis, show strongly enhanced sensitivity towards experimentally induced colitis (Citation28). Next to steroidogenesis LRH-1 is also involved in the regulation of cell cycle progression and epithelial cell renewal. LRH-1 regulates the expression of the cell cycle-regulating genes cyclin D1 and E1, and c-Myc, and deregulated LRH-1 expression may even contribute to the development of intestinal tumors (Citation35,Citation36). Thus, LRH-1 may regulate intestinal epithelial layer integrity and homeostasis via two mechanisms: the production of anti-inflammatory GC and thereby the prevention of immune cell-induced epithelial damage, and the accelerated recovery of the damaged epithelium by increased proliferation of stem and progenitor cells in the intestinal crypts. Of note is also the recent finding that LRH-1 transcriptional activity appears to be somehow linked to cell cycle progression, as cell cycle inhibitors profoundly inhibit LRH-1 activity in intestinal epithelial cells (Citation29). Thereby both the cell cycle- as well as the steroidogenesis-regulating activity of LRH-1 may be restricted to the stem and progenitor cell compartment of the intestinal crypts, enabling a rapid recovery of the epithelial layer after damage.
While the basal synthesis of intestinal GC is relatively low, the initiation of immunological stress and inflammation can strongly boost it (Citation19,Citation20,Citation37,Citation38). What signals, factors, or hormones are involved in this induced steroidogenesis? The intestinal GC synthesis appears to be triggered predominantly by inflammatory mediators, whereas releasing hormones of the HPA axis are less important. While ACTH has a central role in stress-induced release of GC from the adrenals, it fails to trigger intestinal GC synthesis (Citation27). Instead, the pro-inflammatory cytokine TNFα seems to represent a master regulator of intestinal GC synthesis in response to various triggers. Direct stimulation of T cells or macrophages, as well as their activation during induction of experimental colitis, promotes intestinal GC synthesis in a TNFα-dependent manner. Consequently, immune cell-induced intestinal GC synthesis is either strongly reduced or absent in mice lacking TNFα or TNF receptor expression (Citation37,Citation38). While TNFα has also an important role in promoting adrenal GC synthesis, indirectly via the activation of the HPA axis, it appears to promote intestinal GC synthesis via direct activation of intestinal epithelial cells (Citation38). This intestinal steroidogenesis-regulating role of TNFα is at a first glance somewhat surprising, as TNFα and GC are mutually antagonistic. On one hand TNFα is a key pro-inflammatory cytokine involved in the pathogenesis of inflammatory bowel disease (IBD) and efficiently targeted by neutralizing antibodies, such as infliximab (Citation39). Consequently, patients with IBD are also treated with synthetic GC. On the other hand, TNFα is also an omnipresent sensor for inflammation and may therefore represent an ideal regulator of intestinal GC synthesis as a negative feed-back loop response.
Why does the gut produce glucocorticoids?
Given the potent immunoregulatory and immunosuppressive activities of GC in general, we assume that also local GC synthesis in the intestine will likely have such regulatory activities on the activation of local immune cells and the pathogenesis of inflammatory diseases in the intestine. But what is the evidence for this? A formal proof of this idea or hypothesis is strongly hampered by the fact that the capacity of the adrenals to produce GC in response to systemic immune cell activation is so enormous that it becomes difficult to distinguish what is the contribution of local GC production to the immune regulation in the intestinal mucosa versus systemic GC. The initial characterization of intestinal GC synthesis therefore by-passed this problem by investigating the activation of virus-specific T cells in the intestine in adrenalectomized mice. Adrenalectomized animals, devoid of any serum GC, were infected with lymphocytic choriomeningitis virus (LCMV) in the presence or absence of the 11β-hydroxylase and GC synthesis inhibitor metyrapone, and the activation of virus-specific T cells in the intestinal epithelium was analyzed. These studies showed clearly that administration of the well-established GC synthesis inhibitor metyrapone resulted in a significantly enhanced production of pro-inflammatory cytokines and increased expression of activation markers in antigen-specific T cells, clearly indicating that intestinal GC indeed have a immunoregulatory role (Citation19).
But how important is this local production for immune homeostasis or the prevention of inflammatory disorders, such as inflammatory bowel disease (IBD)? Are GC produced in the intestinal epithelium during IBD? Do they counter-regulate inflammatory processes? At present no genetic tools are available to assess such questions, yet a variety of mouse models for experimental colitis exist that allow the assessing of various interesting aspects that support a regulatory role of intestinal GC in this process. The oral administration of dextran sodium sulfate (DSS) results in a macrophage/granulocyte-driven type of intestinal inflammation. In contrast, rectal administration of the hapten TNBS causes a T cell-mediated T helper (Th) 1 type of inflammatory response, whereas the hapten oxazolone promotes a Th2 of response. When analyzing the expression of steroidogenic enzymes and the production of colonic GC, marked differences are noted. Whereas DSS and TNBS trigger the expression of steroidogenic enzymes and promote GC synthesis, oxazolone fails to do so, despite clearly detectable signs of acute inflammation. Thus, inflammatory responses in the gut can initiate local steroidogenesis, but the quality of inflammation may be important and decisive. While Th1 and Th2 responses differ in the expression of a large panel of cytokines and factors, the expression and release of large quantities of TNFα was found to be the most critical differences between GC-inducing and non-GC-inducing types of intestinal inflammation. Indeed, DSS and TNBS failed to promote intestinal GC synthesis in TNFα-deficient mice, whereas therapeutic administration of TNFα to oxazolone-treated mice could restore colonic GC synthesis and significantly improve the intestinal pathology. Since both TNFα-induced colonic GC synthesis and clinical improvement could be blocked by administration of metyrapone, these studies make a strong case that local intestinal GC synthesis contributes indeed to the regulation of intestinal immune responses and the course of IBD in particular (Citation37). These studies also identify the pro-inflammatory cytokine TNFα as an important inducer of intestinal GC synthesis. Thus, TNFα has clearly also an anti-inflammatory role in the pathogenesis of (at least experimental) IBD, in addition to its well-known pro-inflammatory activities. While the restoration of colonic GC synthesis and associated clinical improvement of oxazolone colitis illustrates the potential therapeutic application of triggering intestinal GC synthesis, it is not feasible to treat human IBD patients with TNFα to promote or restore intestinal GC synthesis. However, understanding in detail the molecular mechanisms of the regulation and induction of GC synthesis in intestinal epithelial cells may indeed result in the identification of drugable targets exploiting this autoregulatory mechanism.
Murine models of IBD often only reflect certain aspects of the pathogenesis of either Crohn's disease or ulcerative colitis. Thus, what is the evidence that 1) the human colon produces immunoregulatory GC, and 2) intestinal GC may be involved in the pathogenesis of IBD in human patients? Similar to mouse intestinal tissue, human colonic biopsies can be cultured ex vivo, and cortisol released into the supernatant can be measured. Furthermore, these tissue samples can be simulated unspecifically using phorbolester. This results in the clearly detectable inducible production of GC in a metyrapone-blockable manner, confirming local GC synthesis in colonic tissues (Citation31). Furthermore, steroidogenic enzymes are detected in colonic tissue samples and various human colorectal tumor cell lines as well as primary colorectal tumor samples. The cell lines as well as tissue samples release GC activity, which suppresses the activation of T lymphocytes, demonstrating the immunoregulatory activity of human colonic GC (Citation31).
While these findings confirm GC synthesis in human colonic tissue and colon-derived cells, the role of colonic GC synthesis in the regulation of IBD in human patients is somewhat less clear. Clearly, this issue is more difficult to approach than in mice. A recent gene expression study by Coste et al. (Citation28) in colonic samples from healthy controls or patients with Crohn's disease or ulcerative colitis supports the notion that defective intestinal GC synthesis might contribute to the pathogenesis of human IBD. These studies demonstrated a clear inverse correlation between inflammation (as measured by the expression of inflammatory cytokines) and the expression of LRH-1 and the steroidogenic enzymes CYP11A1 and CYP11B1. It is presently unclear what leads to this inverse expression profile. At the inflamed sites, often massive damage of the intestinal crypts and thus the source of LRH-1 and intestinal GC is seen. Thus, excessive inflammation and associated epithelial damage might result in defective intestinal GC synthesis by depleting the cellular source of intestinal GC. Alternatively, specific mediators produced during chronic IBD might negatively regulate the expression of LRH-1 and steroidogenic enzymes. A profound understanding of the molecular processes in the regulation of intestinal GC synthesis is, however, required to understand these processes better. Nonetheless, these findings illustrate a potential role of local GC synthesis in the pathogenesis of IBD in human patients.
The potentially conflicting role of TNFα in the pathogenesis of IBD and the induction of colonic GC is of special interest with regard to the potential role of intestinal GC in the regulation of IBD. TNFα is a proven disease-promoting factor and well- characterized target in the pathogenesis of human IBD. Neutralizing anti-TNFα antibodies have been applied therapeutically for the past 15 years, and extensive experience has been gained in the optimization of drug administration and dosing (Citation40,Citation41). However, taking a detailed look at the results of a randomized trial, reporting statistically significant effects of anti-TNFα versus placebo treatment, it becomes evident that almost 20% of the patients undergo spontaneous remission over the observed period, as expected, because IBD is a chronic remittent disease. Treatment, on the other hand, is beneficial for about 40% to 60% of the patients (Citation39). While this illustrates an enormous therapeutic success in the treatment of a disease affecting more than 4 million people worldwide, the question remains why this therapy does not work in the remaining 40% or more of the patients. Furthermore, why do some patients get exacerbations during the treatment? Of course in these patients different inflammatory factors might functionally replace the disease-promoting activities of TNFα (Citation42–44). However, it may also be feasible that the treatment of IBD patients could abrogate the autoregulatory induction of intestinal GC synthesis and thereby accelerate inflammatory processes. Further clinical studies will be required to answer these important questions in more detail. Special attention should also be given to the manifestation of intestinal inflammatory problems in patients treated with neutralizing anti-TNFα antibodies for other reasons than IBD.
More recently, intestinal GC synthesis under basal conditions (Citation45) as well as in the context of chronic experimental colitis (Citation46) has been confirmed also by other groups. Of interest is the finding that under basal conditions the intestinal flora also contributes to the regulation of intestinal GC synthesis and that intestinal epithelium-derived GC contribute to the systemic regulation of metabolic processes (Citation45). Thus, intestinal GC synthesis may not only have important regulatory functions under pathophysiological conditions but also contribute to homeostatic processes under steady-state conditions.
Extra-adrenal glucocorticoid synthesis in other mucosal tissues—the lung
While at a first glance the intestine and the lung serve rather different purposes, i.e. the absorption of nutrients versus the exchange of gases, a closer look identifies many common aspects between these two organs. Both are mucosal tissues and as epithelial surfaces located at the borderline between the outside world and the body. Gut and lung (at least in the alveoli) are lined with a single epithelial layer, which permits on the one hand an efficient uptake of nutrients and exchange of gases, respectively, but which on the other hand is also prone to damage, thereby allowing access of potential pathogenic micro-organisms to our body. Thus, not surprisingly, both tissues are home to an extensive and rather complex immune system aimed at defending this contact zone from potential pathogenic invaders while at the same time preventing tissue damage due to exaggerated and devastating inflammatory responses.
Clearly, immune responses in the intestine as well as the lung must be tightly regulated to find this appropriate balance. Having discussed above the proposed role of local GC production in the regulation of intestinal immune homeostasis and the regulation of inflammatory responses, it does not come as much of a surprise that extra-adrenal GC synthesis has also been identified in the lung (Citation22). Much as in the intestinal mucosa, lung GC synthesis is triggered upon stimulation of immune cells, and compared to the intestinal epithelium the lung seems to have even a bigger capacity to produce these immunoregulatory steroids. While at present a clear proof of how local GC synthesis regulates local immune responses in the lung is missing due to the lack of suitable genetic models or means of targeted pharmacological interventions, very likely local GC production will serve similar purposes as in the intestine, i.e. to balance local immune cell activation and inflammatory responses.
Besides many similarities, GC synthesis in these two tissues also shows many differences. The most prominent difference is the enzymatic pathway of GC synthesis in the lung. While there is very good evidence from genetic, metabolic, and pharmacological studies that in the intestine GC are synthesized from cholesterol or at least cholesterol metabolites, such as pregnenolone or progesterone, via an 11β-hydroxylase-dependent pathway, thus very much like in the adrenal glands, various findings support the notion that lung GC synthesis follows different enzymatic pathways. An important aspect is the observation that the expression of 11β-hydroxylase, encoded by Cyp11b1, is hardly detectable in the mouse lung, even upon challenge with LPS or T cell-activating stimuli (Citation22). More importantly, organ cultures of lung tissue fail to convert deoxycorticosterone to corticosterone, indicating that only insufficient 11β-hydroxylase enzymatic activity is present in the lung mucosa. Thus, how are GC produced in the lung tissue in a manner that can be still blocked the by GC synthesis inhibitor metyrapone? Much like the liver, the lung seems to reactivate serum-derived dehydrocorticosterone, an inactive metabolite, to corticosterone in an 11β-steroid dehydrogenase B1 (HSD11B1)-dependent process. Along these lines, ex vivo cultured lung tissue efficiently converts radiolabeled dehydrocorticosterone to corticosterone in a metabolic assay. Though metyrapone has been described as an efficient inhibitor of 11β-hydroxylase, it is also known to inhibit HSD11B1, explaining the potent inhibitory effect of metyrapone on lung GC synthesis.
The different enzymatic pathways used also suggest different transcriptional control of steroidogenesis in intestine and lung. Given the important function of LRH-1 in the transcriptional regulation of enzymes involved in the GC synthesis in the intestinal epithelium, the role of LRH-1 in lung GC synthesis was specifically investigated. While genetic deletion of LRH-1 strongly affects intestinal GC synthesis (Citation20), no important function of this transcription factor could be demonstrated in the lung (Citation22). Most steroidogenic enzyme genes appear to be constitutively expressed in the lung with no apparent induction upon immunological stress, with the exception of Cyp11a1, encoding P450ssc. Since induction of transcription appears not to be the initiating event of immune cell-induced GC reactivation in the lung, the question also arises how lung GC synthesis is triggered and which immune cell-derived factors initiate this process. Whereas TNFα has an important function in promoting immune cell-induced steroidogenesis in the intestine (Citation21), we found no evidence for a similarly dominant role of TNFα in the lung. Although in vivo administration of TNFα alone also promoted lung GC reactivation, this is likely an indirect or non-dominant effect as no defects in immune cell-induced lung GC synthesis were observed in mice deficient in TNF receptor 1 or 2 expression, or double knockout mice. Similarly, no inhibition of GC synthesis was observed in caspase 1-deficient mice, indicating that inflammatory cytokines such as IL-1β or IL-18 are also not critical for lung steroidogenesis (N. Hostettler, C. Benarafa, T. Brunner, unpublished). Of interest is the fact that induction of a Th2 type of inflammation failed to promote GC synthesis in both lung and intestine (Citation22,Citation37).
Possibly, the important initiating trigger in this process is not mediated by sensor cytokines directly on the lung tissue, but possibly on the adrenal glands, which are the relevant source of serum corticosterone being converted to dehydrocorticosterone. A massive increase in serum dehydrocorticosterone could also increase the substrate availability for HSD11B1 in the lung and initiate its metabolism to corticosterone. Along these lines, it is interesting to note that lung GC synthesis largely depends on the presence and function of the adrenal glands, as surgical removal of the adrenal glands abolishes lung GC synthesis (Citation22).
While the molecular events regulating GC reactivation from dehydrocorticosterone in the lung have yet to be investigated, the direct comparison substantiates the idea that, while both lung and intestine can produce substantial amounts of bioactive GC in response to immune cell activation, the regulation is completely different. Likely this represents an adaptation to the local requirements. It is feasible to believe that lung steroidogenesis is more closely linked to that in the adrenal glands, possibly because the lung has an extensive vascularization and, due to the very close contact of lung epithelium and endothelial cells, local inflammation in the lung may rapidly spread and promote a systemic inflammation, ultimately involving also the release of GC from the adrenals. In contrast, in the intestinal mucosa, it may be more important to restrict local inflammatory responses. Of interest is the observation that when the epithelial barrier is broken and intestinal bacteria invade the underlying tissue they often do not spread too far but are removed by phagocytic cells in the lamina propria or draining mesenteric lymph node, as a release of bacteria into the circulation would ultimately cause sepsis and associated shock and become life-threatening. Similarly, an autonomously regulated immunoregulatory GC synthesis in the intestinal epithelium may restrict low-level inflammatory processes to the intestine and thereby prevent repetitive systemic inflammatory responses.
Glucocorticoid synthesis in tumor cells as an immune escape mechanism
The transformation of epithelial cells of the adrenal cortex and associated neoplasia may result in excessive synthesis and release of GC, leading to Cushing's syndrome (Citation47). Given the many parallels of adrenal and extra-adrenal GC synthesis, it would make sense that also tumors derived from the intestinal epithelium could produce bioactive GC and possibly use them to prevent immune surveillance. Indeed, most human colorectal tumor cell lines constitutively express a number of enzymes involved in the synthesis of GC from cholesterol, and cortisol is readily detected in the culture supernatant by radioimmunoassay and bioassays (Citation31). Much as in the normal intestinal epithelium, also GC synthesis in colorectal tumor cells appears to be regulated by LRH-1. Whereas overexpression promotes steroidogenesis, down-regulation significantly attenuates it. Synthesis and release of GC was not only observed in established colorectal tumor cell lines, but importantly also in a series of primary and metastatic colorectal tumors. Of interest was the fact that while normal colonic tissue produced only elevated levels of cortisol upon stimulation, cortisol synthesis in tumor cultures was constitutive (Citation31).
Most tumor samples demonstrated a constitutive and rather high expression of steroidogenic enzymes and the master regulator LRH-1 (Citation31). In particular the high expression of LRH-1 is of interest, since LRH-1 is also critically involved in the transcriptional control of cell cycle-regulating genes, such as cyclin D1 and E1, and c-Myc (Citation35,Citation36). This cell cycle-regulating function of LRH-1 appears to be important for the self-renewal of the intestinal epithelial layer, but also be involved in the development of intestinal tumors. Mice with reduced expression of LRH-1 (LRH-1+/−) demonstrate significantly reduced development of adenomas in the APCmin/+ mouse model of intestinal tumors (Citation36). Given the fact that LRH-1 controls both cell cycle as well the synthesis of GC, this nuclear receptor may regulate the development of colon cancer at two distinct levels, i.e. the actual growth of the tumor by regulating proliferation and the surveillance of tumor development by suppressing immune cells. Targeting of LRH-1 may thus also represent an interesting therapeutic strategy for both processes. Recently identified LRH-1 antagonists have been shown to inhibit the proliferation of LRH-1-expressing tumor cells substantially (Citation48), while their effect on GC synthesis in colorectal tumors has not been investigated yet.
Conclusion
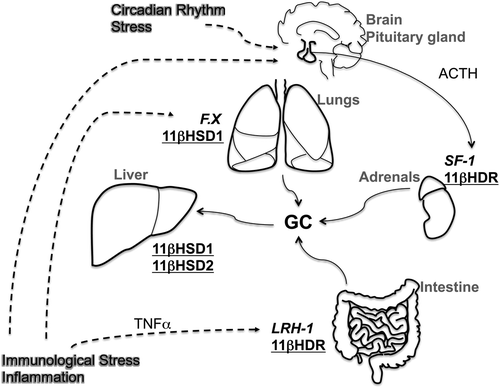
GC synthesis has been demonstrated and confirmed in various extra-adrenal tissues. The different sources of GC in the organism can function in synchronized manner or independently by differential regulation according to local requirements and conditions (). In particular, studies in the intestinal mucosa strongly support the idea that the local synthesis of GC contributes to the maintenance of local immune homeostasis and the regulation of inflammatory processes. TNFα was identified as an important sensor of immunological stress and inducer of intestinal GC synthesis. Similarly, the nuclear receptor LRH-1 appears to be a master regulator of intestinal steroidogenesis. Therapeutic approaches targeting these processes might represent interesting novel strategies for the treatment of patients with chronic intestinal disorders by re-establishing a balanced local immune response.
Figure 3. GC sources and their presumable regulation in the organism. 11βHDR = 11β-hydroxylase; 11βHSD = 11β-hydroxysteroid dehydrogenase; ACTH = adrenocorticotropic hormone; F.X = unknown factor; GC = glucocorticoids; LRH-1 = liver receptor homolog 1; SF-1 = steroidogenic factor 1; TNFα = tumor necrosis factor alpha. Transcription factors are presented in italic, and steroidogenic enzymes are underlined.
Declaration of interest: Work on the regulation of the intestinal glucocorticoid synthesis has been supported by equipment grants from the German Science Foundation (DFG), and grants from the University of Konstanz. The authors report no conflicts of interest.
References
- Szabo S, Tache Y, Somogyi A. The legacy of Hans Selye and the origins of stress research: a retrospective 75 years after his landmark brief “letter” to the editor# of nature. Stress. 2012;15:472–8.
- Hillier SG. Diamonds are forever: the cortisone legacy. J Endocrinol. 2007;195:1–6.
- Benedek TG. History of the development of corticosteroid therapy. Clin Exp Rheumatol. 2011;29(5 Suppl 68):S5–12.
- Gensler LS. Glucocorticoids: complications to anticipate and prevent. Neurohospitalist. 2013;3:92–7.
- Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43.
- Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn’s disease. Aliment Pharmacol Ther. 2001;15:1515–25.
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary- adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71.
- Chesnokova V, Melmed S. Minireview: Neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology. 2002;143:1571–4.
- Gonzalo JA, Gonzalez-Garcia A, Martinez C, Kroemer G. Glucocorticoid-mediated control of the activation and clonal deletion of peripheral T cells in vivo. J Exp Med. 1993;177:1239–46.
- Keeney DS, Ikeda Y, Waterman MR, Parker KL. Cholesterol side-chain cleavage cytochrome P450 gene expression in the primitive gut of the mouse embryo does not require steroidogenic factor 1. Mol Endocrinol. 1995;9:1091–8.
- Provost PR, Tremblay Y. Genes involved in the adrenal pathway of glucocorticoid synthesis are transiently expressed in the developing lung. Endocrinology. 2005;146:2239–45.
- Vacchio MS, Ashwell JD. Glucocorticoids and thymocyte development. Semin Immunol. 2000;12:475–85.
- Godfrey DI, Purton JF, Boyd RL, Cole TJ. Stress-free T-cell development: glucocorticoids are not obligatory. Immunol Today. 2000;21:606–11.
- Ashwell JD, Vacchio MS, Galon J. Do glucocorticoids participate in thymocyte development? Immunol Today. 2000;21:644–6.
- Lechner O, Wiegers GJ, Oliveira-Dos-Santos AJ, Dietrich H, Recheis H, Waterman M, et al. Glucocorticoid production in the murine thymus. Eur J Immunol. 2000;30:337–46.
- Pazirandeh A, Xue Y, Rafter I, Sjovall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J. 1999;13:893–901.
- Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000; 1474:1–4.
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87.
- Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, et al. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med. 2004;200:1635–46.
- Mueller M, Cima I, Noti M, Fuhrer A, Jakob S, Dubuquoy L, et al. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J Exp Med. 2006;203:2057–62.
- Noti M, Sidler D, Brunner T. Extra-adrenal glucocorticoid synthesis in the intestinal epithelium: more than a drop in the ocean? Semin Immunopathol. 2009;31:237–48.
- Hostettler N, Bianchi P, Gennari-Moser C, Kassahn D, Schoonjans K, Corazza N, et al. Local glucocorticoid production in the mouse lung is induced by immune cell stimulation. Allergy. 2012;67:227–34.
- Qiao S, Okret S, Jondal M. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology. 2009;150:4163–9.
- Jia Y, Domenico J, Takeda K, Han J, Wang M, Armstrong M, et al. Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8 + T cell skewing in allergic lung disease. Proc Natl Acad Sci U S A. 2013;110:8152–7.
- Paus R, Botchkarev VA, Botchkareva NV, Mecklenburg L, Luger T, Slominski A. The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann N Y Acad Sci. 1999;885:350–63.
- Parker KL. The roles of steroidogenic factor 1 in endocrine development and function. Mol Cell Endocrinol. 1998;145:15–20.
- Mueller M, Atanasov A, Cima I, Corazza N, Schoonjans K, Brunner T. Differential regulation of glucocorticoid synthesis in murine intestinal epithelial versus adrenocortical cell lines. Endocrinology. 2007;148: 1445–53.
- Coste A, Dubuquoy L, Barnouin R, Annicotte JS, Magnier B, Notti M, et al. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc Natl Acad Sci U S A. 2007; 104:13098–103.
- Atanasov AG, Leiser D, Roesselet C, Noti M, Corazza N, Schoonjans K, et al. Cell cycle-dependent regulation of extra-adrenal glucocorticoid synthesis in murine intestinal epithelial cells. FASEB J. 2008;22: 4117–25.
- Saroff J, Wexler BC. Isoproterenol-induced myocardial infarction in rats. Distribution of corticosterone. Circ Res. 1970;27:1101–9.
- Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C, et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30:2411–19.
- Lu L, Li T, Williams G, Petit E, Borowsky M, Walker WA. Hydrocortisone induces changes in gene expression and differentiation in immature human enterocytes. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G425–32.
- Boivin MA, Ye D, Kennedy JC, Al-Sadi R, Shepela C, Ma TY. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2007;292:G590–8.
- Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–60.
- Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, et al. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15:499–509.
- Schoonjans K, Dubuquoy L, Mebis J, Fayard E, Wendling O, Haby C, et al. Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc Natl Acad Sci U S A. 2005;102:2058–62.
- Noti M, Corazza N, Mueller C, Berger B, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med. 2010;207:1057–66.
- Noti M, Corazza N, Tuffin G, Schoonjans K, Brunner T. Lipopolysaccharide induces intestinal glucocorticoid synthesis in a TNFalpha- dependent manner. FASEB J. 2010;24:1340–6.
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9.
- Magro F, Portela F. Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs. 2010;24(Suppl 1):3–14.
- Dassopoulos T, Sultan S, Falck-Ytter YT, Inadomi JM, Hanauer SB. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145:1464–78 e1–5.
- Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69: 7884–92.
- Kostadinova FI, Baba T, Ishida Y, Kondo T, Popivanova BK, Mukaida N. Crucial involvement of the CX3CR1-CX3CL1 axis in dextran sulfate sodium-mediated acute colitis in mice. J Leukoc Biol. 2010;88: 133–43.
- Mukaida N, Sasakki S, Popivanova BK. Tumor necrosis factor (TNF) and chemokines in colitis-associated cancer. Cancers (Basel). 2011;3:2811–26.
- Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27.
- Huang SC, Lee CT, Chung BC. Tumor necrosis factor suppresses NR5A2 activity and intestinal glucocorticoid synthesis to sustain chronic colitis. Sci Signal. 2014;7:ra20.
- Papotti M, Duregon E, Volante M, McNicol AM. Pathology of the adrenal cortex: a reappraisal of the past 25 years focusing on adrenal cortical tumors. Endocr Pathol. 2014;25:35–48.
- Benod C, Carlsson J, Uthayaruban R, Hwang P, Irwin JJ, Doak AK, et al. Structure-based discovery of antagonists of nuclear receptor LRH-1. J Biol Chem. 2013;288:19830–44.

