Abstract
Diabetic women carry a 2–4 times increased risk of a hypertensive pregnancy compared to non-diabetic people. This risk is related to presence of diabetic nephropathy, but also poor glycaemic control.
Efforts to improve glycaemic control have decreased perinatal morbidity and mortality related to diabetic nephropathy. Despite good glycaemic control, overt nephropathy is associated with a variety of pregnancy complications, such as fetal growth restriction and pre-eclampsia.
General population studies show that women with a history of pre-eclampsia are more prone to develop cardiovascular disease later in life than women with a history of normotensive pregnancy. Furthermore, recent data regarding the long-term effects of hypertensive pregnancies on late diabetic complications indicate that these women should be followed and treatment should be started early. In this review we summarize data on risk factors and long-term effects of hypertensive pregnancies on late diabetic complications that may be of clinical relevance in the prevention of these complications.
Key messages
Hypertensive disorders of pregnancy are the most important risk factors for serious pregnancy-related complications causing maternal and fetal morbidity and mortality.
Pre-eclampsia increases the risk of cardiovascular diseases later in life. Recent studies have observed that pre-eclampsia also associates with diabetic microvascular complications, such as nephropathy and retinopathy in women with diabetes.
A hypertensive pregnancy in women with diabetes is a window into the future health of these women.
Introduction
Hypertensive pregnancy, and especially the proteinuric form (pre-eclampsia), increases the risk of maternal and fetal morbidity and mortality. The World Health Organization (WHO) has estimated that pre-eclampsia annually causes 60,000 maternal deaths and even more deaths of fetuses and newborn infants (Citation1). Hypertensive disorders of pregnancy complicate roughly 5%–10% of all pregnancies (Citation2). Pre-eclampsia in turn occurs in up to 4%–5% of pregnancies, in 10% of first pregnancies, and in 20%–25% of women with a history of chronic hypertension (Citation3). Women with diabetes carry a 2–4 times increased risk of a hypertensive pregnancy and up to 6 times increased risk of pre-eclampsia compared to non-diabetic people. The risk is especially high in patients with diabetic nephropathy (Citation4,Citation5).
How is pre-eclampsia and gestational hypertension defined?
One of the most commonly used definitions of pre-eclampsia is a blood pressure level of ≥ 140/90 mmHg accompanied by proteinuria after 20 weeks of pregnancy (Citation6), while gestational hypertension is defined as an elevated blood pressure in the absence of proteinuria. The American Diabetes Association (ADA) defines gestational hypertension in patients with diabetes as systolic blood pressure of ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg, which is based on the evidence from randomized controlled trials of treatment benefits in non-pregnant diabetes patients (Citation7,Citation8). If blood pressure is elevated before the pregnancy or before 20 weeks of gestation, then the hypertension is considered to be chronic.
In patients with diabetes, albuminuria is considered significant when > 300 mg albumin is excreted into the urine in a 24-h urine collection, or > 200 μg/min in an overnight collection, or the albumin-to-creatinine ratio is > 35 mg/mmol in a single spot urine sample. Microalbuminuria is an early marker of kidney disease and is defined as an albumin excretion rate (AER) 30 to < 300 mg/24 h, or 20 μg/min to < 200 μg/min, or 3.5–35 mg/mmol in a single spot urine sample (Citation9). Notably, AER can increase up to 3-fold during pregnancy due to the enhanced kidney perfusion and glomerulus filtration. Thus microalbuminuria is not used for the diagnosis of pre-eclampsia.
For screening of pre-eclampsia a semi-quantitative single spot urine sample (albumin/creatinine or protein/creatinine ratio) should be performed in all pregnant women. Whenever the protein is repeatedly positive (+), a quantitative measurement (24-h or an overnight collection) of proteinuria should be performed to verify the diagnosis. The diagnosis has been set based on a proteinuria of > 300 mg/24 h. It is of note that a proteinuria of 300 mg/24 h is equivalent to a urinary albumin excretion of 190 mg/24 h.
How to predict pre-eclampsia?
Women at risk of pre-eclampsia should be recognized early in pregnancy in order to start an intensive follow-up of both the mother and the fetus. Research has focused on blood markers, genetic markers, as well as ultrasound and Doppler flow measurements of the uterine artery to predict pre-eclampsia at an early stage (Citation10). Out of the 27 tests studied by Meads el al. only BMI ≥ 34 kg/m², α-fetoprotein, and bilateral uterine artery Doppler-flow notching reached specificity above 90% (Citation11). Furthermore, the placenta protein 13 (PP-13) as well as the soluble fms-like tyrosine kinase-1 (sFlt-1) and the soluble endoglin (sEng) are considered as potential markers, although further studies are warranted (Citation12).
Risk factors for pre-eclampsia
A number of risk factors for gestational hypertension have been recognized (). A woman with a history of pre-eclampsia carries a 7-fold risk of recurrent disease. Cardiovascular risk factors, such as antiphospholipid antibodies (10-fold), diabetes (4-fold), elevated BMI (2-fold), hypertension (1.4-fold), and kidney disease (3-fold) are also known to increase the risk of pre-eclampsia (Citation13). Notably, based on epidemiological studies, smoking has been shown to be a protecting factor (0.7-fold) for pre-eclampsia in child-bearing women (Citation14,Citation15).
Table I. Risk factors for gestational hypertension.
Type 1 diabetes
Hypertensive disorders are common complications in pregnancies of women with diabetes. Patients with type 1 diabetes but without diabetic kidney disease have a 2–3-fold risk of gestational hypertension, but the prevalence of pre-eclampsia is as high as 10%–20% in their first pregnancy (Citation5). This risk increases significantly with the presence of diabetic nephropathy: 42% of patients with type 1 diabetes and microalbuminuria, and 64% of those with macroalbuminuria, developed pre-eclampsia during pregnancy in a Danish study (Citation16). In these studies pre-eclampsia in women with diabetic nephropathy has been defined as blood pressure of ≥ 140/90 mmHg or an increase of ≥ 15% in the systolic or diastolic blood pressure later than 20 weeks of gestation in addition to the persisting proteinuria.
Good glycaemic control protected the women with type 1 diabetes from pre-eclampsia but not from gestational hypertension in our own study (Citation5). The results were recently replicated in a randomized controlled study (Citation17). Women with moderate glycaemic control (HbA1c 6.1%–6.9%) in early pregnancy had a 3-fold higher risk of pre-eclampsia compared to those with good glycaemic control (HbA1c < 6.1%). The risk was 8-fold in women with poor glycaemic control (HbA1c > 8.0%).
Contrasting results have also been published showing that an increase in BMI and worsening of the glycaemic control between 1989 and 2008 did not affect the prevalence of pre-eclampsia, gestational hypertension, or chronic hypertension in patients with type 1 diabetes. However, the proportion of patients with type 1 diabetes who had blood pressure > 130/80 mmHg but < 140/90 mmHg increased over the same time period (Citation18,Citation19).
Although diabetic nephropathy is by far the most important risk factor for pre-eclampsia in women with type 1 diabetes, also other risk factors have been observed and shown to be similar to the known risk factors for non-diabetic participants (). The risk is increased by nulliparity, previous pre- eclampsia, chronic hypertension, diabetic retinopathy, and duration of diabetes (Citation5,Citation20).
Type 2 diabetes
The data regarding risk factors for pre-eclampsia in type 2 diabetes are still scarce, although several factors may be the same as in type 1 diabetes (). However, more studies are certainly needed considering the increasing number of patients with type 2 diabetes (Citation20). Nevertheless, chronic hypertension has been shown to be more common in child-bearing women with type 2 diabetes compared to women with type 1 diabetes. However, these women seem to have less often pre-eclampsia than those with type 1 diabetes, although albuminuria is more prevalent in type 2 diabetes (Citation15). Furthermore, nulliparity, duration of diabetes, poor glycaemic control, elevated BMI, and cardiovascular diseases increase the risk. In contrast, the age of the child-bearing mother seems to affect the risk in type 2 diabetes only (Citation20).
Gestational diabetes
Gestational diabetes is associated with an increased risk of simultaneous hypertensive disorders (Citation21). Notably, controlled clinical trials have shown that the treatment of gestational diabetes modestly decreases the risk of hypertensive disorders in pregnancy, although it is not clear whether this is due to a better blood glucose control or weight reduction (Citation22,Citation23). Furthermore, the most important risk factors for gestational hypertension are shown in . Overweight, impaired glucose tolerance, high fasting plasma glucose, and, above all, insulin resistance are important pathophysiological players. Insulin sensitivity is known to decrease during the latter part of a pregnancy with a concomitant increase in the serum triglyceride concentration. Pre-eclampsia is also a state of sympathetic overactivity, which in the short term increases insulin resistance and in the long term increases the risk of cardiovascular disease later in life (Citation3,Citation24).
Do hypertensive disorders of pregnancy have an impact on later life?
Kidney diseases
Already in 1936 Herrick and Tillman observed that pre-eclampsia had a profound effect on the kidneys during pregnancy as well as later in life (Citation25). Since then our knowledge regarding this intriguing finding has increased, and a meta-analysis (women with and without diabetes) was recently published (Citation26). After 7 years of follow-up microalbuminuria developed in 31% of the women who had had pre-eclampsia compared to 7% of the control women. The estimated glomerular filtration rate (eGFR) did not differ between the groups, but this was probably due to the relatively short follow-up time. A large Norwegian register study recently demonstrated that women with a history of pre-eclampsia had a 5–16-fold increased risk of end-stage renal disease (dialysis or transplantation) (Citation27).
Data regarding pre-eclampsia as a risk factor for kidney disease in patients with type 1 diabetes are also rather scarce. In our own cohort the risk of diabetic nephropathy, after adjusting for associated risk factors, was 8-fold in women with a history of pre-eclampsia compared to those with normal blood pressure during pregnancy () (Citation28). Gestational hypertension did not increase this risk. However, microalbuminuria was quantitatively measured neither before nor during pregnancy. Therefore, we could not rule out the possibility of the presence of microalbuminuria before the pregnancy. It is therefore possible that in women with signs of endothelial dysfunction (microalbuminuria), pre-eclampsia was in fact the first clinical manifestation, which then later was transformed into nephropathy during follow-up.
Table II. Pre-eclampsia (PE) and chronic hyperglycaemia during pregnancy as predictors for diabetic nephropathy later in life in a logistic regression analysis.
Eye diseases
It is a well-known fact that diabetic retinopathy deteriorates temporarily during pregnancy in 17%–70% of women with type 1 diabetes (Citation29,Citation30). Duration of diabetes, poor glycaemic control, and high blood pressure increase the risk. The reasons for this phenomenon are unknown, although markers of endothelial dysfunction and inflammation are increased in women whose eye disease worsens (Citation31).
Pregnancy itself has not been reported to worsen diabetic microvascular complications later in life (Citation32,Citation33). Nevertheless, patients with pre-eclampsia in a Swedish study suffered from a deterioration of retinopathy 6 months after pregnancy compared to women without pre-eclampsia (Citation34). In our cohort, patients with pre-eclampsia and gestational hypertension were analysed separately and followed for 16 years. Severe diabetic retinopathy was more common not only in patients with pre-eclampsia but also in those with gestational hypertension compared to women with a history of normal blood pressure during pregnancy (Citation35).
Cardiovascular disease
Population studies have demonstrated that pre-eclampsia and gestational hypertension increase more than 2-fold the risk of hypertension and cardiovascular disease later in life () (Citation36). In addition to increasing the risk of ischaemic heart disease and stroke, pre-eclampsia also elevates the risk of venous thrombosis.
Figure 1. Pre-eclampsia and risk of fatal and non-fatal ischaemic heart disease events in later life. Modified with permission from Bellamy et al. 2007.
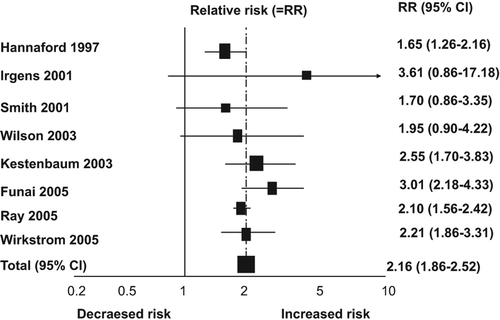
We observed in our cohort of patients with type 1 diabetes that women with a hypertensive pregnancy (pre-eclampsia or gestational hypertension) developed more often hypertension (42%–50%) during a mean follow-up of 11 years, compared to women with normal blood pressure (10%) during pregnancy (Citation28). In the same study, coronary heart disease developed in 12% of the women with pre-eclampsia compared to 2% in the women with normotensive pregnancy (Citation28).
Mortality
Results from a large cohort consisting of almost 630,000 women followed for 13 years demonstrated that pre-eclampsia also predicts mortality (Citation37). The risk was 1.2-fold increased in women with pre-eclampsia during pregnancy compared to women who did not have pre-eclampsia. The most common causes of death were cardiovascular diseases, showing an 8.1-fold higher risk in cardiovascular mortality (Citation37). However, in a large registry study (n = 836,147) exploring the association between pre-eclampsia and cardiovascular mortality, no correlation between a history of pre-eclampsia and all-cause mortality was observed (Citation38). Notably, studies exploring the association between pre-eclampsia and early mortality in women with diabetes are warranted.
How should the existing data be interpreted?
A careful look at the existing data suggests that pre-eclampsia may well serve as a window into the future vascular health of diabetic women. It is possible that the pregnancy itself, as a state of hypervolaemia, acquired thrombophilia, insulin resistance, and low-grade inflammation, could induce pre-eclampsia as the first manifestation of vascular disease, which then progresses to clinical cardiovascular disease only after a few years. On the other hand, it is also possible that some of the women may have had signs of kidney disease (microalbuminuria) already before pregnancy, that enhanced the manifestation of glomerulopathy years after their child-birth. In any case, endothelial dysfunction and its risk factors (dyslipidaemia, insulin resistance, hypertension, subclinical inflammation, increased oxidative stress, and chronic hyperglycaemia) are probably the main factors involved in both pre-eclampsia and diabetic nephropathy (Citation39). The often massive proteinuria observed in pre-eclampsia resolves after delivery, whereas endothelial dysfunction seems to prevail.
An important question for future research is how hypertensive disorders of pregnancy can be reduced in patients with diabetes. Furthermore, controlled studies are certainly warranted to guide clinicians on how to treat women with a history of a hypertensive pregnancy to avoid vascular complications later in life.
In conclusion, the number of pregnant patients with diabetes is continuously increasing, and consequently the number of patients with hypertensive disorders is also increasing. The risk of hypertensive complications is especially enhanced in women with diabetic nephropathy. As hypertensive disorders are known to associate with complications in mothers and fetuses, it is of utmost importance for diabetic patients to plan their pregnancies in advance. Furthermore, given the fact that a proportion of patients with type 1 diabetes are susceptible to complications, we think that women with diabetes should become pregnant at a younger age (e.g. < 30 years) than is the actual tendency in non-diabetic women (> 30 years) as the risk for microangiopathic complications increases with the duration of diabetes and age. Strict control of blood glucose and blood pressure as well as making sure that fundus photographs are taken are important steps in pre-pregnancy planning to minimize the risk of pregnancy-related diseases. Angiotensin-converting enzyme (ACE)-inhibitors/ angiotensin II receptor (ATR)-blockers should be used for renal and retinal protection after the patient has experienced a hypertensive pregnancy. Although prediction of late diabetic complications is difficult, the presence of hypertensive disorders during pregnancy in patients with diabetes may be used as a tool to identify women at risk.
Sources and selection criteria
We searched Medline, the Cochrane Database of Systematic Reviews, and Clinical Evidence online using the search terms ‘pre-eclampsia, gestational hypertension, pregnancy-induced hypertension, hypertensive pregnancy, and diabetes’, as well as recent conference proceedings. We limited studies to those conducted in adults and focused on systematic reviews, meta-analyses, and high-quality randomized controlled trials published during the past 5 years whenever possible. Of the 68 articles reviewed, 39 references were included in the manuscript.
Declaration of interest: The authors report no conflicts of interest.
References
- World Health Organization. World Health Report 2005: Make every mother and child count. Geneva: World Health Organization; 2005. Available at: whqlibdoc.who.int/publications/2006/9241563206_eng.pdf.
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, et al. Williams obstetrics. 23rd ed. Toronto: McGraw Hill Medical; Oct 29th, 2009. 1–1404, [chapter 34].
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–94.
- Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328:915.
- Hiilesmaa V, Suhonen L, Teramo K. Glycaemic control is associated with pre-eclampsia but not with pregnancy-induced hypertension in women with type 1 diabetes mellitus. Diabetologia. 2000;43:1534–9.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gyneol. 2002;99:159–67.
- Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31:1060–79.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al.; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72.
- Mathiesen ER, Oxenboll B, Johansen K, Svendsen PA, Deckert T. Incipient nephropathy in type 1 (insulin-dependent) diabetes. Diabetologia. 1984;26:406–10.
- Skjaerven R, Vatten LJ, Wilcox AJ, Ronning T, Irgens LM, Lie RT. Recurrence of pre-eclampsia across generations: exploring fetal and maternal genetic components in a population based cohort. BMJ. 2005;331:877.
- Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2008;12:iii–iv, 1–270.
- Kaaja R. Predictors and risk factors of pre-eclampsia. Minerva Ginecol. 2008;60:421–9.
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565–72.
- Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181:1026–35.
- Cundy T, Slee F, Gamble G, Neale L. Hypertensive disorders of pregnancy in women with Type 1 and Type 2 diabetes. Diabet Med. 2002; 19:482–9.
- Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Mølvig J, Mathiesen ER. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care. 2001;24:1739–44.
- Holmes VA, Young IS, Patterson CC, Pearson DW, Walker JD, Maresh MJ, et al.; Diabetes and Pre-eclampsia Intervention Trial Study Group. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care. 2011;34:1683–8.
- Klemetti M, Nuutila M, Tikkanen M, Kari MA, Hiilesmaa V, Teramo K. Trends in maternal BMI, glycaemic control and perinatal outcome among type 1 diabetic pregnant women in 1989-2008. Diabetologia.2012;55:2327–34.
- Klemetti M, Teramo K, Nuutila M, Tikkanen M, Hiilesmaa V, Laivuori H. Blood pressure levels but not hypertensive complications have increased in type 1 diabetes pregnancies during 1989Diabet Med. 2013;30:1087–93.
- Colatrella A, Loguercio V, Mattei L, Trappolini M, Festa C, Stoppo M, et al. Hypertension in diabetic pregnancy: impact and long-term outlook. Best Pract Res Clin Endocrinol Metab. 2010;24:635–51.
- Mayer-Davis EJ, Bell RA, Dabelea D, D’Agostino R Jr, Imperatore G, Lawrence JM, et al; SEARCH for Diabetes in Youth Study Group. The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:S99–101.
- Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, et al. Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;340:c1395.
- Falavigna M, Schmidt MI, Trujillo J, Alves LF, Wendland ER, Torloni MR, et al. Effectiveness of gestational diabetes treatment: a systematic review with quality of evidence assessment. Diabetes Res Clin Pract. 2012;98:396–405.
- Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia – a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–5.
- Herrick WW, Tillman AJB. The mild toxemias of pregnancy. Their relation to cardiovascular and renal disease. Am J Obstet Gynecol. 1936;31:832–44.
- McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55:1026–39.
- Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–9.
- Gordin D, Hiilesmaa V, Fagerudd J, Rönnback M, Forsblom C, Kaaja R, et al.; FinnDiane Study Group. Pre-eclampsia, but not pregnancy-induced hypertension is a risk factor for diabetic nephropathy in type 1 diabetic women. Diabetologia. 2007;50:516–22.
- Klein BE, Moss SE, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13:34–40.
- Loukovaara S, Immonen I, Teramo KA, Kaaja R. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care. 2003;26:1193–8.
- Loukovaara S, Immonen I, Koistinen R, Hiilesmaa V, Kaaja R. Inflammatory markers and retinopathy in pregnancies complicated with type 1 diabetes. Eye (Lond). 2005;19:422–30.
- Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084–91.
- Vérier-Mine O, Chaturvedi N, Webb D, Fuller JH. Is pregnancy a risk factor for microvascular complications? The EU-RODIAB Prospective Complications Study. Diabet Med. 2005;22:1503–9.
- Lövestam-Adrian M, Agardh CD, Aberg A, Agardh E. Pre-eclampsia is a potent risk factor for deterioration of retinopathy during pregnancy in type 1 diabetic patients. Diabet Med. 1997;14:1059–65.
- Gordin D, Kaaja R, Forsblom C, Hiilesmaa V, Teramo K, Groop PH; FinnDiane Study Group. Pre-eclampsia and pregnancy-induced hypertension are associated with severe diabetic retinopathy in type 1 diabetes later in life. Acta Diabetol. 2013;50:781–7.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974–85.
- Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–17.
- Skjaerven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677.
- Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–7.

