Abstract
Angioedema refers to a localized, transient swelling of the deep skin layers or the upper respiratory or gastrointestinal mucosa. It develops as a result of mainly two different vasoactive peptides, histamine or bradykinin. Pathophysiology, as well as treatment, is different in each case; nevertheless, the resulting signs and symptoms may be similar and difficult to distinguish. Angioedema may occur at any location. When the affected area involves the upper respiratory tract, both forms of angioedema can lead to an imminent upper airway obstruction and a life-threatening emergency. Emergency physicians must have a basic understanding of the pathophysiology underlying this process. Angioedema evaluation in the emergency department (ED) should aim to distinguish between histamine- and bradykinin-induced angioedema, in order to provide appropriate treatment to patients. However, diagnostic methods are not available at the ED setting, neither to confirm one mechanism or the other, nor to identify a cause. For this reason, the management of angioedema should rely on clinical data depending on the particular features of the episode and the patient in each case. The history-taking should be addressed to identify a possible etiology or triggering agent, recording complete information for an ulterior diagnostic study in the outpatient clinic. It is mandatory quickly to recognize and treat a potential life-threatening upper airway obstruction or anaphylaxis. This review focuses on the underlying mechanisms and management of histamine- and bradykinin-induced angioedema at the emergency department and provides an update on the currently available treatments.
Key messages
Angioedema develops mainly as a result of the release of two different vasoactive peptides, histamine or bradykinin.
Nevertheless clinical presentation may be similar.
Thus, angioedema evaluation in the emergency department (ED) should aim to distinguish between histamine- and bradykinin-induced angioedema, in order to provide patients with appropriate treatment, which is substantially different in each case.
Introduction
The term angioedema refers to a localized, transient swelling of the deep skin layers or the upper respiratory or gastrointestinal mucosa. This condition involves the reticular (deep) dermis and subcutaneous and submucosal tissues. Isolated angioedema can sometimes manifest with symptoms of pain and tenderness, whereas itching may not be present (Citation1,Citation2).
Angioedema develops as a result of mainly two different vasoactive peptides: histamine or bradykinin. Pathophysiology of angioedema resulting from the release of bradykinin is distinct from that caused by histamine; nevertheless, the resulting signs and symptoms may be similar. Both substances induce vascular leakage and the consequent non-pitting interstitial edema, which results in transient localized swelling. Angioedema may occur at any location; however, it most commonly involves the head, neck, lips, mouth, tongue, larynx, and pharynx along with peripheral, abdominal, or genital areas (Citation1,Citation3,Citation4).
Angioedema can progress rapidly. In cases where the affected areas involve the pharyngolaryngeal region, it could constitute a medical emergency. Swelling can occur within minutes in the case of histamine-released angioedema compared with a typically slower onset in bradykinin-mediated angioedema (Citation5). Nevertheless, both forms of angioedema can lead to an imminent upper airway obstruction and a life-threatening condition. Emergency physicians should have a basic understanding of the pathophysiology of this process. This review focuses on the underlying mechanisms and the emergency department management of histamine- and bradykinin-induced angioedema.
Pathophysiology of angioedema
Two main forms of angioedema can be distinguished:
Histamine-mediated angioedema consists of a mast cell-dependent reaction. Mast cell activation and degranulation results in a release of preformed mediators, such as histamine, and newly formed mediators such as leukotrienes. These bioactive mediators are responsible for the edema and swelling.
On the other hand, bradykinin-mediated angioedema occurs due to the increased production of bradykinin, because of a lack of regulation of the contact pathway, or a decrease in bradykinin degradation. The resulting high levels of bradykinin lead to edema.
There are other mechanisms that may induce angioedema. Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cyclo-oxygenase and consequently prostaglandin synthesis, switching arachidonic acid metabolism to the lipoxygenase pathway, and the generation of leukotrienes, which serve as mediators that may result in angioedema (Citation6).
The end-point of angioedema is an increase of vascular permeability in the skin and submucosa. This causes plasma extravasation and the consequent tissue swelling. Angioedema can be classified based on its pathophysiology (Citation7), as shown in .
Table I. Classification of angioedema (modified from Caballero et al. (Citation49)).
1. Histamine-mediated angioedema
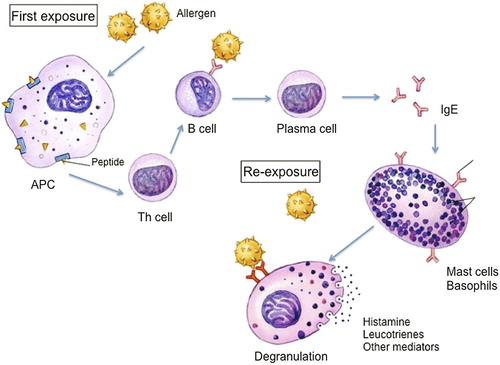
This angioedema occurs due to mast cell activation and degranulation. The best-known mechanism of mast cell activation is through a type I IgE-mediated hypersensitivity immune response (). Susceptible individuals become sensitized after exposure to an allergen. Antigen presenting cells take up the allergens and break them into peptides that are presented to the cell surface via MHC-2 molecules (Citation8). The complex is recognized by T-helper lymphocyte receptors, which results in T-cell activation and Th2 cytokines release including interleukin (IL)-4, IL-5, IL-13 (Citation9). These interleukins promote IgE synthesis and eosinophil differentiation and migration. They also cause B lymphocytes to differentiate into specific IgE-producing plasma cells able to recognize the original sensitizing allergen. The specific antibody binds to the high-affinity IgE receptors (FcϵRI) in the mast cell and basophil surface. Upon re-exposure to the allergen, the antigen-binding sites of the specific IgE recognize the peptide. The cross-linking by the antigen of two IgE molecules bound to their high-affinity receptors in the mast cell surface leads to the transduction of an intracellular signaling that results in mast cell activation and degranulation. Thus, preformed and newly generated bioactive mediators are released. Released histamine (Citation10) binds to selective receptors (i.e. H1 receptor) on the vascular endothelium leading to vasodilation and increased vascular permeability (Citation11).
The existence of autoantibodies against mast cell IgE receptor (FcϵRI) or mast cell or basophil-bound IgE is another possible cause of mast cell activation and histamine release (Citation12), underlying chronic autoimmune urticaria and angioedema. Other immunologic mechanisms that do not involve IgE are implicated in anaphylaxis and mast cell activation (such as through complement or IgG mast cell receptors). In addition, mast cells could also be activated and degranulated by non-immunologic triggering mechanisms (Citation13) such as physical factors, opioids, and ethanol or by an unknown mechanism. Nevertheless this angioedema responds to antihistamine treatment (idiopathic histaminergic angioedema) (Citation14).
2. Bradykinin-mediated angioedema
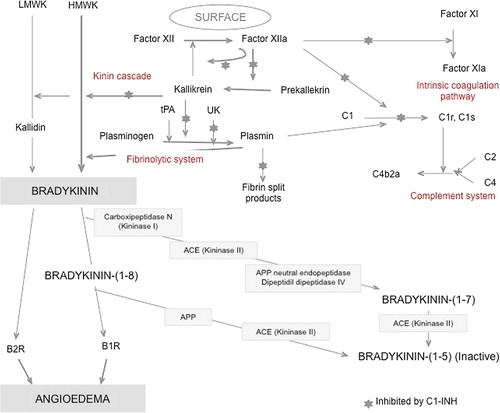
Kinins are a group of active peptides that are released into body fluids and tissues, following the enzymatic action of kallikreins on kininogens. This occurs through the proteolytic cascade of events called the kallikrein-kinin system () also referred as the ‘contact activation pathway’. The contact activation pathway is initiated when factor XII binds to damaged tissue and becomes activated in FXIIa. FXIIa converts prekallikrein into plasma kallikrein, and they autoactivate through a positive feedback loop. Plasma kallikrein cleaves high-molecular-weight kininogen (HMWK) into bradykinin (Citation15). Bradykinin then binds to B2-receptors, inducing vasodilation and increased endothelial permeability, leading to the characteristic swelling of an angioedema attack (Citation16).
Figure 2. The contact system, showing the generation and degradation of bradykinin (reproduced with permission from: Caballero T, et al. J Investig Allergol Clin Immunol. 2011;21:333–47). ACE = angiotensin-converting enzyme; APP = aminopeptidase P; B1R = bradykinin B1 receptor; B2R = bradykinin B2 receptor; C1-INH = C1 esterase inhibitor; HMWK = high-molecular-weight kininogen; LMWK = low-molecular-weight kininogen; tPA = tissue plasminogen activator; UK = urokinase.
2.1. Hereditary and acquired angioedema with C1-inhibitor deficiency
Hereditary angioedema (HAE) is a rare autosomal dominant disease characterized by a quantitative (type I) or qualitative (type II) deficiency in C1 esterase inhibitor (C1-INH). The condition is caused by a mutation of the C1-INH SERPING1 gene, located on the chromosome 11q (Citation17). C1-INH has an anti-inflammatory effect, as it is a key inhibitor of many enzymes in the kallikrein-kinin cascade controlling the production of bradykinin (). The deficiency in C1-INH results in the uncontrolled activation of the entire cascade. This generates an excessive amount of bradykinin. Due to its vasodilator properties, there is an increase in vascular permeability, which leads to plasma extravasation and, thus, to localized swelling (Citation18,Citation19).
Acquired angioedema (AAE) results from a deficiency in C1-INH, secondary to an underlying condition such as lymphoproliferative disorders (Citation20), autoimmune diseases (Citation21), vasculitis (Citation22), or infections (Citation23). C1-INH deficiency occurs as a result of increased consumption by paraprotein or immune complexes or by direct cleavage by C1-INH autoantibodies (Citation24,Citation25).
2.2. Hereditary angioedema without C1-inhibitor deficiency (HAE III)
Some HAE III patients have been described to be affected by a point mutation of the coagulation factor XII gene (F12) (Citation26–31). This mutation leads to an increased amidolytic activity of coagulation FXII (Citation32) that causes overactivation of the kallikrein-kinin cascade and, thus, can increase the production of bradykinin. However, the increase in bradykinin has not been shown yet. Recently, Defendi et al. (Citation33) showed an increase in spontaneous amidase activity in this type of angioedema that supports bradykinin involvement in the pathogenesis of angioedema attacks in HAE with normal C1-INH. There is a preponderance in female patients, probably explained by the fact that FXII transcription is enhanced by estrogens (Citation32). Endogenous (pregnancy) or exogenous estrogens (oral contraceptives (OCP) or replacement hormonal therapy (RHT)) trigger angioedema attacks in these patients (Citation34–36).
2.3. Angiotensin-converting enzyme inhibitor-induced angioedema
Angiotensin-converting enzyme inhibitors (ACEi) are the mainstay in the treatment of many conditions such as hypertension, myocardial infarction, heart failure or type I diabetic nephropathy, which makes them a widely utilized drug (Citation36). Angiotensin-converting enzyme (ACE) plays a major role in the renin-angiotensin-aldosterone system. ACE is involved in two proteolytic mechanisms: conversion of angiotensin I to angiotensin II and degradation of bradykinin. ACE destroys bradykinin by removing the C-terminal Phe-Arg dipeptide, followed by removal of Ser-Pro, leaving the inactive pentapeptide Arg-Pro-Pro-Gly-Phe (Citation37).
ACEi cause a reduction in bradykinin degradation, and thus increased levels of bradykinin lead to swelling, pain, and inflammation. ACEi-induced angioedema most commonly involves the orofacial region, and up to 25%–39% of the cases may involve the upper airway (Citation38). ACEi-induced angioedema can develop years after the initiation of treatment (Citation39). Angiotensin receptor blockers (ARBs) may also induce angioedema but at a lower frequency (Citation3). A recent meta-analysis has shown that the incidence of angioedema related to these drugs, in controlled trials, is not significantly different from placebo (Citation40). Also the incidence of angioedema in patients treated with ARBs that previously reported ACEi induced angioedema is similar to placebo (Citation41).
During ACE inhibition, other enzymes assume the degradation of bradykinin: neutral endopeptidase (NEP), aminopeptidase (APP), kininase I (carboxypeptidase N), and dipeptidyl- peptidase IV (DPP-IV). Kininase I cleaves bradykinin yielding the active metabolite des-Arg9-bradykinin which is inactivated by APP and DPP-IV. Bradykinin stimulates the release of substance P from sensory nerves. ACE also degrades substance P. Nevertheless, under ACE inhibition, DPP-IV and aminopeptidase N sequentially degrade substance P. An association between a decrease in amount and activity of DPP-IV in patients with ACEi-angioedema has been shown (Citation42,Citation43). This suggests that DPP-IV inhibitors, like vildagliptin, saxagliptin, or sitagliptin, used in the treatment of diabetes in patients concurrently taking ACEi, may increase the risk for the development of angioedema. In line with this, a recent meta-analysis has shown that although there is no association between the use of vildagliptin (DPP-IV inhibitor) and angioedema, among individuals taking ACEi, the use of vildagliptin was associated with an increased risk (OR: 4.57; 95% CI: 1.57–13.28) (Citation44). For this reason, the use of DPP-IV inhibitors should be monitored in patients taking ACEi.
2.4. Idiopathic bradykinin-mediated angioedema
Bradykinin-induced idiopathic angioedema is a diagnosis of exclusion for cases where neither exogenous trigger nor underlying abnormality can be identified (Citation45) and that do not respond to antihistamine treatment (Citation14). It has been shown that non-histaminergic angioedema can be related to an increase of bradykinin levels (bradykinergic idiopathic angioedema) (Citation46). This is supported by the responsiveness to bradykinin receptor antagonists (Citation47,Citation48).
3. Non-histamine-, non-bradykinin-mediated angioedema
Other forms of angioedema are provoked by the release of vasoactive mediators other than histamine or bradykinin. This is the case of reactions to acetyl-salicylic acid (ASA) and non-steroidal anti-inflammatory drugs (NSAIDs), where respiratory symptoms, angioedema, urticaria, or shock may develop (Citation6). It is postulated that cyclo-oxygenase inhibition deflects arachidonic acid through the lipoxygenase pathway, with excessive leukotriene (LT) production. Cysteinil leukotrienes LTC4 and LTD4 have vasoactive properties that lead to edema formation (Citation49).
Diagnosis of angioedema at the emergency department
Angioedema evaluation in the emergency department (ED) should aim to distinguish between histamine- and bradykinin-induced angioedema, in order to provide appropriate treatment to patients. Since we do not have available diagnostic tests to distinguish histaminergic from bradykinergic angioedema that could be implemented in an ED setting, diagnosis should rely on clinical data (). The history-taking should also be addressed to identify a possible etiology or triggering agent, recording complete information for an ulterior diagnostic study in the outpatient clinic. It is mandatory quickly to recognize and treat a potential life-threatening upper airway obstruction or anaphylaxis.
Table II. Differential features of bradykinin and histamine-mediated angioedema.
1. Medical history
History is the most important component of the diagnostic evaluation of patients with angioedema (Citation1).
A.1. Symptoms and clinical features
Angioedema causes an evident swelling or tumefaction of subcutaneous or submucosal tissues (Citation50,Citation51). It may be present at different locations (face, lips, eyelids, tongue, larynx, extremities, genitalia, buttocks, or gastrointestinal tract). When it is localized in the face or neck or in the upper airway the patient should be closely monitored. There is not a specific location that could help to distinguish between the different types of angioedema, although ACEi-induced angioedema is often localized to the head, lips, mouth, tongue, larynx, pharynx, and subglottal regions (Citation2).
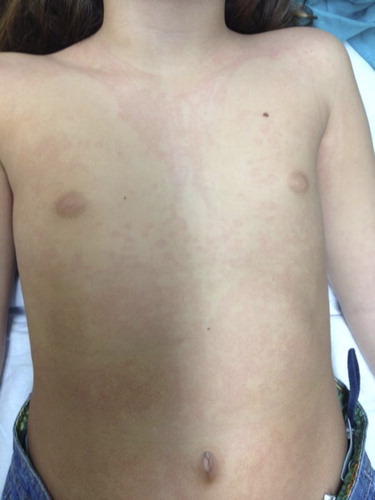
A.1.1. Skin or peripheral angioedema. Bradykinin-induced angioedema is typically not associated with urticaria. It is non-erythematous, it does not associate pruritus or increased temperature, and it is circumscribed with poorly defined borders (Citation51–53). Some patients experience symptoms that predict the attack (prodrome), including mood swings, anxiety, asthenia, itching, paresthesia, or erythema marginatum-like exanthema, which consists of erythematous patches with circular borders and a clear center, that spread outward drawing polycyclic rings (). The rash does not itch or burn, and it should not be confused with urticaria (Citation51,Citation54–56).
Figure 3. The picture shows an erythema marginatum preceding an angioedema acute attack in a patient with type I HAE. The erythematous rash with circular polycyclic border and a clear center should not be confused with urticaria.
Histaminergic angioedema is erythematous and itching. It is often accompanied by urticaria. However, it can present isolated without urticaria, as differential diagnosis with bradykinin-induced angioedema. Other symptoms of anaphylaxis may be associated as the result of other organ or system involvement in a systemic reaction (Citation51,Citation52).
A.1.2. Angioedema affecting upper airway. Patients who complain of dyspnea, hoarseness, voice change, odynophagia, or have stridor on examination are likely to have laryngeal edema that can be life-threatening (Citation57,Citation58).
A.1.3. Gastrointestinal involvement. It may manifest as acute abdominal pain caused by edema in the gastrointestinal wall and free fluid in the peritoneal cavity (Citation54). It can range from slight discomfort to intense abdominal pain and cramping that is refractory to analgesic treatment and progresses to abdominal distension, nausea, vomiting, and constipation due to obstruction of the gastrointestinal tract (Citation51,Citation59). For this reason, patients frequently have had unnecessary surgery. Massive fluid accumulation in the intestinal wall and lumen and in the peritoneal cavity as well as vasodilatation can cause diarrhea and hypovolemia, producing orthostatic hypotension, dehydration, syncope, and hypovolemic shock (Citation51,Citation60,Citation61).
Some 70% to 80% of patients with HAE present with recurrent abdominal attacks. Abdominal symptoms may also be mediated by histamine, in the context of anaphylaxis (Citation54).
A.2. Assessment of time of onset and duration
Bradykinin-mediated angioedema has a slower and progressive onset and usually reverts within 48 to 72 hours, although it can persist for up to five days (Citation51,Citation52).
ACEi-induced angioedema can occur within days or weeks following treatment initiation, or it can also occur after a prolonged course of several years (Citation62).
In contrast, histaminergic angioedema has an abrupt onset of a few minutes and usually subsides within 24–48 hours, but relapses are common and unpredictable (Citation52). A period of six weeks has arbitrarily been chosen to divide patients between acute and chronic histaminergic urticarial/angioedema. In contrast to chronic urticaria, acute urticaria and/or angioedema are more likely to have an identifiable etiology (Citation1).
A.3. Possible triggers
In patients with bradykinin-mediated angioedema, the attack may be triggered by infections, trauma, dental interventions, endoscopy or surgery, emotional stress, ACEi, endogenous (menses or pregnancy) or exogenous (OCP or RHT) hormones, or DPP-IV inhibitors in patients taking ACEi (Citation51).
In patients with histaminergic angioedema, an etiologic allergenic (foods, drugs, insect venom, latex, Anisakis simplex), or non-allergenic agent (some drugs that can induce IgE- but also non-IgE-mediated reactions like iodinated contrast media, vancomycin, opioids, infections, or a physical stimulus like cold or pressure) should be investigated (Citation1).
A.4. Personal or family history of angioedema or atopy
Family history may provide diagnostic data. If the patient or relatives are already diagnosed with HAE type I, II, or III, the angioedema is likely bradykinin-induced. A prevalence of de novo mutations as high as 25% of cases of HAE-C1-INH has been described. So the absence of a family history of angioedema should not exclude this diagnosis (Citation63,Citation64).
If the patient or relatives associate atopic diseases like allergic rhinitis, asthma, or atopic dermatitis, angioedema would be likely histamine-mediated. However, drug or food allergic reactions are possible in non-atopic individuals.
A.5. Response to treatment of previous angioedema episodes
The patient must be asked about previous angioedema episodes, what treatment was administered, and the response of the episodes to that treatment. While histaminergic angioedema resolves with optimal doses of antihistamines, steroids, and epinephrine, bradykinin-mediated angioedema is unresponsive to those treatments but resolves with plasma-derived human (pdh)-C1INH concentrate or icatibant (B2-receptor antagonist).
B. Physical examination
Vital signs: Physical exam starts with an assessment of the airway and cardiovascular status. Oxygen saturation, blood pressure, respiratory rate, and heart rate should be determined, and, in case of respiratory and cardiovascular symptoms or evidence of hypoxia or hypotension, continuous pulse oximetry and electrocardiogram monitoring are indicated.
Upper airway: Special attention should be paid to any evidence of swelling of the lips, the uvula, or the tongue. In patients with laryngeal symptoms or signs, like voice changes, hoarseness, stridor, and dyspnea, otorhinolaryngology consultation for nasopharyngolaryngoscopy (NPL) should be obtained in order to evaluate upper airway compromise and the severity of the attack (Citation57).
Skin: Complete skin body surface exam should be performed, assessing the presence of swelling, urticaria, or erythema marginatum, and describing the location and the extent of the affected area.
Cardiopulmonary auscultation: This should be carried out in order to determine the presence of stridor, wheezing, or arrhythmia in the context of anaphylaxis.
Abdomen: In case of gastrointestinal attacks, abdominal distension may exist as well as diffuse abdominal tenderness or rebound. Abdominal auscultation may reveal hypoactive or hyperactive bowel sounds, and percussion may show shifting dullness (Citation59).
C. Diagnostic tests
Routine laboratory studies are not indicated in the evaluation of patients with acute angioedema of peripheral location at the ED. In case of abdominal or upper airway attacks or anaphylaxis some complementary studies may be helpful:
Blood cell count: There may be elevation of the hematocrit and leukocytosis due to hemoconcentration, especially in abdominal attacks or anaphylactic shock with massive extravasation of fluid. It must be taken into account in differential diagnosis with acute abdomen, as leukocytosis is not specific of acute abdomen (Citation51).
Arterial blood gas: Cases with airway compromise may show hypoxia or respiratory acidosis.
Electrocardiogram: This is indicated in cases of anaphylaxis for the assessment of arrhythmia or ischemia.
Abdominal ultrasonography (Citation65) or computed tomography scan (Citation66) may show intestinal wall and mucosal thickening consistent with edema, fluid accumulation in dilated bowel loops, and ascites. Abdominal X-ray may show various degrees of obstruction with or without air–fluid levels and dilated intestinal loops (Citation59,Citation66).
Mast cell activation markers, like serum tryptase levels, measured 1–4 hours after the onset of the episode, can detect mast cell-dependent angioedema (IgE-mediated or non-IgE-mediated histamine release) (Citation67). If that measurement is not available at the ED, the sample should be centrifuged, serum separated, and kept frozen until laboratory availability.
Differential diagnosis of angioedema
Histamine- versus bradykinin-induced angioedema: bradykinin-induced angioedema should be considered in the following cases (Citation51):
Patients or relatives diagnosed with HAE
Peripheral angioedema without urticaria and/or pharyngolaryngeal edema that does not respond to treatment with optimal doses of epinephrine, antihistamines, and corticosteroids
Recurrent abdominal pain: bradykinin-induced abdominal angioedema attacks should be considered within the differential diagnosis of acute or recurrent abdominal pain
Patients under treatment with ACEi presenting angioedema which does not respond to conventional treatment with epinephrine, antihistamines, and corticosteroids
Abdominal angioedema attacks and acute abdomen: Abdominal involvement in angioedema is often a challenge to diagnose, especially in patients who do not manifest angioedema in other locations or other anaphylactic symptoms as well as in cases of first-time attacks. Acute onset abdominal pain is the most common presenting symptom. Differential diagnosis with acute surgical abdomen is made, since abdominal rebound, as well as leukocytosis (due to hemoconcentration) may be present. Misdiagnosis may lead to unnecessary surgical intervention (Citation51,Citation53,Citation59). On the other hand, patients diagnosed with HAE with previous abdominal attacks could also suffer acute surgical abdomen. Response to specific treatment with pdhC1-inhibitor concentrate or icatibant at the ED is very helpful in differential diagnosis.
Anisakis simplex can cause acute abdominal attacks through an IgE-mediated allergic mechanism or due to a direct local inflammatory reaction in the gut mucosa. Patients should be asked about recent fish ingestion, especially raw or hardly cooked fish.
Other causes of edema: a number of other causes should be differentiated including the following (Citation52):
Hydrostatic edema
Oncotic edema
Gleich syndrome (episodic angioedema associated with eosinophilia)
Ascher syndrome (episodic swelling of eyelids and lips associated with a euthyroid goiter)
Melkersson–Rosenthal syndrome (intermittent but often persistent granulomatous swelling of the lips or cheek, fissured or plicated tongue, and Bell palsy)
Dermatitis
Cellulitis
Venous obstructive diseases (superior vena cava syndrome, deep vein thrombosis)
Filariasis
Treatment of angioedema
In all forms of angioedema, airway location is the first priority in an acute attack.
All cases with laryngeal edema are considered a medical emergency, and if there are signs of airway compromise an intubation must be performed (Citation3,Citation68). In case of angioedema involving the tongue, oral intubation is difficult and often impossible to perform and fiber-optic nasotracheal intubation is the preferred method (Citation68). It should be cautioned that physical manipulation of the airways during evaluation in HAE patients could potentially increase the amount of edema present and result in further compromise of the airway.
A way to measure the severity and risk of airway compromise is the Ishoo staging system that can predict the risk of airway obstruction depending on the anatomic site of angioedema presentation, allowing appropriate triage in ED () (Citation58).
Table III. Staging system of airway obstruction risk from Ishoo et al. (Citation58).
Supplementary oxygen should be given to all patients with angioedema involving the airway, especially if the patient is hypoxic.
An algorithm for the management of acute angioedema is presented in .
Figure 4. Algorithm for management of acute angioedema in the emergency department. Note: Non-responders are considered when there is not an improvement 30–60 minutes after antihistamines, steroids, and/or epinephrine treatment. *Doses and treatment in each type of angioedema as described in . In those cases that a patient with ACEi treatment presents the first attack of angioedema, and the localization or the history of the evolution of the angioedema does not seem to be related to ACEi, first it is preferred to treat with antihistamines, steroids, and epinephrine to confirm that it is a histamine-mediated angioedema or it is a bradykinin angioedema. **In those cases that patient has presented previous episodes of angioedema without response to antihistamines, steroids, and epinephrine, or in those cases that suspicion of ACEi induced angioedema is elevated, they should be treated directly as a bradykinin-mediated angioedema as shown in . Indications for treatment of acute episodes depend on the severity and location of the angioedema episodes. One should treat all episodes of glottic edema, those that affect the cervicofacial or pharyngolaryngeal region, and also abdominal episodes. Peripheral episodes should be treated based on the impact on the patient's quality of life. HAE = hereditary angioedema; ACEI = angiotensin-converting enzyme inhibitors; ICU = intensive care unit.
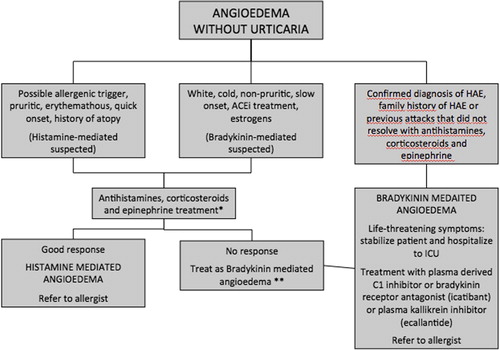
Table IV. Treatment profile in each type of angioedema.
Since there is large number of causes of angioedema, approaches for the management of acute attacks in each type are described.
Angioedema, regardless of cause, has traditionally been treated as a histaminergic reaction with antihistamines, corticosteroids, and epinephrine. For allergic or immunologic-induced angioedema, these would be considered the mainstay of treatment as specified below (Citation69). In the case of bradykinin-mediated angioedema, these medications are useless, and new approaches for treatment are now available (Citation5).
1. Histamine-mediated angioedema
Emergency management focuses on treating the acute attack. For any type of allergic or drug-induced angioedema, removal of the offending agent and assessing the patient quickly is the first step (Citation70).
The first-line treatment of acute angioedema will be antihistamines and glucocorticoids. If the patient presents a systemic reaction (anaphylaxis), including gastrointestinal symptoms, respiratory symptoms, or hypotension, epinephrine is the first-line most effective treatment, positioning the patient appropriately, in Trendelenburg ().
1.1. Antihistamines
First-generation H1 antagonists are the only antihistamines available for intravenous infusion (Citation69). The recommended dosage is:
Dexchlorpheniramine: 5 mg (1 vial) in adults and 0.15–0.3 mg/kg in children.
Diphenhydramine: 1.25 mg/kg/6 hours
Second-generation H1 antagonists (cetirizine, ebastine, loratadine, fexofenadine, rupatadine) may also be given, although this would be limited to the oral route as there are no parenteral versions available (Citation71,Citation72).
They are safe and well tolerated with very few and mild side effects (mainly somnolence).
1.2. Glucocorticosteroids (GCS)
There are no high-quality investigations assessing the use of GCS for angioedema due to any pathological process (Citation73). The optimal dose and duration of GCS treatment used for acute urticaria and angioedema has not been systematically studied, and recommendations vary among specialists and countries (Citation71). In case of respiratory symptoms, the use of steroids is beneficial in adults and children (Citation74).
The recommended dosage is (Citation5):
Hydrocortisone 200 mg in adults and 10–15 mg/kg in children.
Methylprednisolone: 1–2 mg/kg in adults and in children.
GCS used for the acute attack are well tolerated, and they have few side effects.
1.3. Epinephrine
After rapid assessment, if a patient suffers an anaphylaxis, intramuscular (IM) epinephrine injection in the mid-anterolateral thigh should be administered promptly in order to control symptoms and sustain blood pressure (Citation75), placing the patient on the back (or in a position of comfort if there is respiratory distress and/or vomiting). Epinephrine may be life-saving for patients with acute laryngeal edema or anaphylaxis (Citation5,Citation69).
The recommended doses are the following (Citation75): Epinephrine 1:1000 (1 mg/mL) via IM, 0.3–0.5 mg in adults; 0.01 mg/kg (up to 0.3 mg) in children. This dose can be repeated every 5–15 minutes, as needed. Failure promptly to inject it is potentially associated with fatality. If cardiac arrest is imminent or has already occurred, or in patients requiring multiple doses of IM epinephrine, intravenous epinephrine is indicated (1–4 μg/min) under ECG and blood pressure monitoring.
Transient pharmacologic side effects after a recommended dose of epinephrine by any route of administration include pallor, tremor, anxiety, palpitations, dizziness, and headache. Serious adverse effects such as ventricular arrhythmias, ischemia, hypertensive crisis, and pulmonary edema potentially occur after an overdose of epinephrine by any route of administration, although typically they are reported after intravenous epinephrine dosing, with a solution appropriate for intramuscular injection (1:1000 instead of 1:100,000) (Citation75,Citation76).
2. Bradykinin-mediated angioedema
This type of angioedema does not respond to antihistamines, corticosteroids, or epinephrine (Citation23). Indications for the treatment of acute episodes depend on the severity and the location of the angioedema episodes. All the episodes of glottic edema, those affecting the cervicofacial or pharyngolaryngeal region, and also abdominal episodes should be treated. Peripheral episodes should be treated on the basis of the impact on the patient's quality of life.
2.1. With C1-INH deficiency
2.1.1. Hereditary angioedema (HAE). Consensus approaches for management of HAE recommend that HAE attacks should be treated as early as possible (Citation51,Citation77). Patients with HAE are unlikely to respond to antihistamines or corticosteroids. In the acute attack it is important not to delay the administration of treatment, especially if the location of the attack is life-threatening.
Plasma-derived human C1 esterase inhibitor concentrate (pdhC1INH) or recombinant human C1 esterase inhibitor concentrate (rhC1INH). C1-INH replacement therapy functions to restore the lacking C1-INH (Citation5,Citation77). The recommended dosage of the currently available drugs is (Citation2):
Berinert® (CSL Behring, Marburg, Germany). (pdhC1INH): 20 U/kg body weight i.v.
Cinryze® (Viropharma, Brussels, Belgium). (pdhC1INH): 1000 UI i.v. (only in children > 12 years and adults)
Ruconest® (Pharming NV, Leiden, Netherlads). (rhC1INH: Conestat alfa): 50 U/kg body weight up to 4200 U (adults).
Doses can be repeated as needed, usually after 1 hour.
Possible side effects: they are well tolerated. The main concern is the possible risk of transmission of infections given that they are plasma-derived, although no viral borne transmission has been reported to date.
Considerations: As the recombinant drug (Conestat alfa) is produced by recombinant DNA technology in the milk of transgenic rabbits, it could induce an allergic reaction in patients with rabbit allergy. Thus, specific IgE anti-rabbit epithelium determination is recommended prior to its use.
Bradykinin receptor antagonist (icatibant acetate). It is a synthetic decapeptide, a highly specific second-generation antagonist of the bradykinin B2 receptor (B2R), which inhibits the vasodilatation produced by bradykinin. Its effectiveness has been shown in clinical trials and in patients series (Citation78,Citation79). It has been demonstrated that early blockade, particularly within the first hour of attack onset, significantly reduces attack duration and time to attack resolution (Citation78).
Dosage: Firazyr® (Shire Orphan Therapies GmbH, Berlin, Germany) (icatibant acetate) 30 mg (prefilled 30 mg/3 mL syringes). It should be administered subcutaneously. If an adequate response does not occur, re-injection is indicated after 6 hours have elapsed. The administration of more than three doses within a 24-hours period or more than eight doses in 1 month is not recommended. It should not be used in patients with active ischemic heart disease or those who had an ischemic stroke in the preceding 2 weeks (Citation80). There is no information about its use in patients younger than 18 years or women who are pregnant or breastfeeding (Citation77).
Possible side effects: No serious adverse reactions have been reported. The only significant side effect is injection site reactions, such as erythema, edema, pruritus, and pain (Citation78,Citation79).
Plasma kallikrein inhibitor (ecallantide). Ecallantide is a plasma kallikrein inhibitor that is effective against attacks of HAE (Citation81). It is only approved by the FDA. It is not available in Europe (Citation77).
The recommended dose is: Kalbitor® (Dyax Crop. Cambridge, Mass, US) 30 mg (three vials of 10 mg/mL) injected subcutaneously in abdominal site.
Possible side effects: Hypersensitivity reactions have been reported (Citation82), including an episode of anaphylaxis (Citation83). For this reason, it is recommended to guard the patient during a few hours after the ecallantide administration.
Antifibrinolytic agents. These drugs can be effective in an angioedema attack, but they are only recommended when the previously described treatments are not available. There are no data based on controlled clinical trials. High intravenous or oral doses of tranexamic acid (Amchafibrin®, Rottapharm, Barcelona, Spain) have been used (Citation52,Citation77). This has only proven effective in prodromal phases of the attack (Citation84).
The recommended dosage for tranexamic acid is: Amchafibrin®: 15 mg/kg/4 hours i.v.
In the case that none of these drugs is available, fresh frozen plasma could be an alternative option (Citation85).
2.1.2. Acquired angioedema. The main point is to control the underlying disease. The treatment of acute attacks is the same as for HAE-C1INH, although the doses of pdhC1INH needed may be higher because of the presence of anti-C1INH autoantibodies. There is little experience with icatibant acetate, although this agent could be used in case of resistance to pdhC1INH (Citation86).
This type of angioedema does not respond to antihistamines, corticosteroids, or epinephrine.
2.2. Without C1-INH deficiency
2.2.1. Hereditary. The main therapeutic measure is prophylaxis avoiding estrogens (Citation51,Citation87). There is no consensus on treatment, but isolated cases or small series have been reported in which tranexamic acid (1–2 g/6 hours), pdhC1INH, and icatibant acetate have been used off-label with good results (Citation87).
2.2.2. Angioedema induced by angiotensin-converting enzyme inhibitors. The main therapeutic measure is to avoid antihypertensive drugs of the ACEi group (Citation88).
ARBs should not be systematically avoided in patients with ACEi-induced angioedema, although their use should be monitored (Citation51).
The mechanism underlying ACEi-induced angioedema (a bradykinin excess) is similar to that underlying HAE. For this reason, agents shown to be effective in HAE, including the bradykinin receptor antagonist icatibant, are currently being investigated in clinical studies as treatment for acute ACEi-induced angioedema. The effectiveness of icatibant acetate has been described in published series, although it has been used off-label (Citation89,Citation90). One case of successful tranexamic acid treatment has been published (Citation85).
In the case of angioedema presenting in patients taking DPP-IV inhibitors, the main therapeutic measure is to avoid the drug. Nevertheless, as the mechanism underlying DPP-IV-induced angioedema (a bradykinin excess) is similar to that underlying ACEi and HAE, agents that have been shown to be effective in these conditions, including the bradykinin receptor antagonist icatibant, could be useful in this type of angioedema (Citation44).
2.2.3. Idiopathic bradykinin-mediated angioedema. The idiopathic bradykinin group does not respond to antihistamine prophylaxis or treatment (Citation14) and can be related to an increase of bradykinin levels (Citation46). For this reason, it has to be treated similarly to that underlying ACEi and HAE, as is shown in .
3. Non-histamine- and non-bradykinin-mediated angioedema
3.1. NSAIDs (non-steroidal anti-inflammatory drugs)
NSAID hypersensitivity is thought to be related to cyclo- oxygenase-1 (COX-1) inhibition, and symptoms are the same as in a histamine allergic reaction, but commonly occur 1 hour after taking the drug. Therefore, the treatment in this group should be the same as in the histamine-mediated angioedema (Citation91).
Severity assessment and requirements of intensive care unit (ICU)
The main severe outcome in angioedema attacks is the upper airway compromise that can lead to life-threatening airway obstruction. In the case of anaphylaxis, hypotension and shock may also occur.
Therefore, patients with airway angioedema in stages III and IV of the Ishoo classification should be managed in the ICU (Citation58). Also patients with a severe anaphylaxis (with hypotension, hypoxia, or shock) unresponsive to epinephrine should be considered to be managed in the ICU (Citation92).
Duration of monitoring in the health care setting
After apparent resolution of symptoms, duration of monitoring in a medically supervised setting should be individualized. Patients with a peripheral angioedema without any other organ involved should be monitored in ED for 4 to 6 hours after the peak clinical expression of angioedema. Patients with mild reactions with no progression of the symptoms during the period of observation may be discharged (Citation93). If angioedema is associated with anaphylaxis and epinephrine is required, the patient should be monitored for at least 6–12 hours (Citation94). If the patient presented cardiovascular symptoms, 12–24 hours of monitoring might be required (Citation92). This is based on the concern for biphasic anaphylaxis.
In addition, at time of discharge they should be advised that, if possible, the suspected trigger(s) needs to be avoided, and referral to an allergy specialist is strongly recommended (Citation93,Citation95). If the angioedema was due to an allergic response, a prescription for three days of steroids and antihistamines should be provided. Patients treated with epinephrine should be prescribed an epinephrine autoinjector (Citation93,Citation96), mainly if the trigger cannot be easily avoided (hymenoptera venom, foods). There are many trademarks of epinephrine autoinjectors currently available (EpiPen®, Mylan Specialty, Basking Ridge, NJ, USA; Jext®, ALK, Copenhagen, Denmark; Anapen®, Lincoln Medical Ltd, Salisbury, Wiltshire, UK; Adrenaclick®, Amedra Pharmaceuticals, LCC, Horsham, PA, USA; Allerject®, Sanofi, Laval, Quebec, Canada; AuviQ®, Sanofi, Bridgewater, NJ, USA; Altellus®, Meda Pharma, San Fernando de Henares, Madrid, Spain). Available doses include a pediatric presentation, for patients weighing less than 30 kg, which contains 0.15 mL of epinephrine 1:1000 dilution (0.15 mg); and the adult formulation, recommended for patients weighing 30 kg or more, which contains 0.3 mL of epinephrine 1:1000 dilution (0.3 mg). It is important to train the patient regarding storage conditions and autoadministration (Citation95), according to manufacturer directions.
Conclusion
Angioedema management at the ED is a challenge for the physician in a setting where etiologic diagnostic tests are not available. The knowledge of different mechanisms underlying this condition is essential to provide the appropriate treatment to patients. While histaminergic angioedema responds to classical treatment with antihistamines, corticosteroids, and epinephrine, bradykinin-mediated angioedema does not resolve with these drugs, and new therapies are now available to treat this potentially life-threatening condition. The better knowledge of this pathology as well as the availability of these new therapies at the ED will improve the correct attention for these patients and their quality of life, avoiding unnecessary surgeries, the need of orotracheal intubation, and the frustration of the patients when their attacks are not efficiently aborted.
Notice of correction
In the original version of this article, published online ahead of print on 12 August 2014, one of the members of the SGBA was omitted from the contributor list. M. Piñero-Saavedra has now been added to the list in this version.
Acknowledgements
M.P., A.P.-G., and A.S.-C. contributed equally to the manuscript and are listed in alphabetical order.
Declaration of interest: Dr M. Pedrosa has received sponsorship for educational purposes and has taken part in clinical trials sponsored by Jerini AG/Shire, CSL-Behring, Pharming NV, and Viropharma.
Dr A. Prieto-García has received sponsorship for educational purposes, has been paid for consultancy services, and has taken part in clinical trials sponsored by Shire, CSL-Behring, and Viropharma.
Dr A. Sala-Cunill has received sponsorship for educational purposes, has been paid for consultancy services, and has taken part in clinical trials sponsored by Shire, CSL-Behring, Pharming NV, and Viropharma.
References
- WintersM. Clinical practice guideline: initial evaluation and management of patients presenting with acute urticaria or angioedema. American Academy of Emergency Medicine web site. 2006. Available at: http://www.aem.org/em-resources/position-statements/2006/clinical-practice-guidelines (accessed 6 February 2013).
- KaplanAP, GreavesMW. Angioedema. J Am Acad Dermatol. 2005;53:373–88.
- BasM, AdamsV, SuvoravaT, NiehuesT, HoffmannTK, KojdaG. Nonallergic angioedema: role of bradykinin. Allergy. 2007;62:842–56.
- WeldonD. Differential diagnosis of angioedema. Immunol Allergy Clin North Am. 2006;26:603–13.
- BernsteinJA, MoellmanJ. Emerging concepts in the diagnosis and treatment of patients with undifferentiated angioedema. Int J Emerg Med. 2012;5:39.
- StevensonDD. Aspirin and NSAID sensitivity. Immunol Allergy Clin North Am. 2004;24:491–505.
- CicardiM, AbererW, BanerjiA, BasM, BernsteinJA, BorkK, et al. Classification, diagnosis and approach to treatment in angioedema: Consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602–16.
- StoneKD, PrussinC, MetcalfeDD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80.
- ComminsSP, BorishL, SteinkeJW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S53–72.
- SchwartzL, MetcalfeD, MillerJ, EarlH, SullivanT. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–6.
- DurhamSR. Allergic inflammation: cellular aspects. Allergy. 1999;54(Suppl 5):18–20.
- GrigoriadouS, LonghurstHJ. An approach to the patient with angio-oedema. Clin Exp Immunol. 2009;155:367–77.
- TheoharidesTC, KempurajD, TagenM, ContiP, KalogeromitrosD. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78.
- CicardiM, BergamaschiniL, ZingaleLC, GioffréD, AgostoniA. Idiopathic nonhistaminergic angioedema. Am J Med. 1999;106:650–4.
- BjörkqvistJ, Sala-CunillA, RennéT. Hereditary angioedema: a bradykinin-mediated swelling disorder. Thromb Haemost. 2013;109: 368–74.
- KaplanAP, GhebrehiwetB. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47:2161–9.
- BlanchA, RocheO, López-GranadosE, FontánG, López-TrascasaM. Detection of C1 inhibitor (SERPING1/C1NH) mutations in exon 8 in patients with hereditary angioedema: evidence for 10 novel mutations. Hum Mutation. 2002;20:405–6.
- DavisAE. The pathophysiology of hereditary angioedema. Clin Immunol. 2005;114:3–9.
- NussbergerJ, CugnoM, CicardiM. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621–2.
- CastelliR, DeliliersD, ZingaleLC, PoglianiE, CicardiM. Lymphoproliferative disease and acquired C1 inhibitor deficiency. Haematologica. 2007;92:716–18.
- Barilla-LaBarcaM, GioffreD, ZanichelliA, CicardiM, AtkinsonJP. Acquired C1 esterase inhibitor deficiency in two patients presenting with a lupus-like syndrome and anticardiolipin antibodies. Arthritis Rheum. 2002;47:223–6.
- FarkasH, SzongothM, BélyM, VargaL, FeketeB, KarádiI, et al. Angiooedema due to acquired deficiency of C1-esterase inhibitor associated with leucocytoclastic vasculitis. Acta Derm Venereol. 2001;81:298–300.
- FarkasH, CsepregiA, NemesánszkyE. Acquired angioedema associated with chronic hepatitis C. J Allergy Clin Immunol. 1999;103:711–12.
- AlsenzJ, BorkK, LoosM. Autoantibody-mediated acquired deficiency of C1 inhibitor. N Engl J Med. 1987;316:1360–6.
- PonceIM, CaballeroT, RecheM, PiteiroAB, López-SerranoMC, FontánG, et al. Polyclonal autoantibodies against C1 inhibitor in a case of acquired angioedema. Ann Allergy Asthma Immunol. 2002; 88:632–7.
- MarcosC, López LeraA, VarelaS, LiñaresT, Alvarez-EireMG, Lopez-TrascasaM. Clinical, biochemical, and genetic characterization of type III hereditary angioedema in 13 Northwest Spanish families. Ann Allergy Asthma Immunol. 2012;109:195–200.e2.
- MartinL, Raison-PeyronN, NöthenMM, CichonS, DrouetC. Hereditary angioedema with normal C1 inhibitor gene in a family with affected women and men is associated with the p.Thr328Lys mutation in the F12 gene. J Allergy Clin Immunol. 2007;120:975–7.
- BouilletL, PonardD, RoussetH, CichonS, DrouetC. A case of hereditary angio-oedema type III presenting with C1-inhibitor cleavage and a missense mutation in the F12 gene. Br J Dermatol. 2007;156:1063–5.
- BorkK. Hereditary angioedema with normal C1 inhibitor activity including hereditary angioedema with coagulation factor XII gene mutations. Immunol Allergy Clin North Am. 2006;26:709–24.
- DewaldG, BorkK. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 2006;343:1286–9.
- Gómez-TraseiraC, López-LeraA, DrouetC, López-TrascasaM, Pérez-FernándezE, FavierB, et al. Hereditary angioedema caused by the p. Thr309-Lys mutation in the F12 gene: a multifactorial disease. J Allergy Clin Immunol. 2013;6:986–9.
- CichonS, MartinL, HenniesHC, MüllerF, Van DriesscheK, KarpushovaA, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79: 1098–104.
- DefendiF, CharignonD, GhannamA, BarosoR, CsopakiF, Allegret-CadetM, et al. Enzymatic assays for the diagnosis of bradykinin-dependent angioedema. PLoS One. 2013;8:e70140.
- BorkK, FischerB, DewaldG. Recurrent episodes of skin angioedema and severe attacks of abdominal pain induced by oral contraceptives or hormone replacement therapy. Am J Med. 2003;114:294–8.
- BouilletL, LonghurstH, Boccon-GibodI, BorkK, BucherC, BygumA, et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol. 2008;199:484.e1–4.
- CasasJP, ChuaW, LoukogeorgakisS, VallanceP, SmeethL, HingoraniAD, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–33.
- SheikhIA, KaplanAP. Mechanism of digestion of bradykinin and lysylbradykinin (kallidin) in human serum. Role of carboxipeptidase, angiotensin converting enzyme and determination of final degradation products. Biochem Pharmacol. 1989;38:993–1000.
- SlaterEE, MerrillDD, GuessHA, RoylancePJ, CooperWD, InmanWH, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–70.
- Sánchez-BorgesM, González-AveledoLA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2:195–8.
- MakaniH, MesserliFH, RomeroJ, Wever-PinzonO, KorniyenkoA, BerriosRS, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383–91.
- CaldeiraD, DavidC, SampaioC. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2012;12:263–77.
- ByrdJB, ShreevatsaA, PutlurP, ForetiaD, McAlexanderL, SinhaT, et al. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol. 2007;120:403–8.
- ByrdJB, TouzinK, SileS, GainerJV, YuC, NadeauJ, et al. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor–associated angioedema. Hypertension. 2008;51:141–7.
- BrownNJ, ByiersS, CarrD, MaldonadoM, AnnB. Dipeptidyl peptidase IV inhibitor use associated with increased risk of ACE inhibitor- associated angioedema. Hypertension. 2009;54:516–23.
- KaplanAP. Angioedema. World Allergy Organ J. 2008;1:103–13.
- CugnoM, NussbergerJ, CicardiM, AgostoniA. Bradykinin and the pathophysiology of angioedema. Int Immunopharmacol. 2003;3: 311–17.
- MontinaroV, LoizzoG, ZitoA, CastellanoG, GesualdoL. Successful treatment of a facial attack of angioedema with icatibant in a patient with idiopathic angioedema. Am J Emerg Med. 2013;31:1295.e5–6.
- Del CorsoI, PuxedduI, SardanoE, GeraciS, BreggiaM, RocchiV, et al. Treatment of idiopathic nonhistaminergic angioedema with bradykinin B2 receptor antagonist icatibant. Ann Allergy Asthma Immunol. 2012;108:460–1.
- JenneckC, JuergensU, BuechelerM, NovakN. Pathogenesis, diagnosis, and treatment of aspirin intolerance. Ann Allergy Asthma Immunol. 2007;99:13–21.
- CaballeroT, BaezaML, CabañasR, CamposA, CimbollekS, Gómez-TraseiraC, et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol. 2011;21: 333–47.
- CaballeroT, BaezaML, CabañasR, CamposA, CimbollekS, Gómez-TraseiraC, et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part II. Treatment, follow-up, and special situations. J Investig Allergol Clin Immunol. 2011;21:422–3.
- WilkersonRG. Angioedema in the emergency department: an evidence-based review. Emerg Med Pract. 2012;14:1–21.
- AgostoniA, CicardiM. Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine. 1992;71:206–15.
- AgostoniA, Aygören-PürsünE, BinkleyKE, BlanchA, BorkK, BouilletL, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114(3 Suppl): S51–131.
- CarreerFM. The C1 inhibitor deficiency. A review. Eur J Clin Chem Clin Biochem. 1992;30:793–807.
- FarkasH, HarmatG, FáyA, FeketeB, KarádiI, VisyB, et al. Erythema marginatum preceding an acute oedematous attack of hereditary angioneurotic oedema. Acta Derm Venereol. 2001;81:376–7.
- BentsianovBL, ParhiscarA, AzerM, Har-ElG. The role of fiberoptic nasopharyngoscopy in the management of the acute airway in angioneurotic edema. Laryngoscope. 2000;110:2016–19.
- IshooE, ShahUK, GrilloneGA, StramJR, FuleihanNS. Predicting airway risk in angioedema: staging system based on presentation. Otolaryngol Head Neck Surg. 1999;121:263–8.
- NzeakoUC. Diagnosis and management of angioedema with abdominal involvement: a gastroenterology perspective. World J Gastroenterol. 2010;16:4913.
- CohenN, SharonA, GolikA, ZaidensteinR, ModaiD. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol. 1993;16:237–9.
- BorkK.[Hypovolemic shock caused by ascites in hereditary angioedema]. Med Klin (Munich). 1998;93:554.
- SabroeR, BlackA. Angiotensin-converting enzyme (ACE) inhibitors and angio-oedema. Br J Dermatol. 1997;136:153–8.
- TosiM. Molecular genetics of C1 inhibitor. Immunobiology. 1998;199:358–65.
- PappalardoE, CicardiM, DuponchelC, CarugatiA, ChoquetS, AgostoniA, et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol. 2000;106:1147–54.
- PedrosaM, CaballeroT, Gómez-TraseiraC, OlveiraA, López- SerranoMC, López-SerranoC. Usefulness of abdominal ultrasonography in the follow-up of patients with hereditary C1-inhibitor deficiency. Ann Allergy Asthma Immunol. 2009;102:483–6.
- SadeghiN, Van DaeleD, HainauxB, EngelholmL, MichelO. Hereditary angio-edema involving the gastrointestinal tract: CT findings. Eur Radiol. 2001;11:99–101.
- SchwartzLB, YungingerJW, MillerJ, BokhariR, DullD. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551–5.
- Papadopoulou-AlatakiE. Upper airway considerations in hereditary angioedema. Curr Opin Allergy Clin Immunol. 2010;10:20–5.
- FrigasE, ParkMA. Acute urticaria and angioedema: diagnostic and treatment considerations. Am J Clin Dermatol. 2009;10:239–50.
- SimonsFER, ArdussoLRF, BilòMB, DimovV, EbisawaM, El-GamalYM, et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12:389–99.
- Sánchez-BorgesM, AseroR, AnsoteguiIJ, BaiardiniI, BernsteinJA, CanonicaGW, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5:125–47.
- ZuberbierT, AseroR, Bindslev-JensenC, Walter CanonicaG, ChurchMK, Giménez-ArnauAM, et al. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–43.
- ChooK, SimonsFE, SheikhA. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev. 2012;4:CD007596.
- RoweB, SpoonerC, DucharmeF, BretzlaffJ, BotaG. Early emergency department treatment of acute asthma with systemic corticosteroids (Review). Cochrane Database Syst Rev. 2001;(1):CD002178.
- SimonsFER. Anaphylaxis: recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124:625–36.
- SimonsFER, ArdussoLRF, BilòMB, El-GamalYM, LedfordDK, RingJ, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127:587–93.e1–22.
- CicardiM, BorkK, CaballeroT, CraigT, LiHH, LonghurstH, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67:147–57.
- MaurerM, AbererW, BouilletL, CaballeroT, FabienV, KannyG, et al. Hereditary angioedema attacks resolve faster and are shorter after early icatibant treatment. PLoS One. 2013;8:e53773.
- CicardiM, BanerjiA, BrachoF, MalbránA, RosenkranzB, RiedlM, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363:532–41.
- European Public Assessment Report (EMEA 925383/2011) for Firazyr (Icatibant). 2011. Available at: http://www.ema.europa.eu/ema (accessed 18 August 2013).
- CicardiM, LevyR, McNeilD, LiHH, ShefferAL, CampionM, et al. Ecallantide for treatment of acute attacks of hereditary angioedema. N Engl J Med. 2010;363:523–31.
- LumryWR, BernsteinJA, LiHH, MacGinnitieAJ, RiedlM, SoteresDF, et al. Efficacy and safety of ecallantide in treatment of recurrent attacks of hereditary angioedema: open-label continuation study. Allergy Asthma Proc. 2013;34:155–61.
- CaballeroT, López-SerranoMC. Anaphylactic reaction and antibodies to DX-88 (kallikrein inhibitor) in a patient with hereditary angioedema. J Allergy Clin Immunol. 2006;117:476–7.
- ShefferAL, AustenKF, RosenFS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med. 1972;287:452–4.
- PremattaM, GibbsJG, PrattEL, StoughtonTR, CraigTJ. Fresh frozen plasma for the treatment of hereditary angioedema. Ann allergy Asthma Immunol. 2007;98:383–8.
- CastelliR, ZanichelliA, CicardiM, CugnoM. Acquired C1-inhibitor deficiency and lymphoproliferative disorders: a tight relationship. Crit Rev Oncol Hematol. 2013;87:323–32.
- BorkK. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol. 2010;6:15.
- FlatteryMP, SicaDA. Angiotensin-converting enzyme inhibitor-related angioedema: recognition and treatment. Prog Cardiovasc Nurs. 2007;22:47–51.
- BasM, GreveJ, StelterK, BierH, StarkT, HoffmannTK, et al. Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme inhibitors: a case series. Ann Emerg Med. 2010; 56:278–82.
- BasM, KojdaG, StelterK. [Angiotensin-converting enzyme inhibitor induced angioedema: new therapy options]. Anaesthesist. 2011; 60:1141–5.
- DoñaI, Blanca-LópezN, Cornejo-GarcíaJA, TorresMJ, LagunaJJ, FernándezJ, et al. Characteristics of subjects experiencing hypersensitivity to non-steroidal anti-inflammatory drugs: patterns of response. Clin Exp Allergy. 2011;41:86–95.
- Working Group of Resucitation Council (UK). Resuscitation Council (UK) Emergency treatment of anaphylactic reactions. Guidelines for healthcare providers. 2013;(286360).
- Cardona DahlV.[Guideline for the management of anaphylaxis]. Med Clin (Barc). 2011;136:349–55.
- UnsworthDJ. Following up patients after treatment for anaphylaxis. Practitioner. 2012;256:21–4, 3.
- CampbellRL, LukeA, WeaverAL, St SauverJL, BergstralhEJ, LiJT, et al. Prescriptions for self-injectable epinephrine and follow-up referral in emergency department patients presenting with anaphylaxis. Ann Allergy Asthma Immunol. 2008;101:631–6.
- SheikhA, SimonsF, BarbourV, WorthA. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev. 2012;(8): CD008935.