Abstract
Background. The cardiovascular safety of many glucagon-like peptide-1 agents (GLP-1 agents) is unclear. In this study, we assess the effects of the GLP-1 agents on left ventricular function in patients with type 2 diabetes (T2DM) and/or cardiovascular disease (CVD).
Methods. PubMed, EMBASE, and the Cochrane Library were searched for the relevant publications up to May 2013 without restriction by language. All clinical controlled trials assessing left ventricular function and cardiovascular outcomes with the GLP-1 agents were selected for eligibility. Fourteen trials (415 patients) were identified as eligible between 1966 and 2013. Twelve of the studies were randomized controlled trials (RCT).
Results. The results showed that GLP-1 agent treatment in patients with T2DM and/or CVD led to significantly improved regional left ventricular contractile parameters (including peak left systolic tissue velocity and strain) and global left ventricular performance (including stroke volume, ejection fraction, and left ventricular chambers) compared with patients receiving placebo.
Conclusions. GLP-1 agent treatment in T2DM and/or CVD patients is associated with a modest but significant increase in the odds of left ventricular contractile parameters and left ventricular performance compared with patients having received placebo, which may be indicative of additional cardiovascular benefits for these patients.
Key messages
In addition to glycemic control and a low risk of hypoglycemia, GLP-1 may favorably affect several cardiovascular risk factors.
Among patients with T2DM and/or CVD, use of GLP-1 agents is associated with a modest but statistically significant increase in the odds of left ventricular contractile parameters and left ventricular performance.
Introduction
The worldwide epidemic of type 2 diabetes mellitus (T2DM) has brought increasing attention to its common complications such as cardiovascular diseases (CVD). The risk of CVD and heart failure is increased 2–5-fold in patients with T2DM compared with patients without diabetes mellitus (Citation1). Therefore, the medications used in diabetes should not only focus on glucose-lowering strategies, but also on cardioprotection. Glucagon-like peptide-1 agents (GLP-1 agents), including analogues, receptor agonists (RA), and dipeptidyl peptidase-4 (DPP-4) inhibitors, are currently emerging as new antidiabetic agents for the glucose-dependent insulin secretion and cytoprotective actions in islet β-cells. GLP-1 has also been proven to suppress inappropriately elevated postprandial glucagon secretion, slow gastric emptying, and reduce food intake (Citation2,Citation3). In addition to glycemic control and a low risk of hypoglycemia, GLP-1 may favorably affect several cardiovascular (CV) risk factors, such as blood pressure, lipid profiles, and body weight (Citation4–8). Furthermore, preliminary data in humans suggest that GLP-1 could have direct effects on improving myocardial function (Citation9). Nonetheless, specific effects of GLP-1 agents on cardiovascular risks remain unclear. In fact, to date, no meta-analysis assessing the effects of GLP-1 agents on left ventricular function in patients with CVD and T2DM is available. Therefore, our objective was to assess systematically the effects of GLP-1 agents on cardiovascular outcomes and left ventricular function, in patients with T2DM and CVD.
Materials and methods
Data sources and searches
We conducted a systematic literature search of PubMed (1966 to May 2013), EMBASE (1974 to May 2013), and the Cochrane Library 2013 for studies reporting on cardiovascular effects of GLP-1 agents. In addition, we also searched the reference lists of the relevant publications, reviewed scientific meeting abstracts (the American Diabetes Association, the Society for Research on GLP-1 agents, and other major diabetes and endocrinology or major cardiology and cardiothoracic surgery scientific meetings) from 2003 to 2013. Other completed but still unpublished trials were identified and retrieved from the www.clinicaltrials.gov, www.novonordisk-trials.com, and www.clinicalstudyresults.org. Reference lists of all relevant studies were reviewed irrespective of language, and attempts were made to correspond with authors of relevant trials. For these electronic searches, we employed versions of Medical Subject Headings (MeSH) and main key words (glucagon-like peptide-1, exenatide, liraglutide, exendin-4, DPP-4 inhibitor, and cardioprotective, cardioprotection, myocardial, heart, cardiovascular, cardiac or cardio).
Study selection
The selections of relevant abstracts and publications were performed by two of the authors (R.L. and M.Y.) independently, based on the criteria described below, and were confirmed by a third investigator. Clinical trials were included if they met the following criteria: 1) randomized controlled trials (RCT) or un-randomized controlled trials using either cross-over or parallel designs, conducted in humans, and published in any language; 2) subjects treated with GLP-1 agents; 3) regular baseline intervention was performed equally; 4) full-text articles of controlled trials assessing GLP-1 effects on cardiovascular system; 5) end-points including hemodynamic parameters, regional and global left ventricular function, heart rates, blood pressure, stroke volume, side effects; and 6) randomized trials were included. Excluded were studies that assessed the correlation between endogenous GLP-1 levels and cardiovascular effects, studies that did not report the outcomes of interest, or for which it was impossible to assess the outcomes from the published results, or studies that did not have controls.
Data extraction and quality assessment
The primary clinical outcome of interest was the effect of GLP-1 agents on regional and global left ventricular function. Secondary outcomes were hemodynamic parameters including heart rate, blood pressure, and systemic vascular resistance. Furthermore, in data on 6-minute walk test, side effects were also evaluated. Two reviewers extracted data from each study independently, including study title, first author, publication year, institution, population demography, study design, follow-up period, inclusion and exclusion criteria, and main outcomes. Duplicate reports were merged. If outcomes from the same patients were published in multiple articles with different follow-up periods, we extracted the outcomes from the first study and the follow-up studies of the patients. When studies from the same institution reported the same outcomes at similar follow-up periods, either the better quality or the most informative reports were selected. The quality of the included randomized clinical trials was assessed by three categories ranging from A (high quality) to C (low quality) (Citation10).
These categories included the randomization procedure, the use of intention-to-treat analysis, drop-out rate, allocation concealment, and the extent to which valid outcomes were described. Any disagreement regarding study quality was resolved by discussion among the authors.
Statistical analysis
This meta-analysis was carried out according to the Cochrane Collaboration recommendations guidelines (Citation11). For categorical variables, the relative risk (RR) was employed, which compares the event ratio between the treatment group (GLP-1 agents) and the control group (placebo). A RR of less than 1 was in favor of the treatment group. If the event was adverse, statistical significance was assumed at the P < 0.05 level. Continuous data were expressed as the weighted mean difference (WMD), and an overall WMD was calculated. The actual measures of effect of all continuous variables were the differences between the study and control arms. A fixed effect model was chosen on the presumption that variation in the individual trial results occurred around a true mean. Otherwise, the random model was adopted. The heterogeneity of effects was calculated using chi-square and I2; an I2 of more than 50% was considered to indicate heterogeneity, then the random model was adopted. All statistical analyses were carried out with Stata 10 (StataCorp, College Station, TX, USA). We examined each study for potential selection bias and detection bias. In order to verify possible bias associated with inadequate allocation or randomization, the quality of studies was evaluated. A funnel plot of primary outcomes or important secondary outcomes was examined to assess potential publication bias. In addition, the association between variance and effect size was analyzed by the Begg adjusted rank correlation test. Sensitivity analyses were conducted to estimate the strength of outcomes and to explore the influence of trial design and methods on the effect size.
Results
Trial flow and characteristics
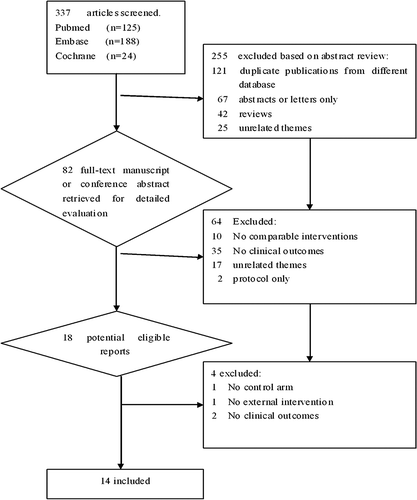
We identified a total of 81 relevant articles assessing safety and the cardiovascular effects of the GLP-1 agents in humans. A total of 18 clinical trials (Citation5,Citation9,Citation12–27) met our inclusion criteria. Four of these (Citation14,Citation17,Citation18,Citation26) were excluded from this analysis for the predetermined reasons: one for being without a control group, one for evaluating the correlation between GLP-1 agents and postprandial cardiovascular parameters alone, another two for missing out relative outcomes. Hence, the results from 14 trials including 415 participants (published between 1966 and 2013) were reviewed (). The processes of trial retrieval and selection are summarized in . Finally, predominantly 12 RCTs and two case control studies (Citation9,Citation12) were included for further extractions and analysis (). The age of trial participants ranged from 34 to 66 years. The majority of studies were carried out with GLP-1 agents for a short term (within 72 hours) except for two trials for 3–4 months. To reduce heterogeneity in the analysis resulting from the various durations of treatment and the wide range of follow-ups, a comparison of subgroups with respect to treatment and follow-up duration was performed. The most common adverse effects were gastrointestinal, such as nausea, diarrhea, vomiting, and asymptomatic hypoglycemia primarily in non-diabetic subjects.
Table I. Characteristics of 14 clinical controlled trials evaluating the cardiovascular effects of GLP-1 administration.
The overall quality was assessed on a three-point scale according to the Cochrane handbook: the majority of included articles were scored as B (moderate quality); three studies scored as A (high quality) ().
Table II. Quality assessment of randomized clinical studies inclusion.
Hemodynamic parameters
Heart rate
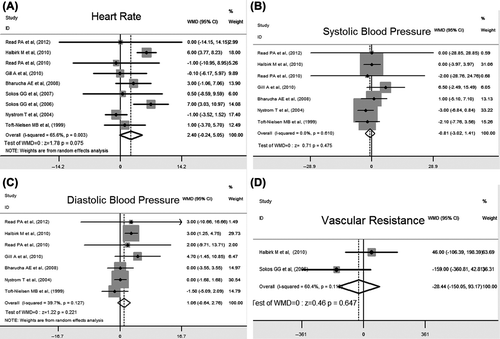
In this meta-analysis, a total of nine studies provided heart rate data, in which the duration of GLP-1 agent treatment ranged widely from 30 minutes to 12 weeks. The overall pooled data did not show a statistically significant effect on heart rates in GLP-1 agent-treated cases (WMD 2.40, 95% CI: – 0.24 to 5.05, P = 0.075) ().
Blood pressure
We included seven trials measuring the changes of blood pressure. In these trials, blood pressure was represented as systolic (SBP) and diastolic blood pressure (DBP), or mean blood pressure (MBP). However, pooling of results showed that the changes of both SBP and DBP failed to reach statistical significance by GLP-1 agent treatment (WMD – 0.81, 95% CI: – 0.32 to 1.41, P = 0.475 and WMD 1.06, 95% CI: – 0.64 to 2.76, P = 0.221). No statistical heterogeneity was observed ( and ). In addition, two studies observing MBP and its change also failed to reach statistical significance.
Systemic vascular resistance
It was remarkable that systemic vascular resistance was reported as the outcome of GLP-1 agent treatment in many trials. Calculated systemic vascular resistance was reduced significantly after GLP-1 agent treatment for five weeks (Citation9). However, GLP-1 agent infusion for 48 hours did not affect systemic vascular resistance in one of the studies (Citation13). The pooled data did not show a statistically significant effect on systemic vascular resistance in GLP-1 agent-treated cases, but heterogeneity was significant (I2 = 60.4%). However, due to a small size, it was impossible to do a post hoc analysis ().
Left ventricular performance
Of the 14 studies, eight provided data on ejection fraction (EF) responding to GLP-1 agent treatment, with some assessing time (from immediately to 3 months), and some assessing cardiac function (from normal to EF less than 40%). Compared to the control arm, patients treated with GLP-1 agents had a better effect for improved left ventricular performance. As heterogeneity was significant (I2 = 90.3%), the conclusion was obtained by a random-effect model showing a combined WMD of 4.88 (95% CI 1.25 to 8.50, P = 0.008) (). To investigate the cause of heterogeneity in pooling results, subgroup analyses, including various treatment durations, follow-up periods, cardiac function, and revascularization states, were further performed. However, similar results were obtained from subgroup analysis by various treatment durations (from 30 min to 4 weeks). Subgroup analyses revealed that short-term GLP-1 treatment could also improve left ventricular performance with elevated EF (WMD 3.07, 95% CI: 0.44 to 5.7, P = 0.022), whereas longer treatment duration had larger WMD (5.21) compared with short-term treatment (WMD 3.07, P < 0.001) ().
Figure 3. Forest plot showing results from meta-analysis of trials reporting global left ventricular performance.
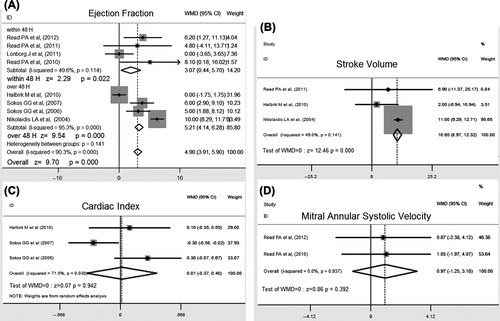
Stroke volume (SV)
To confirm further the effect of GLP-1 on left ventricular performance, SV was also analyzed in our meta-analysis. Compared with controls, treatment of GLP-1 agents was associated with significantly increased odds of SV from three studies (WMD 10.65, 95% CI: 8.97 to 12.32, P = 0.0001), showing a significant advantage for GLP-1 agent therapy with respect to LV performance ().
Cardiac index
Based on the pooled results of three studies, there were no statistically significant effects of GLP-1 agent treatment on cardiac index (WMD 0.01, 95% CI: – 0.37 to 0.40, P = 0.942) (), with statistical heterogeneity (I2 = 71.5%). One study (Citation25) reported outcomes achieved in a different manner, i.e. the control group had 2-fold to 4-fold greater requirements for intravenous agents, including inotropic agents, vasopressors, and vasodilators, compared with the GLP-1 agent-treated group. Hence, to reduce heterogeneity in the analyses, a comparison of subgroups without this study (Citation25) was performed, and it showed the same result without any heterogeneity (P = 0.382).
Mitral annular systolic velocity
The mitral annular systolic velocity, an index for assessment of global LV function, was reported in two studies. The pooled data from these two studies showed that GLP-1 infusion had no significant alteration in mitral annular systolic velocity ().
Left ventricular contractile capacity
Peak left systolic tissue velocity
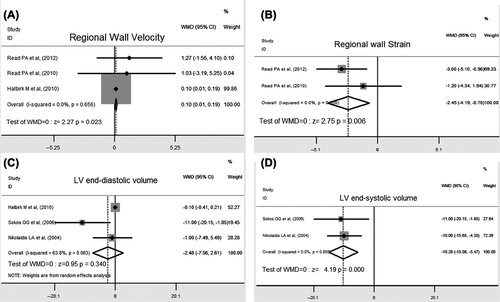
Myocardial Doppler velocities are correlated with systolic shortening as measured by sonomicrometry and more specific for myocardial properties. Our pooled data from three trials showed that GLP-1 agents significantly increased peak left systolic tissue velocity compared with control (WMD 0.10, 95% CI: 0.01 to 0.19, P = 0.023), indicating improved myocardial performance in the areas of the left ventricle. There was no evidence of substantial heterogeneity among the included trials (I2 = 0%) ().
Peak left systolic tissue strain
Myocardial Doppler strain is a good local measure of contractility, excluding the effects of tethering from adjacent segments and translational movement in tissue velocity. Compared with the controls, GLP-1 agents significantly improved regional contractile property, as indicated by strain (WMD – 2.45, 95% CI: – 4.19 to – 0.70, P = 0.006), without heterogeneity ().
Left ventricular chamber volume
Clinically, it is important that left ventricular function was assessed by left ventricular end-diastolic volume (EDV) and end-systolic volume (ESV). Pooled results of three studies showed that GLP-1 agent infusion significantly decreased ESV (WMD – 10.28, 95% CI: – 15.08 to – 5.47, P < 0.01) (), and moderately decreased EDV without statistical significance. In addition, there was no heterogeneity for this pooled result ().
Clinical manifestation
Six-minute walk test
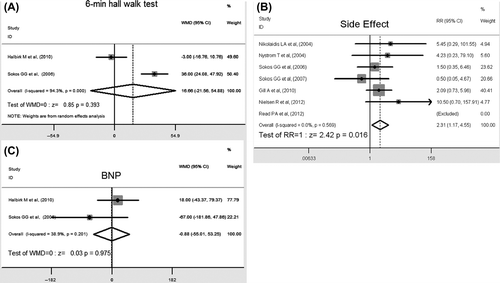
As shown in , the 6-minute walk distance reported in two studies was not increased in patients treated with GLP-1s, which is not in accordance with an elevation in EF (WMD 6.66, 95% CI: – 21.56 to 54.88, P = 0.393).
Side effects
Data of diverse adverse effects were available for quantitative evaluation in seven clinical trials. The most common adverse effects were gastrointestinal, such as nausea, diarrhea, vomiting, and asymptomatic hypoglycemia primarily in non-diabetic subjects (RR 2.31, 95% CI: 1.17 to 4.55, P = 0.016) () and (OR = 2.82, 95% CI: 1.25 to 6.66, P = 0.013). However, life-threatening adverse events were rarely reported.
B-type natriuretic peptide (BNP)
It was assumed that the improvement in LV hemodynamics was associated with a decreased BNP in the GLP-1 agent-treated group. While the given treatment duration and BNP values varied widely in the included studies, there was no change in BNP levels among groups (WMD –0.88, 95% CI: –55.01 to 53.65, P = 0.975), suggesting that GLP-1 agent treatment failed to affect circulating BNP ().
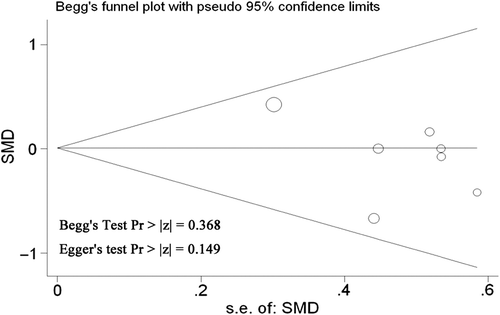
Publication bias and sensitivity analysis
Both graphical and statistical bias was performed by Begg's rank correlation analysis of funnel plot for examining publication bias. Begg's linear regression test was performed for the quantitative evaluation of the symmetry of the meta-analysis funnel plot. P values of Begg's test were 1.0, and their 95% CI of intercept included zero in Begg's publication bias plots. The result indicated that the meta-analysis funnel plots were symmetrical without publication bias. Sensitivity analysis was performed by using different sample size or effect models in the present study. We did not identify any marked difference in the RR and heterogeneity for the outcome of interest using both random and fixed effect models ().
Discussion
GLP-1, secreted from intestinal endocrine L cells in response to nutrients, exerts incretin-like actions stimulating insulin secretion, which may be associated with cytoprotective actions of GLP-1 when interacting with its receptor (GLP-IR) on β-pancreatic cells. However, the reduction of cardiovascular morbidity and mortality is one of the main aims of long-term treatment of hyperglycemia in T2DM. Therefore, the possibility of an increased cardiovascular risk associated with some hypoglycemic treatments is somewhat paradoxical. In order to determine cardiovascular safety of any drug, large-scale, long-term trials should be performed prior to marketing; unfortunately, this effort is economically prohibitive for pharmaceutical companies. As more studies concentrating on the clinical efficacy of GLP-1 agents are completed, the advantages and cardioprotective benefits of these drugs make them more marketable.
Extrapolating from preclinical studies, it is possible that GLP-1 receptors located in the human heart and vasculature may play a protective role with respect to CV disease (Citation28–30). In animal models, GLP-1 reduced infarct size after coronary artery ischemia (Citation31), improved left ventricular ejection volume in heart failure (Citation29), improved glucose uptake in the myocardium, and induced nitric oxide-independent vasorelaxation in the endothelium (Citation31). However, the possibility of any beneficial action of GLP-1 on CV events has not been confirmed by specifically designed randomized clinical trials. To date, in meta-analyses of GLP-1, CV effects recorded as adverse events in randomized clinical trials designed for other purposes were represented only as an additional source of information. However, in this meta-analysis, we analyzed the CV effects of GLP-1 agents from both clinical trials designed for CV benefits and for other purposes.
For most GLP-1 trials, heart rate and blood pressure are reported inconsistently (Citation13,Citation26,Citation32). The difference in heart rate and blood pressure between trials may be related to the composition of the study population, the different study protocol and end-point, the type of GLP agents, treatment duration, and follow-up duration. Therefore, it is currently unclear whether or not GLP-1 agents affect heart rate and blood pressure. However, the overall pooled data in our meta-analysis did not show a statistically significant effect on heart rate, blood pressure, or systematic vascular resistance in GLP-1 agent-treated cases, suggesting that hemodynamic parameters were not affected by GLP-1 agent treatment.
Recently, when the current work had been completed, a meta-analysis assessed the effects of GLP-1 RA on major CV events (Citation33). The results of this study showed that GLP-1 RA treatment, but not DPP-4 inhibitor, was associated with a significant reduction in major CV events compared with placebo and pioglitazone. However, unlike us, they did not analyze the effects of GLP-1 agents on left ventricular function, and in that study T2DM patients were analyzed alone.
To evaluate further whether GLP-1 agents have cardioprotective effects, we analyzed the changes of left ventricular contractile capacity and performance in GLP-1 agent-treated subjects including T2DM, T2DM with CVD, CVD, or controls. The pooled results of this meta-analysis demonstrated that GLP-1 agent treatment increased regional wall contractility assessed by velocity and strain particularly in the ischemic area under stress. GLP-1 agent treatment also led to elevated EF and SV, and decreased left ventricular chambers.
Although this study demonstrated a beneficial effect of GLP-1 agent on cardiac function, this effect is modest. Recently, the SAVOR-TIMI study (Citation34) has shown that saxagliptin, a DPP-4 inhibitor, did not decrease the rate of ischemic events, whereas it increased the rate of hospitalization for heart failure. The reason for this difference is not clear but it may be related to: 1) the difference in study selection (for example, studies by Scirica et al. focused on saxagliptin, whereas we included all GLP-1 agents); 2) the difference in the study population; the study by Scirica et al. included T2DM patients with one or more risk factors, such as dyslipidemia, hypertension, or active smoking. In our study, selected populations included healthy subjects, coronary artery disease (CAD) patients, and T2DM patients with or without CAD. Because of these discrepancies, further studies are needed to confirm the role of GLP-1 agents in cardioprotection. Importantly, several limitations need to be considered in the current study. First, other unpublished literature on relevant pharmaceutical websites was not searched, which may have led to potential publication bias and selection bias. Second, some trials included in this review were not specifically designed to evaluate cardiovascular outcomes. Some of the studies used low dose and short duration of GLP-1 treatment with small patient numbers, and they reported few cardiovascular events. Hence they failed to cover all clinically important CVD issues. Lastly, we did not investigate the distribution of clinical and methodological variables in detail that might be potential sources of either heterogeneity or inconsistency in every comparison-specific group of trials, although our pooled studies used the random effect model and only showed slight inconsistencies. Therefore, application of our results should take into account any limitations of the analysis and the specific clinical situations. In addition, it should also be noted that diastolic blood pressure has an increasing trend for GLP-1 agent treatment (P = 0.221) which together with a decrease in end-diastolic volume may suggest detrimental effects on diastolic function. Importantly, we found that when the duration of GLP-1 agent treatment is longer than two days, its effect on left ventricular function is visible. Therefore, it is possible that a longer treatment period is required to achieve any effect of GLP-1 agents. More long-term and large-scale randomized trials should be considered to confirm the role of GLP-1 agents in cardioprotection.
In conclusion, our results show that among patients with T2DM and/or CVD, use of GLP-1 agents is associated with a modest but statistically significant increase in left ventricular contractility and left ventricular performance compared with patients receiving other diabetes agents or placebo, which may provide additional cardiovascular benefits for these patients, possibly due to a mechanism independent of body weight reduction. However, a double-blinded random controlled study is needed.
Acknowledgements
R.L. and L.L. contributed equally to this work.
Declaration of interest: The authors report no conflicts of interest.
This work was supported by research grants from the National Natural Science Foundation of China (81270913, 81070640, 81100567, 81300702, 81100567, and 81300670), Doctoral Fund of Ministry of Education of China (20105503110002, 20125503110003), and Natural Science Foundation Key Project of CQ cstc (cstc2012 jjB10022).
References
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40.
- Wang Y, Ling L, Mengliu Y, Liu H, Boden G, Gang Y. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) versus insulin in inadequately controlled patients with type 2 diabetes mellitus: a meta-analysis of clinical trials. Diabetes Obes Metab. 2011;13:972–81.
- Ling L, Zhongyu M, Rui L, Muengliu Y, Hua L, Gangyi Y. Liraglutide prevents hypoadiponectinemia-induced insulin resistance and alterations of gene expression involved in glucose and lipid metabolism. Mol Med. 2011;17:1168–78.
- Bojanowska E, Stempniak B. Effects of glucagon-like peptide-1 (7-36) amide on neurohypophysial hormone secretion induced by acute hyperosmotic challenge. Neuropeptides. 2003;37:45–50.
- Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, et al. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R874–80.
- Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–47.
- Ratner RE, Maggs D, Nielsen LL, Stonehouse AH, Poon T, Zhang B, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419–28.
- Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86.
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Library I. Wiley; 2011 ISBN: 0470699515.
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5.
- Halbirk M, Nørrelund H, Møller N, Holst JJ, Schmitz O, Nielsen R, et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1096–102.
- Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34:90–5.
- Gejl M, Søndergaard HM, Stecher C, Bibby BM, Møller N, Bøtker HE, et al. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1165–9.
- Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6.
- Gitt AK, Bramlage P, Binz C, Krekler M, Deeg E, Tschope D. Comorbidity hypoglycaemia and appropriate selection of antidiabetic pharmacotherapy in diabetic patients with heart failure in clinical practice in Germany. Results of the DiaRegis registry. Herz. 2012;37:294–300.
- Hlebowicz J, Lindstedt S, Bjorgell O, Dencker M. The effect of endogenously released glucose, insulin, glucagon-like peptide 1, ghrelin on cardiac output, heart rate, stroke volume, and blood pressure. Cardiovasc Ultrasound. 2011;9:43.
- Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–9.
- Nielsen R, Wiggers H, Halbirk M, Bøtker H, Holst JJ, Schmitz O, et al. Metabolic effects of short-term GLP-1 treatment in insulin resistant heart failure patients. Exp Clin Endocrinol Diabetes. 2012;120:266–72.
- Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–15.
- Read PA, Hoole SP, White PA, Khan FZ, O’Sullivan M, West NE, et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4:266–72.
- Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2012;98:408–13.
- Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201.
- Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ Jr, Maher TD, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–9.
- Thrainsdottir I, Malmberg K, Olsson A, Gutniak M, Ryden L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–3.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22: 1137–43.
- Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–24.
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50.
- Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83.
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51.
- Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15:737–49.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:38–47.
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.