Abstract
Introduction. The higher incidence of gastrointestinal (GI) bleeding with the non-vitamin K oral anticoagulants (NOACs) may be related to pre-existing malignancies; diagnostic measures triggered by these bleedings could lead to early detection of these malignancies.
Methods. We retrieved the preferred terms on GI bleeding and GI cancer reported as adverse events (AEs) from phase III studies in patients with atrial fibrillation for each NOAC on ClinicalTrials.gov. We also analyzed the RE-LY trial database.
Results. From ClinicalTrials.gov, AE-GI bleeding incidence was: dabigatran 110 mg b.i.d. (D110: 1.42% versus 1.37%), dabigatran 150 mg b.i.d. (D150: 1.93% versus 1.37%), rivaroxaban (3.52% versus 2.68%), and apixaban (1.93% versus 1.59%), compared with warfarin, respectively. The incidence of AE-GI cancer was similar between the NOACs (D110 [0.79%], D150 [0.61%], rivaroxaban [0.83%], and apixaban [0.69%]), but numerically higher compared with warfarin (0.37%; 0.73%; 0.57%, respectively). In the RE-LY database, the same pattern was seen for dabigatran, with an association between GI bleeding and GI cancer diagnosis.
Conclusion. Anticoagulant-related GI bleeding may represent the unmasking of pre-existing malignancies leading to increased detection of GI cancer. This may be especially in the first month of treatment and could explain the numerically higher numbers of GI malignancies observed with NOACs.
Key messages
In patients receiving oral antithrombotic agents (e.g. vitamin K antagonists [VKAs] or antiplatelet agents), the ‘anticoagulation gastrointestinal (GI) stress-test’ triggers GI bleeding. This is also true when the non-VKA oral anticoagulants (NOACs) are used. The ‘anticoagulation GI stress-test’ may increase the sensitivity of screening tests for identification of early-stage GI cancers.
This is supported by data from clinical trials as well as adverse event reporting data published in ClinicalTrials.gov.
A screening test for occult blood in stools and/or hemoglobin values, preferably within 2 to 4 weeks of initiating treatment with NOACs, might allow for early detection of GI pathologies, with the potential for appropriate and more successful treatment, especially if the cause of bleeding is a malignancy. However, neither approach has been scientifically evaluated in clinical studies.
Introduction
Gastrointestinal (GI) bleeding is a common adverse event associated with conventional anticoagulants such as the vitamin K antagonists (VKA) (e.g. warfarin) (Citation1–3). Most such bleeding events are reported to occur within the first year of therapy (Citation4,Citation5). Patients who experienced a GI bleeding with warfarin treatment were more likely to be diagnosed with a pre-existing GI cancer (Citation6–9).
Based on clinical trial results, the incidence of GI major bleeding is reported to be higher with some of the non-VKA oral anticoagulants (NOACs, previously referred to as new or novel oral anticoagulants) such as rivaroxaban and dabigatran, when compared with warfarin (Citation10,Citation11). But it is of note that the higher incidence of GI bleeding was ‘compensated’ by a constantly lower rate of intracranial bleeding episodes (Citation12–14). This difference is of clinical significance as the fatality index is relatively low for GI bleeding compared with intracranial hemorrhage (ICH); the reported mortality rate for ICH is 38% (Citation15) in an observational study and 36% in the RE-LY study (Citation16), and the mortality rate is 6% for GI bleeding (Citation15).
However, GI bleeding could be a surrogate for GI lesions (e.g. neoplasia). In an animal model, apixaban and dabigatran significantly increased GI bleeding in Apcmin/+ mice (which are prone to develop multiple adenomas through the entire intestinal tract) but not in wild-type mice, highlighting an underlying predisposition to be the ‘trigger’ for GI bleeding (Citation17). The GI cancer incidence from the phase III trials of the NOACs has not yet been formally published, but data are available as adverse event (AE) results from ClinicalTrials.gov.
We hypothesized that the majority of early GI bleeding with NOACs would be related to a pre-existing malignancy, and that the intense diagnostic measures triggered by the GI bleeding (e.g. endoscopies) may lead to earlier detection of a possible GI neoplasm. As patients with early-stage colorectal cancer (CRC) or precancerous lesions are mostly asymptomatic, and survival is closely related to the stage of cancer when diagnosed (Citation18), such early detection via GI bleeding might paradoxically lead to better prognosis and survival. To investigate this hypothesis further, we used different available data sets to evaluate the link between NOACs, GI bleeding, and the incidence of GI cancer.
Methods
For each of the NOACs, we retrieved the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms (PTs) in the AE data section on all GI bleeding and GI cancer, both reported as AEs from ClinicalTrials.gov for the completed phase III studies in patients with atrial fibrillation (AF). The AEs in ClinicalTrials.gov are described in a tabular summary of all anticipated and unanticipated serious AEs and a tabular summary of anticipated and unanticipated other AEs exceeding a specific frequency threshold. For each serious or other AE, it includes the AE term, affected organ system, number of participants at risk, and number of participants affected, by study arm or comparison group. As double-counting of patients (e.g. GI bleeding events or GI cancer at different locations in one patient) cannot be excluded, we did not perform any statistical comparisons of data from ClinicalTrials.gov. Here, we present the actual number of bleeding events in these studies.
In the original clinical trials, bleeding events (as a specific predefined safety end-point) were assessed on a separate report form outside of the AE reporting followed by central adjudication for major bleeding events. Therefore, it was not required to report all GI bleeding on an AE form. As a consequence, the GI bleeding incidence based on AE information from ClinicalTrials.gov differs from the adjudicated annual major GI bleeding rates published for the trials. This difference is dependent on the requirements of each individual trial protocol, which also makes comparisons between the different trials inappropriate. Rates for any GI bleeding including minor bleeds based on the separate bleeding assessment are not published.
There are four phase III studies of stroke prevention in patients with AF registered on ClinicalTrials.gov for the NOACs. These are: the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) study (Citation19); the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) study (Citation20); the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study (Citation21); and the Global Study to Assess the Safety and Effectiveness of Edoxaban (DU-176b) versus Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation (ENGAGE AF-TIMI 48) study (Citation22). As of 12 February 2014, the data for serious AEs associated with GI bleeding or GI cancer were available for dabigatran, rivaroxaban, and apixaban; results were not yet posted for edoxaban.
In addition, we analyzed information from the RE-LY clinical trial database on GI malignancy cases with an extended set of predefined PTs, using the safety set as treatment population and ‘start of treatment to end of study’ as observation period, since a malignancy may be detected outside the treatment period. This analysis is patient-based, hence statistical analysis using time-to-event methodology for first ‘any GI bleeding’ and first ‘any GI cancer’ is used for the comparison of dabigatran (both doses) and warfarin. The analysis was restricted to the first 6 months of treatment in order to describe the odds of early detection of GI cancer. By selecting this short time-frame, the analysis describes malignancies that definitely existed pre-treatment. Cox proportional hazard regression was used to analyze the hazards for GI bleeding and GI cancer with the NOACs compared with VKA. This analysis was also performed by means of P value for interaction in the different groups of patients who were VKA-experienced or VKA-naïve when entering the study, to understand whether this would be a potential confounder. Additional descriptive analyses were carried out using odds ratios for number and time to GI cancer diagnosis after the first of any GI bleeding event.
Results
Incidence of GI bleeding and GI cancer reported on ClinicalTrials.gov
In the RE-LY study (Citation19), overall, PTs related to GI bleeding were reported as AEs in 1.42% (85/5983) of patients on dabigatran 110 mg twice daily (D110), 1.93% (117/6059) of patients on dabigatran 150 mg twice daily (D150), and 1.37% (82/5998) of patients on warfarin (). The overall incidence of PTs associated with GI cancer was 0.79% (47/5983) in patients on D110, 0.61% (37/6059) in patients on D150, and 0.37% (22/5998) in patients on warfarin ().
Table I. Incidence of GI bleeding (reported as adverse events) with NOACs and warfarin in the phase III studies in patients with AF (ClinicalTrials.gov).
Table II. Incidence of GI cancer (reported as adverse events) with NOACs and warfarin in the phase III studies in patients with AF (ClinicalTrials.gov).
In the ROCKET AF study (Citation20), the PTs associated with GI bleeding were reported as AEs in 3.52% (250/7111) of patients on rivaroxaban and in 2.68% (191/7125) of patients on warfarin (). The overall incidence of PTs associated with GI cancer was 0.83% (58/7111) in patients on rivaroxaban and 0.73% (52/7125) in patients on warfarin ().
In the ARISTOTLE study (Citation21), the PTs associated with GI bleeding were reported as AEs in 1.93% (175/9052) of patients on apixaban (combined presentation for the dosages of 5.0 mg or 2.5 mg twice daily) and in 1.59% (145/9088) of patients on warfarin (). The overall incidence of PTs associated with GI cancer was 0.69% (63/9052) in patients on apixaban and 0.57% (52/9088) in patients on warfarin ().
GI bleeding
Overall, based on the data on ClinicalTrials.gov, the incidence of PTs associated with GI bleeding in AEs as well as in GI major bleeding was numerically higher for both D110 and D150 compared with warfarin (ClinicalTrials.gov and RE-LY trial); for rivaroxaban and apixaban (ClinicalTrials.gov) the major GI bleeding incidence was numerically higher when compared with warfarin (, ).
Table III. Incidence and hazard ratio of major GI bleeding with NOACs and warfarin based on bleeding assessment in phase III studies in patients with AF.
Lower GI tract bleeding occurred in more patients on rivaroxaban or dabigatran (either dose) compared with warfarin; the incidence was similar between apixaban and warfarin (). Looking at the MedDRA-coded AE data, each of the individually reported PTs (according to MedDRA) associated with a GI bleeding AE occurred in < 1% of patients on NOACs (or warfarin), except GI hemorrhage, which was reported in 1.24% of patients on rivaroxaban (Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.952327).
GI cancer
The incidence of GI cancer was numerically higher for all three NOACs compared with warfarin (, Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.952327). GI cancer overall was reported in more patients on dabigatran compared with warfarin, although this difference was driven by the slightly lower overall incidence of GI cancer in the warfarin arm of the RE-LY trial (0.37%) compared with the warfarin arms in ROCKET AF (0.73%) and ARISTOTLE (0.57%). The incidence of GI cancer overall was similar between the three NOACs: dabigatran 110 mg twice daily (0.79%), dabigatran 150 mg twice daily (0.61%), rivaroxaban (0.83%), and apixaban (0.69%) ().
GI bleeding and detection of GI cancer with dabigatran and warfarin in RE-LY
In the RE-LY clinical trial database, significantly more ‘any GI bleeding’ (i.e. major plus minor GI bleeding) events on treatment were reported in the separate bleeding assessment for the pooled dabigatran treatment arms compared with warfarin (hazard ratio [HR] 1.47; 95% confidence interval [CI] 1.32, 1.64) over the complete RE-LY treatment period. As Bytzer et al have shown for major GI bleeding (Citation23), in our analysis, the main difference in any GI bleeding detection was in the early phase after start of treatment, namely in the first 6 months (). Hence, the HR (95% CI) for any GI bleeding for the pooled dabigatran treatment arms compared with warfarin is higher: 2.18 (1.83, 2.59) ().
Figure 1. Time to onset from treatment initiation until any GI bleeding and any GI malignancy (days) for dabigatran and warfarin within the first 6 months of treatment—RE-LY database. GI = gastrointestinal; RE-LY = Randomized Evaluation of Long Term Anticoagulant Therapy.
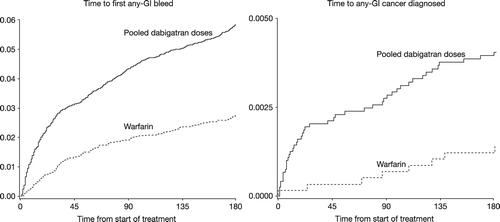
Table IV. Hazard ratio of any GI bleeding and any GI cancer within first 6 months of treatment—RE-LY database.
In RE-LY, 199 GI cancer events (based on an extended list of predefined PTs) were reported in 181 patients. The GI malignancy was located in the upper GI tract for 61 patients, in the lower GI tract for 114 patients, and in an unspecified location for 6 patients. The most frequently reported tumors were colon cancer (n = 83), esophageal carcinoma (n = 15), rectal cancer (n = 13), and gastric cancer (n = 11).
In parallel to the GI bleeding rates, malignancies of any GI location were significantly more frequent within the pooled dabigatran arms (n = 137, P = 0.0068) compared with warfarin (n = 43). The majority of patients with a GI malignancy were diagnosed within the first year of the study in the dabigatran groups (97/138 patients; 70.3%) and warfarin group (22/43 patients; 51.1%); the rest were diagnosed in the second and third year of the study. It is of note that in parallel to the observations with any GI bleeding, the hazard graph and 6-month HR (2.94; 95% CI 1.39, 6.23) () for GI cancer also showed that the main difference between dabigatran and warfarin is in the early phase after start of treatment ().
A malignant GI tumor in any location was detected significantly more often in patients who experienced GI bleeding compared with patients who had not experienced one of the events (), also supported by the finding of twice as high odds ratios for lower GI tumors in patients who had any GI bleeding (Supplementary Table III available online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.952327). Looking at the temporal relationship of GI bleeding and any GI cancer diagnosis from all patients with any GI bleeding and any GI cancer, 85.4% had the GI bleeding before the GI malignancy was diagnosed (median time ∼22 days before) (Supplementary Table IV, available online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.952327, data on file). This was seen without a difference between dabigatran and warfarin (Supplementary Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.952327).
Table V. Patients with and without any GI bleeding and odds ratio of GI malignancy (RE-LY study).
The median time-span between the first occurrence of any GI bleeding and the detection of lower GI tract malignancy was shorter for the dabigatran groups (17 days for D110 and 19 days for D150) compared with the warfarin group (41.5 days) (Supplementary Table V available online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.952327). A perianal bleeding observed with dabigatran was the initial starting-point for the discovery of a clinically silent tumor in the lower GI tract in several cases.
Discussion
In patients receiving antithrombotic therapy, the ‘anticoagulation GI stress-test’ seems to trigger GI bleeding, which is a well-known observation for patients treated with warfarin and antiplatelet agents. In our analysis we showed for the first time that this also holds true for patients with AF treated with NOACs. Additionally, our study supports the hypothesis that the putative imbalance in GI cancer between warfarin and NOACs might be due to a higher rate of unmasking (e.g. via initiation of endoscopic diagnostics) of pre-existing malignancies based on a higher GI bleeding rate of the NOACs. Based on this observation, with all the limitations of our evaluation, the ‘anticoagulant GI stress-test’ seems to increase the sensitivity of screening for the identification of early-stage GI cancers. Hence, the more sensitive screening test for occult blood in stool (if compared to any clinically observed GI bleeding), preferably executed within 2 to 4 weeks of starting treatment with dabigatran or other NOACs, may lead to an earlier detection of GI cancer. This might enable early treatment especially in patients with a malignancy.
As any GI bleeding has naturally a higher frequency compared with major GI bleeding, it therefore reflects a better model for screening via fecal occult blood test systems. This is the reason why we analyzed ‘any GI bleeding events’ in the RE-LY data set. As already discussed, to our knowledge these events are not reported publicly for apixaban and rivaroxaban. In a time-to-event/diagnosis analysis, it became apparent that any GI bleeding and also GI cancer were detected earlier in dabigatran-treated patients than in warfarin-treated patients (). This difference in bleeding rates between dabigatran and warfarin was also observed in the adjudicated major bleeding events reported by Bytzer et al. (Citation23). Unlike VKAs, the active moiety of the NOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) is present in the gastrointestinal tract lumen. This might be the underlying aspect for the imbalance of GI bleeding of NOACs compared with warfarin. Overall, the detection of GI cancer might be due to GI diagnostic procedures being performed earlier (post-GI bleeding) in the dabigatran-treated patients in comparison with those on warfarin. This early occurrence of GI bleeding and detection of a GI malignancy, both more frequent with dabigatran compared with warfarin, was seen in VKA-naïve as well as in VKA-experienced patients, with no indication of an interaction (data on file).
Several studies have shown the pre-existence of a malignancy in patients with GI bleeding who are on conventional anticoagulant or antiplatelet therapy (Citation6–9). Patients on clopidogrel or warfarin who experienced a GI bleeding were six times more likely to be diagnosed with GI cancer compared with those without bleeding, and the risk for occurrence of GI cancer was higher within 6 months of the first GI bleeding (Citation6). The GI bleeding after warfarin administration occurred anytime from immediately after warfarin administration to after 2 years of continuous warfarin treatment. None of the patients experiencing a GI bleeding had previous GI symptoms or rectal bleeding (Citation8,Citation9). The diagnostic evaluation of GI bleeding during warfarin treatment led to identification of previously unknown lesions in approximately one-third of cases, resulting in earlier treatment, better prognosis, and possibly increased 5-year survival (Citation9). In a prospective study, during a 6-month period, 12% of the patients who received anticoagulants had occult GI bleeding compared with 3% of those who had not received anticoagulants (Citation7). These results suggest that the majority of patients with a GI bleeding triggered by conventional anticoagulants or antiplatelet treatment have an unknown and pre-existing GI malignancy or other GI pathological conditions. VKA (and heparins) are unlikely to cause cancer; in fact, they have been reported to have anti-metastatic potential in preclinical studies (Citation24,Citation25) and antineoplastic effects in humans (Citation26–29), and to improve survival in patients with cancer and venous thromboembolism (Citation30).
Similarly, the observed increase in GI cancers with apixaban, dabigatran, or rivaroxaban is very unlikely to be the carcinogenic effect of treatment with the NOACs. The NOACs have not shown any carcinogenic or mutagenic potential in preclinical safety investigations (Citation31–33). There are no specific studies that have shown an increased GI cancer risk with NOACs. In fact, treatment with thrombin inhibitors (e.g. dabigatran etexilate) is suggested to prevent tumor progression, as thrombin has been linked to angiogenesis and tumor progression (Citation34). The diagnosis of GI cancer mainly within 1 year of exposure in the RE-LY study is suggestive of pre-existing malignancies in these patients, as it generally takes about 5 to 10 years for the development of malignant pathologies (Citation35).
Fecal occult blood test (FOBT) is a widely used screening test for GI bleeding with a possible diagnosis of CRC in a later work-up and could serve as the first-line screening test for the screening of asymptomatic individuals (Citation35), and reduces CRC mortality when used as a screening tool (Citation36,Citation37). Randomized controlled trials show that FOBT screening helps reduce CRC mortality in the general population (Citation38–41). A biennial FOBT screening reduces the relative risk for mortality from CRC to less than 0.70 (Citation42).
Anticoagulants and antiplatelet medications have been suggested to lower the positive predictive value of the FOBT (Citation43,Citation44). However, systematic reviews and data from mass screening programs have shown that neither anticoagulants nor antithrombotics diminish the positive predictive value of the FOBT to locate GI pathology (Citation4,Citation44–46), or the yield of colonoscopy among patients with a positive FOBT result (Citation47). Similarly, anticoagulants alone or in combination with antiplatelet drugs did not reduce the positive predictive value of the fecal immunochemical test (FIT) for advanced neoplasia (Citation48). Dabigatran, its prodrug dabigatran etexilate, as well as other excipients in its marketed formulation have also been shown not to cross-react with commonly used FOBTs (Citation49). This information on cross-reactivity is not available for the other NOACs.
Therefore, for patients who are on an anticoagulant, and specifically those on a NOAC, it is recommended to screen with a FOBT, e.g. hemoccult test within the first month after treatment initiation and then annually (Citation50). Additionally the evaluation of full blood counts (hemoglobin)—specifically a drop of hemoglobin from baseline after initiation of anticoagulation—can also identify patients at risk (Citation51). Neither approach has been the subject of a prospective scientific evaluation so far. If suggestive of GI bleeding, e.g. positive for occult blood, an appropriate work-up of the GI tract should be carried out in order to exclude a possible GI tract anomaly (e.g. GI neoplasia). Alternatively, in those unwilling to undergo a full GI tract work-up, FITs in patients with a positive FOBT could be used for the diagnosis of CRC. However, it must be acknowledged that the endoscopic GI work-up is more sensitive than these methods in ensuring correct diagnosis (Citation52).
Limitations
Our observations are limited by the retrospective nature and by the use of PT assessment in the ClinicalTrials.gov database, where possible double-counting of reported events cannot be excluded. Nevertheless these rules applied for all evaluated anticoagulants including NOACs and warfarin.
Conclusions
GI bleeding events with NOACs or VKAs can identify GI cancers at an early stage, if proper diagnostic procedures are carried out immediately after a GI bleeding. Therefore, a routine screening with FOBT and/or check of hemoglobin values in patients initiated or chronically treated with anticoagulants should be considered as a screening tool for early detection of GI bleeding.
Supplementary Tables I–V
Download PDF (105.6 KB)Acknowledgements
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and received no compensation related to the development of the manuscript. Medical writing assistance, supported financially by Boehringer Ingelheim Pharma GmbH & Co. KG, was provided by Lakshmi Venkatraman, PhD, of PAREXEL during the preparation of this article.
Declaration of interest: Dr Clemens was and Drs Brueckmann and Noack are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. They had a role in the design, data analysis, and statistical input for the study.
Dr Konstantinides is a consultant for AstraZeneca; Bayer; and Boehringer Ingelheim Pharmaceuticals, Inc.; and has been on the speakers bureau for AstraZeneca; Bayer; Boehringer Ingelheim Pharmaceuticals, Inc.; Bristol-Myers Squibb; Eli Lilly; Pfizer, Inc.; and SERVIER. Dr Konstantinides has received grants for clinical research from: Bayer; and Boehringer Ingelheim Pharmaceuticals, Inc.
Dr Lip is a consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Medtronic, Portola, and Boehringer Ingelheim and has been on the speakers bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, and Sanofi Aventis.
Dr Strack has nothing to disclose.
All authors were involved in writing and reviewing the manuscript, were involved at all stages of manuscript development, and have approved the final version.
References
- Hallas J, Dall M, Andries A, Andersen BS, Aalykke C, Hansen JM, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. BMJ. 2006;333:726.
- Ghate SR, Biskupiak J, Ye X, Kwong WJ, Brixner DI. All-cause and bleeding-related health care costs in warfarin-treated patients with atrial fibrillation. J Manag Care Pharm. 2011;17:672–84.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis. 2011;31:419–23.
- Barada K, Abdul-Baki H, El Hajj II, Hashash JG, Green PH. Gastrointestinal bleeding in the setting of anticoagulation and antiplatelet therapy. J Clin Gastroenterol. 2009;43:5–12.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–28.
- Asiimwe A, Li JJ, Weerakkody G, Vangerow H, Delisle F, Benoit K, et al. Diagnoses of gastrointestinal cancers after gastrointestinal bleeding in patients receiving clopidogrel or warfarin. Curr Drug Saf. 2013;8: 261–9.
- Jaffin BW, Bliss CM, LaMont JT. Significance of occult gastrointestinal bleeding during anticoagulation therapy. Am J Med. 1987;83:269–72.
- Michaels MM. Bleeding from occult tumors during anticoagulant therapy. Circulation. 1962;25:804–6.
- Norton SA, Armstrong CP. Lower gastrointestinal bleeding during anticoagulant therapy: a life-saving complication? Ann R Coll Surg Engl. 1997;79:38–9.
- Bloom BJ, Filion KB, Atallah R, Eisenberg MJ. Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. 2014;113:1066–74.
- Goodman SG, Wojdyla DM, Piccini JP, White HD, Paolini JF, Nessel CC, et al. Factors associated with major bleeding events: insights from the ROCKET AF Trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol. 2014;63:891–900.
- Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–72.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
- Gallagher AM, van Staa TP, Murray-Thomas T, Schoof N, Clemens A, Ackermann D, et al. Population-based cohort study of warfarin-treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open. 2014;4:e003839.
- Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511–17.
- Wei H, Shang J, Keohane C, Wang M, Li Q, Ni W, et al. A novel approach to assess the spontaneous gastrointestinal bleeding risk of antithrombotic agents using Apc(min/+) mice. Thromb Haemost. 2014;111:1121–32.
- Merck Manual. Colorectal cancer. Available at: http://www.merckmanuals.com/home/digestive_disorders/tumors_of_the_digestive_system/colorectal_cancer.html (accessed 23 January 2014).
- RE-LY (ClinicalTrials.gov). Randomized evaluation of long term anticoagulant therapy (RE-LY) with dabigatran etexilate. Available at: http://clinicaltrials.gov/ct2/show/results/NCT00262600?term=dabigatran& recr=Completed&rslt=With&cond=atrial+fibrillation&phase=123&rank=3§=X43015 (accessed 21 January 2014).
- ROCKET AF (ClinicalTrials.gov). An efficacy and safety study of rivaroxaban with warfarin for the prevention of stroke and non-central nervous system systemic embolism in patients with non-valvular atrial fibrillation. Available at: http://clinicaltrials.gov/ct2/show/results/NCT00403767?term=rivaroxaban&recr=Completed&rslt=With&cond= atrial+fibrillation&phase=123&rank=1 (accessed 21 January 2014).
- ARISTOTLE (ClinicalTrials.gov). Apixaban for the prevention of stroke in subjects with atrial fibrillation (ARISTOTLE). Available at: http://clinicaltrials.gov/ct2/show/results/NCT00412984?term=apixaban& recr=Completed&rslt=With&cond=atrial+fibrillation&phase=123& rank=1§=X43015 (accessed 21 January 2014).
- EngageAFTIMI48 (ClinicalTrials.gov). Global study to assess the safety and effectiveness of edoxaban (DU-176b) vs standard practice of dosing with warfarin in patients with atrial fibrillation (EngageAFTIMI48). Available at: http://clinicaltrials.gov/ct2/show/NCT00781391?term= edoxaban&recr=Completed&cond=atrial+fibrillation&rank=1 (accessed 21 January 2014).
- Bytzer P, Connolly SJ, Yang S, Ezekowitz M, Formella S, Reilly PA, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol. 2013;11:246–52.
- Banke IJ, Arlt MJ, Mueller MM, Sperl S, Stemberger A, Sturzebecher J, et al. Effective inhibition of experimental metastasis and prolongation of survival in mice by a potent factor Xa-specific synthetic serine protease inhibitor with weak anticoagulant activity. Thromb Haemost. 2005;94:1084–93.
- Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb Haemost. 2006;96:816–21.
- Akl EA, Kamath G, Kim SY, Yosuico V, Barba M, Terrenato I, et al. Oral anticoagulation may prolong survival of a subgroup of patients with cancer: a Cochrane systematic review. J Exp Clin Cancer Res. 2007;26:175–84.
- Cunningham MS, Preston RJ, O’Donnell JS. Does antithrombotic therapy improve survival in cancer patients? Blood Rev. 2009;23:129–35.
- Letai A, Kuter DJ. Cancer, coagulation, and anticoagulation. Oncologist. 1999;4:443–9.
- Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ Jr, Forcier RJ, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer. 1984;53:2046–52.
- Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902–11.
- Apixaban CDER review. Center for drug evaluation and research pharmacology reviews. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202155Orig1s000PharmR.pdf (accessed 2 May 2014).
- Dabigatran SmPC. Pradaxa summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf (accessed 2 March 2014).
- Rivaroxaban EPAR. Committee for medicinal products for human use assessment report. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000944/WC500120736.pdf (accessed 2 May 2014).
- Tsopanoglou NE, Maragoudakis ME. Role of thrombin in angiogenesis and tumor progression. Semin Thromb Hemost. 2004;30:63–9.
- Wu C-W, Sung JJ-Y. Colorectal cancer screening: are stool and blood based tests good enough? Chin Clin Oncol. 2013;2:8.
- Holme Ø, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database of Systematic Reviews. Available at: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD009259.pub2/references. (Accessed 6 March 2014).
- Kershenbaum A, Flugelman A, Lejbkowicz F, Arad H, Rennert G. Excellent performance of Hemoccult Sensa in organised colorectal cancer screening. Eur J Cancer. 2013;49:923–30.
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7.
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal- occult-blood test. Lancet. 1996;348:1467–71.
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71.
- Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–14.
- Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50:29–32.
- Clarke P, Jack F, Carey FA, Steele RJ. Medications with anticoagulant properties increase the likelihood of a negative colonoscopy in faecal occult blood test population screening. Colorectal Dis. 2006;8:389–92.
- Sawhney MS, McDougall H, Nelson DB, Bond JH. Fecal occult blood test in patients on low-dose aspirin, warfarin, clopidogrel, or non- steroidal anti-inflammatory drugs. Dig Dis Sci. 2010;55:1637–42.
- Konrad G, Katz A. Are medication restrictions before FOBT necessary? Practical advice based on a systematic review of the literature. Can Fam Physician. 2012;58:939–48.
- Mandelli G, Radaelli F, Paggi S, Terreni N, Gola G, Gramegna M, et al. Anticoagulant or aspirin treatment does not affect the positive predictive value of an immunological fecal occult blood test in patients undergoing colorectal cancer screening: results from a nested in a cohort case-control study. Eur J Gastroenterol Hepatol. 2011;23:323–6.
- Ashraf I, Paracha S, Paracha S, Arif M, Choudhary A, Godfrey JD, et al. Antithrombotics during fecal occult blood testing: a meta-analysis and systematic review. The Internet Journal of Gastroenterology. 2013;13.
- Bujanda L, Sarasqueta C, Lanas A, Quintero E, Cubiella J, Hernandez V, et al. Effect of oral anticoagulants on the outcome of faecal immunochemical test. Br J Cancer. 2014;110:1334–7.
- Goss AM, van Ryn J, Schurer J, Clemens A. Assessment of cross- reactivity in three different fecal occult blood test systems with dabigatran and dabigatran etexilate: identification of useful test methods. Blood. 2012;120: Abstr 4360.
- Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–47.
- Johannsdottir GA, Onundarson PT, Gudmundsdottir BR, Bjornsson ES. Screening for anemia in patients on warfarin facilitates diagnosis of gastrointestinal malignancies and pre-malignant lesions. Thromb Res. 2012;130:e20–e25.
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer Systematic review and meta-analysis. Ann Intern Med. 2014;160:171–81.

