Abstract
Background. The best treatment option for drug-eluting stent (DES) restenosis has not been established. We performed a meta-analysis to assess the clinical efficacy of drug-coated balloon (DCB) for the treatment of DES restenosis.
Methods. Trials were identified through a literature search from January 2005 through April 2014. All randomized controlled trials were eligible for inclusion if they compared DCB with a control treatment (plain old balloon angioplasty [POBA] or DES) in patients with DES restenosis.
Results. Five studies and a total of 864 patients were included in this analysis. Most end-points were significantly reduced for DCB compared with the control groups. For major adverse cardiac events, the relative risk (RR) was 0.49 (P = 0.012); for target lesion revascularization, it was 0.50 (P = 0.044); for recurrent restenosis, it was 0.41 (P = 0.002). There was a lower mortality for DCB (RR 0.29; P = 0.017). The incidence of myocardial infarction was numerically lower, but without statistical significance (RR 0.76; P = 0.55). The DCB effect was more pronounced when compared with POBA than when compared with DES.
Conclusions. This meta-analysis showed that DCB was superior to POBA and comparable to DES for treatment of DES restenosis. The findings in this meta-analysis cannot be extrapolated to DCB in general, because all DCB used in trials included was a single brand of paclitaxel-coated balloon.
Key messages
The best treatment for drug-eluting stent restenosis has not been established.
Paclitaxel-coated balloon was superior to plain balloon angioplasty and comparable to drug-eluting stent implantation for drug-eluting stent restenosis.
Drug-eluting stents (DES) have emerged as an improved alternative to bare metal stents (BMS) by demonstrating reduced occurrence of restenosis and subsequent need for repeat revascularization (Citation1,Citation2). However, the unrestricted use of DES for various indications and lesions has led to a sizeable population of patients facing a novel interventional therapeutic dilemma of DES in-stent restenosis (ISR) (Citation3–5). Treatment of DES restenosis remains challenging, and the best treatment option has not yet been well established (Citation6,Citation7). Drug-coated balloon (DCB) is a promising option to treat ISR as it avoids additional stent layers (Citation8,Citation9). For bare-metal stent restenosis, DCB has been shown to be superior to plain old balloon angioplasty (POBA) and non-inferior to paclitaxel-eluting DES (Citation10–14). However, the effects of DCB on DES restenosis are less well known. We performed a meta-analysis to assess the clinical efficacy of DCB for the treatment of DES restenosis.
Methods
Search strategy
The published literature was scanned by a comprehensive search of electronic databases (PubMed, MEDLINE, EMBASE, and ISI Web of Science) to identify relevant articles from January 2005 to April 2014. Search terms included ‘randomized’ (or ‘randomised’), ‘coronary’, ‘restenosis’, ‘drug-coated balloon’ (or ‘drug-eluting balloon’), and ‘drug-eluting stent’. In addition, abstract lists of conference proceedings from 2005 to 2014 scientific meetings of the American College of Cardiology, the European Society of Cardiology, the Transcatheter Cardiovascular Therapeutics, and the American Heart Association were searched. We also considered published review articles, editorials, and internet-based sources of information to assess potential information on studies of interest.
Study selection
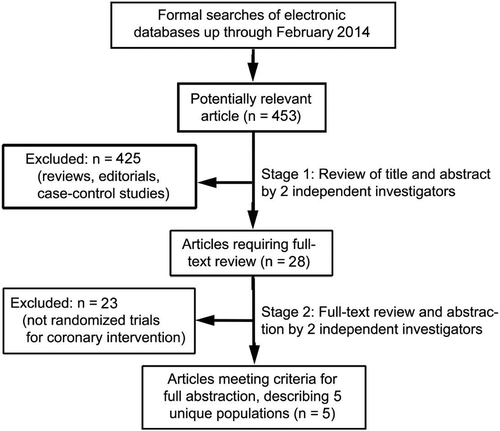
To be selected for this meta-analysis, studies comparing DCB with a control treatment (POBA or DES implantation) in patients with DES restenosis had to be randomized and have their results reported by the trial investigators. All studies meeting the requirements, regardless of the language or form of publication, were considered to be eligible for this meta-analysis. Finally, five trials were included in this meta-analysis (Citation15–19). A flow diagram depicting the overall search strategy is demonstrated in .
Data abstraction
Data abstraction was independently performed by two investigators. In addition to pertinent data on the outcomes of interest, we gathered information on study characteristics, patient characteristics, and treatment information. Disagreements were resolved by consensus. Data were managed according to the intention-to-treat principle.
Study end-points
The primary end-point in the present study was a composite of major adverse cardiac events (MACE). The secondary end-points included all-cause mortality, myocardial infarction (MI), target lesion revascularization (TLR), and recurrent binary restenosis (≥ 50% diameter stenosis).
Statistical analysis
Statistical analysis was performed using Stata software version 9.0 (Stata Corp, College Station, TX, USA). Relative risk (RR) with 95% confidence intervals (CI) were calculated as summary statistics. The pooled RR was calculated with the DerSimonian and Laird method for random effects (Citation20,Citation21). To assess heterogeneity across trials, we used Cochran's test and means of I2 statistic (Citation22). We assessed publication bias with respect to the primary outcomes of interest using a funnel plot as well as the adjusted rank correlation test according to the method of Begg and Mazumdar (Citation23). A sensitivity analysis was performed by assessing the contribution of individual studies to the summary effect estimate with respect to the primary outcomes (Citation24).This was done by excluding each trial one at a time and computing meta-analysis estimates for the remaining studies. Results were considered statistically significant at P < 0.05.
Results
Eligible studies
Of the 453 potentially relevant articles initially screened, a total of 5 randomized clinical trials were finally included in this meta-analysis, involving 864 patients (396 in the DCB group, 468 in the control group) (Citation15–19). Characteristics in the individual trials are shown in . The DCB studied in all five trials was a single brand of paclitaxel-coated balloon (PCB) (SeQuent Please, B. Braun, Melsungen, Germany) (Citation15–19). For the control, the plain balloon angioplasty was used in four trials (Citation15–18), and the paclitaxel-eluting stent (PES) was used in two trials (Citation18,Citation19).
Table I. Characteristics of randomized controlled trials included in the meta-analysis.
Primary end-point
Data on a composite of MACE were available in all 864 patients (100%). The definition of MACE differed slightly among the trials (). The risk for this primary end-point was significantly reduced for DCB compared with the control treatments (POBA or DES) (19.7% versus 32.5%, RR 0.49 [0.28 to 0.85]; P = 0.012) by the random effect model ().
Figure 2. Risk ratios of major adverse cardiac events (primary end-point) associated with drug-coated balloon versus the control treatment in patients with drug-eluting stent restenosis. The size of the data marker is proportional to the weight of the individual studies, measured as the inverse of the variance in the study by the Mantel–Haenszel procedure.
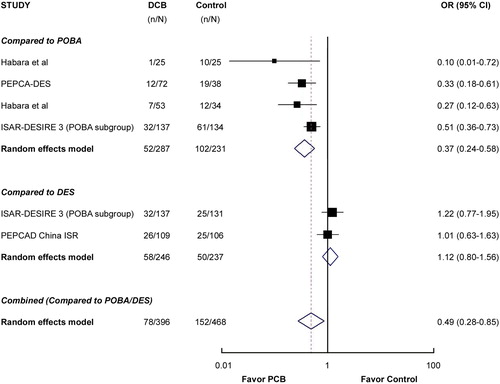
The PEPCAD China ISR (Citation19) and one arm of the ISAR-DESIRE 3 trial (Citation18) compared DCB with a DES, while four trials (including one arm of ISAR-DESIRE 3) (Citation15–18) used POBA as comparator. As shown in , the rate of MACE was much lower for DCB as compared with POBA (RR 0.37 [0.24 to 0.58]; P < 0.001), but it was not significantly different when compared with DES (RR 1.12 [0.80 to 1.56]; P = 0.52).
Secondary end-points
As shown in , mortality was significantly reduced for the DCB group as compared to the control group (POBA or DES) (RR 0.29 [0.11 to 0.80]; P = 0.017). The difference was statistically significant (RR 0.27 [0.08 to 0.96]; P = 0.042) when comparing DCB with POBA, but not when comparing DCB with DES (RR 0.41 [0.12 to 1.42]; P = 0.16).
Figure 3. Risk ratios of death (A), myocardial infarction (B), target lesion revascularization (C), and recurrent binary restenosis (D) associated with drug-coated balloon versus the control treatment in patients with drug-eluting stent restenosis. The size of the data marker is proportional to the weight of the individual studies, measured as the inverse of the variance in the study by the Mantel–Haenszel procedure.
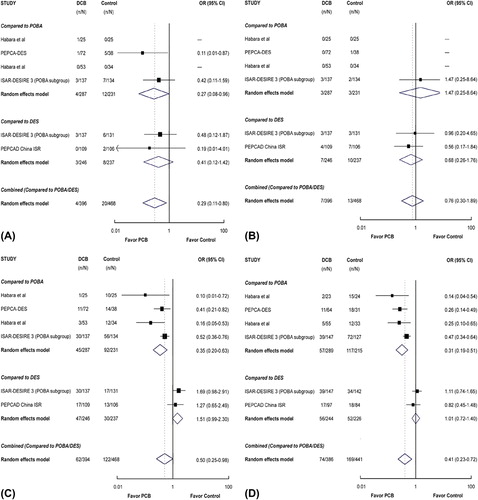
As shown in , the risk of MI was numerically lower for DCB as compared with the control group (POBA or DES), but this difference was not statistically significant (RR 0.76 [0.30 to 1.89]; P = 0.55). Nor was the difference statistically significant when comparing DCB either with POBA (RR 1.47 [0.25 to 8.64]; P = 0.67) or with DES (RR 0.68 [0.26 to 1.76]; P = 0.42).
As shown in , TLR was significantly reduced for the DCB group as compared to the control group (POBA or DES) (RR 0.50 [0.25 to 0.98]; P = 0.044). Interestingly, the rate of TLR for DCB was significantly lower than that for POBA (RR 0.35 [0.20 to 0.63]; P < 0.001), but numerically higher than that for DES in spite of the lack of statistical significance (RR 1.51 [0.99 to 2.30]; P = 0.057).
Data on angiographic follow-up were available in 827 ISR lesions. In the pooled estimate (), the risk of ISR was significantly reduced for the DCB group as compared to the control group (POBA or DES) (RR 0.41 [0.23 to 0.72]; P = 0.002). The difference was statistically significant when comparing DCB with POBA (RR 0.31 [0.19 to 0.51]; P < 0.001), but not when comparing DCB with DES (RR 1.01 [0.72 to 1.40]; P = 0.96).
Discussion
This is the first meta-analysis specially focusing on the clinical effectiveness of DCB for the treatment of DES restenosis. Our data show that DCB angioplasty was superior to POBA and comparable to DES in terms of both angiographic restenosis rate and clinical event risks in patients with ISR after DES implantation.
By dramatically reducing the risk of in-stent restenosis and the need for target revascularization, DES have become the mainstay in the treatment of patients with coronary artery disease. However, the incidence of restenosis remains high due to the adoption of DES treatment in increasingly complex subsets of patients and lesions. Moreover, restenosis after DES implantation is associated with a different clinicopathological entity and relatively poor therapeutic effect compared with restenosis after BMS implantation (Citation25,Citation26). Several percutaneous treatment options are currently available for DES restenosis, including plain balloon angioplasty, repeat DES implantation, vascular brachytherapy, and DCB angioplasty. DCB is an attractive option to treat ISR, as it obviates the need for another metal implantation and has the potential of shortened dual antiplatelet therapy duration without increasing the risk of thrombosis (Citation8,Citation9).
For BMS restenosis, DCB is superior to plain balloon angioplasty, with a sustained benefit for up to five years (Citation10,Citation11,Citation13,Citation17). In the present meta-analysis, the use of DCB is also superior to plain balloon angioplasty in patients with restenosis after DES implantation. Furthermore, DCB angioplasty not only reduces the incidence of repeated restenosis and the need for TLR, but also lowers the mortality. The superiority of DCB to plain balloon angioplasty in our study and previous reports suggests that plain balloon angioplasty alone has a restricted role for DES restenosis as well as BMS restenosis, at least as a default treatment strategy for these patients.
The use of DES implantation for treatment of restenotic lesions after BMS implantation has been common in current clinical practice. And repeated DES is also shown to be effective and safe for DES restenosis in randomized controlled trials as well as in observational registries (Citation27–29). In this meta-analysis, DCB angioplasty was comparable to repeated DES implantation with respect to both the angiographic end-points and the clinical outcomes for treatment of DES restenosis. It is interesting to note that despite similar rates of recurrent binary restenosis (RR 1.01), the number of patients undergoing TLR was numerically higher in patients treated with DCB angioplasty than with DES implantation (RR 1.51). The most likely explanation for this finding is that the presence of existing multiple stent layers in the DES group might have discouraged the operator from repeat intervention. This reflects the significant limitation of use of repeated stenting for treatment of ISR. When an additional stent is deployed to treat coronary ISR, the second strut layer results in excessive stiffening of the coronary vessel, and injury to the ostium of side branches may occur. Moreover, once repeated restenosis occurs after DES implantation in the ISR, further management will become more and more challenging.
In the present analysis, all DCB used in trials included were of a single brand of paclitaxel-coated balloon (SeQuent Please). It is known from the literature that certain DCB products do not inhibit restenosis or to a very modest extent (Citation30,Citation31). Therefore, the findings in this meta-analysis cannot be extrapolated to DCB in general. In addition, all DES used in the control group were the first-generation PES in our analysis. A recent non- randomized study compared the DCB with the second-generation everolimus-eluting stent (EES) in patients with DES restenosis and showed a favorable effect of DCB as compared with EES (Citation32). Further large, well-conducted randomized trials are needed to provide additional insights into the relative safety and efficacy of the DCB in comparison with the new-generation DES for the treatment of DES restenotic lesions.
In conclusion, this meta-analysis demonstrated that DCB angioplasty was superior to POBA and comparable to DES implantation for treatment of patients presenting DES restenosis. DCB could be the preferred interventional strategy for these patients by avoiding additional implantation of metal layers.
Declaration of interest: This work was supported by the National Natural Science Foundation (No. 81101133) and the Shanghai Outstanding Young Scientist Foundation from the Shanghai Municipal Health Bureau (No. XYQ2011001). The authors report no conflicts of interest.
References
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 346:1773–80.
- Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31.
- Lemos PA, Serruys PW, van Domburg RT, Saia F, Arampatzis CA, Hoye A, et al. Unrestricted utilization of sirolimus-eluting stents compared with conventional bare stent implantation in the “real world”: the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation. 2004;109:190–5.
- Mauri L, Silbaugh TS, Wolf RE, Zelevinsky K, Lovett A, Zhou Z, et al. Long-term clinical outcomes after drug-eluting and bare-metal stenting in Massachusetts. Circulation. 2008;118:1817–27.
- Kastrati A, Dibra A, Mehilli J, Mayer S, Pinieck S, Pache J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–300.
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–907.
- Minha S, Pichard AD, Waksman R. In-stent restenosis of drug-eluting stents. Future Cardiol. 2013;9:721–31.
- Waksman R, Pakala R. Drug-eluting balloon: the comeback kid? Circ Cardiovasc Interv. 2009;2:352–8.
- Choo GH. Drug-eluting balloons: future potential indications and applications. EuroIntervention. 2011;7(Suppl K):K112–18.
- Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355:2113–24.
- Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, et al. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2008;97:773–81.
- Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119:2986–94.
- Scheller B, Clever YP, Kelsch B, Hehrlein C, Bocksch W, Rutsch W, et al. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. 2012;5: 323–30.
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García Del Blanco B, Seidelberger B, Iñiguez A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol. 2014;63:1378–86.
- Habara S, Mitsudo K, Kadota K, Goto T, Fujii S, Yamamoto H, et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv. 2011; 4:149–54.
- Rittger H, Brachmann J, Sinha AM, Waliszewski M, Ohlow M, Brugger A, et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the PEPCAD-DES study. J Am Coll Cardiol. 2012;59:1377–82.
- Habara S, Iwabuchi M, Inoue N, Nakamura S, Asano R, Nanto S, et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am Heart J. 2013;166:527–33.
- Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381:461–7.
- Xu B, Gao R, Wang J, Yang Y, Chen S, Liu B, et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the PEPCAD China ISR trial. JACC Cardiovasc Interv. 2014;7:204–11.
- Egger M, Ebrahim S, Smith GD. Where now for meta-analysis? Int J Epidemiol. 2002;31:1–5.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Byrne RA, Joner M, Tada T, Kastrati A. Restenosis in bare metal and drug-eluting stents: distinct mechanistic insights from histopathology and optical intravascular imaging. Minerva Cardioangiol. 2012; 60:473–89.
- Steinberg DH, Gaglia MA Jr, Pinto Slottow TL, Roy P, Bonello L, De Labriolle A, et al. Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. Am J Cardiol. 2009;103491–5.
- Alfonso F, Pérez-Vizcayno MJ, Dutary J, Zueco J, Cequier A, García-Touchard A, et al. Implantation of a drug-eluting stent with a different drug (switch strategy) in patients with drug-eluting stent restenosis. Results from a prospective multicenter study (RIBS III [Restenosis Intra-Stent: Balloon Angioplasty Versus Drug-Eluting Stent]). JACC Cardiovasc Interv. 2012;5:728–37.
- Latib A, Mussardo M, Ielasi A, Tarsia G, Godino C, Al-Lamee R, et al. Long-term outcomes after the percutaneous treatment of drug-eluting stent restenosis. JACC Cardiovasc Interv. 2011;4:155–64.
- Mehilli J, Byrne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, et al. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol. 2010;55:2710–16.
- Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart. 2010;96:1291–6.
- Bondesson P, Lagerqvist B, James SK, Olivecrona GK, Venetsanos D, Harnek J. Comparison of two drug-eluting balloons: a report from the SCAAR registry. EuroIntervention. 2012;8:444–9.
- Almalla M, Schröder J, Pross V, Marx N, Hoffmann R. Paclitaxel-eluting balloon versus everolimus-eluting stent for treatment of drug-eluting stent restenosis. Catheter Cardiovasc Interv. 2014;83:881–7.