Abstract
Background. Reports on the association of prediabetes with all-cause mortality and cardiovascular mortality are inconsistent.
Objective. To evaluate the risk of all-cause and cardiovascular mortality in association with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT).
Methods. Prospective cohort studies with data on prediabetes and mortality were included. The relative risks (RRs) of all-cause and cardiovascular mortality were calculated and reported with 95% confidence intervals (95% CIs).
Results. Twenty-six studies were included. The risks of all-cause and cardiovascular mortality were increased in participants with prediabetes defined as IFG of 110–125 mg/dL (IFG 110) (RR 1.12, 95% CI 1.05–1.20; and RR 1.19, 95% CI 1.05–1.35, respectively), IGT (RR 1.33, 95% CI 1.24–1.42; RR 1.23, 95% CI 1.11–1.36, respectively), or combined IFG 110 and/or IGT (RR 1.21, 95% CI 1.11–1.32; RR 1.21, 95% CI 1.07–1.36, respectively), but not when IFG was defined as 100–125 mg/dL (RR 1.07, 95% CI 0.92–1.26; and RR 1.16, 95% CI 0.94–1.42, respectively).
Conclusions. Prediabetes, defined as IFG 110, IGT, or combined IFG 110 and/or IGT, was associated with increased all-cause and cardiovascular mortality.
Key messages
Prediabetes, defined as impaired fasting glucose (IFG) of 110–125 mg/dL, impaired glucose tolerance (IGT), or combined IFG of 110–125 mg/dL and/or IGT, was associated with increased all-cause and cardiovascular mortality.
The current American Diabetes Association definition of IFG of 100–125 mg/dL was not associated with increased risk of all-cause or CVD mortality.
These results reaffirmed the importance of screening prediabetes defined as the WHO proposal.
Introduction
‘Prediabetes’ is a general term that refers to an intermediate stage between normoglycaemia and overt type 2 diabetes mellitus (T2DM). It includes two groups of individuals: those with impaired glucose tolerance (IGT) and those with impaired fasting glucose (IFG) (Citation1). In 2003, the American Diabetes Association (ADA) redefined the fasting plasma glucose (FPG) concentration range for diagnosing IFG from 110–125 mg/dL to 100–125 mg/dL in order better to identify individuals at risk of future T2DM (Citation2). However, this change has been contentious and was not adopted by the World Health Organization (WHO) Expert Group (Citation3) or other international guidelines (Citation4,Citation5). One of the main arguments against this change is that it greatly increases the number of individuals labelled with IFG, without a clear association with clinical events and mortality. Since the 2003 ADA proposal, some studies have demonstrated that the new IFG category is associated with increased cardiovascular and all-cause mortality (Citation6,Citation7), while others have not shown this association (Citation8–11).
Although IGT and IFG are often lumped together, they have distinct pathophysiology and aetiology, and may have differing associations with clinical events and mortality (Citation1,Citation12). Some studies have shown that IGT was a stronger predictor of cardiovascular and all-cause mortality than IFG (Citation9,Citation13), but it was not documented in other studies (Citation14–16).
Considering these inconsistent results, a meta-analysis of prospective cohort studies may help to clarify this issue. Therefore, the objective of the present study was to evaluate the putative association between different definitions of prediabetes with all-cause and cardiovascular mortality.
Methods
Search strategy and selection criteria
The search strategy was performed in accordance with the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group (Citation17). Electronic databases (PubMed, EMBASE, and the Cochrane Library) were searched for prospective cohort studies to 2 October 2013, using the terms ‘blood glucose’, ‘impaired glucose tolerance’, ‘impaired fasting glucose’, ‘prediabetes’, ‘pre-diabetes’, ‘prediabetic state’, ‘hyperglycaemia’, or ‘borderline diabetes’ and ‘mortality’, ‘death’, ‘deaths’, or ‘fatal’. We restricted the search to human studies. No language restrictions were applied. We also manually reviewed the references cited in potentially relevant studies. The detailed search strategy used for PubMed is presented in online Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.955051. The search strategies were similar for other databases, but were adapted where necessary.
Studies were included if they met the following criteria: 1) prospective cohort studies involving participants aged ≥ 18 years; 2) blood glucose and other cardiovascular risk factors were evaluated at baseline; 3) follow-up duration ≥ 2 years with assessment of cardiovascular or all-cause mortality; 4) the multivariate-adjusted relative risk (RRs, including study-specific relative risk ratios or hazard ratios) and 95% confidence intervals (CIs) were reported for events associated with prediabetes relative to normoglycaemia. Prediabetes was defined as IFG (FPG of 100–125 mg/dL (IFG 100) or 110–125 mg/dL (IFG 110)) (Citation2,Citation18) and/or IGT (2-h plasma glucose (2-h PG) level of 140–199 mg/dL during an oral glucose tolerance test) (Citation3). Normoglycaemia was defined as FPG < 100 or < 110 mg/dL, and/or normal glucose tolerance (NGT).
Studies were excluded if: 1) enrolment depended on having a particular condition or risk factor (e.g. cardiovascular disease, chronic kidney disease); 2) the RR was only adjusted for age and sex; or 3) duplicate publications. If more than one report derived from the same cohort, only the most recent one was included.
Data extraction and quality assessment
Two investigators (Yi.H. and X.C.) conducted independent literature searches using the strategy described above to identify potentially relevant articles. Full manuscripts of potentially relevant studies were obtained and reviewed according to the predefined criteria. Data, including participant characteristics, follow-up duration, and outcome assessment, were recorded in a standard format. Discrepancies were resolved by discussion with other investigators (W.M. and Yul.H.). The principal authors were contacted for any additional data if required.
We assessed the quality of each study according to whether there was adequate adjustment for potential confounders (at least six of seven factors: age, sex, hypertension or systolic blood pressure or antihypertensive drug, body mass index or other measure of overweight/obesity, physical activity, cholesterol concentration or lipid-lowering medication use, and smoking) (Citation19).
Data synthesis and analysis
The primary outcomes were the risks of all-cause and cardiovascular mortality. Subgroup analyses of primary outcomes were conducted according to ethnicity (Asian versus non-Asian), sex (male versus female), participant’s age (mean age < 55 years versus ≥ 55 years), follow-up duration (< 10 years versus ≥ 10 years), possibility of enrolling patients with T2DM (none enrolled versus might be enrolled), and adequate adjustment for risk factors (yes versus no). For each study, we extracted the multivariate-adjusted RRs (with 95% CIs) for analyses. We logarithmically transformed these values in each study and calculated the corresponding standard errors (SEs) to stabilize the variance and normalize the distribution (Citation20). The inverse variance method was used to combine the log RRs and SEs. We used chi-square and I2 statistics to test for heterogeneity, where 25%, 50%, and 75% represented low, moderate, and high heterogeneity (Citation21). The results of studies were pooled using fixed-effects models, if appropriate, after consideration of heterogeneity among studies. Alternatively, a random-effects model was used. We assessed publication bias by inspecting funnel plots for each outcome in which the ln (RR) was plotted against SE.
We also conducted sensitivity analyses in which the pooled RR was recalculated by omitting one study at a time. All P values are two-tailed, and the statistical significance was set at 0.05. All analyses were performed with RevMan software version 5.1 for Windows (The Cochrane Collaboration, Copenhagen, Denmark).
Results
Studies retrieved and characteristics
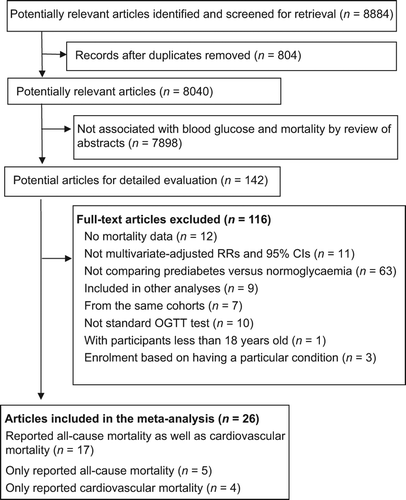
The initial search retrieved 8884 manuscripts. After screening of the abstracts, we found 142 that qualified for full review. Our final primary analysis included data for 280,185 participants derived from 26 prospective cohort studies () (Citation6–11,Citation13–16,Citation22–37). Twenty-five of the primary papers were published in full, and one was in abstract form (Citation34).
Of the 26 studies, 17 reported all-cause and cardiovascular mortality (Citation7,Citation11,Citation13–16,Citation22,Citation24–26,Citation28,Citation29,Citation31,Citation32,Citation34–36), while 5 only reported all-cause mortality (Citation6,Citation8,Citation27,Citation30,Citation33), and 4 only reported cardiovascular mortality (Citation9,Citation10,Citation23,Citation37). Therefore, there were 22 and 21 studies for analyses of all-cause and cardiovascular mortality, respectively. The mean follow-up of the included studies was 11.3 years. All studies were derived from the general population. Four studies only enrolled males (Citation7,Citation15,Citation22,Citation24), while the other studies included males and females. All of the studies excluded people with FPG ≥ 126 mg/dL, except for one that only measured non-fasting glucose at baseline (Citation22). That study reported the association between IGT and mortality, and NGT was used as a reference; therefore, it is possible that a few patients with FPG ≥ 126 mg/dL were enrolled in the IGT or NGT groups (Citation22). Seven studies only measured FPG at baseline without performing oral glucose tolerance tests; therefore, these studies possibly enrolled patients with 2-h PG ≥ 200 mg/dL (Citation6,Citation7,Citation10,Citation23,Citation24,Citation28,Citation32). Of the 26 studies, 19 were conducted in Europe or the United States (Citation6–9,Citation11,Citation13,Citation14,Citation16,Citation22–26,Citation28,Citation30,Citation31,Citation33,Citation36,Citation37), while 6 were conducted in Asia or included Asian migrants (Citation10,Citation15,Citation27,Citation29,Citation32,Citation34), and 1 was conducted in Africa (Citation35). Asians accounted for 27.8% of all participants in the 26 studies. The follow-up duration ranged from 5 to 18.8 years. According to the predefined quality assessment criteria, eight studies did not meet our criteria for adequate adjustment of potential confounders (Citation9,Citation10,Citation23,Citation27,Citation28,Citation31,Citation32,Citation35). The characteristics of the 26 studies are presented in . Confounders adjusted and quality assessments of the included studies are presented in Supplementary Table II to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.955051.
Table I. Study characteristics.
Association between prediabetes and all-cause mortality
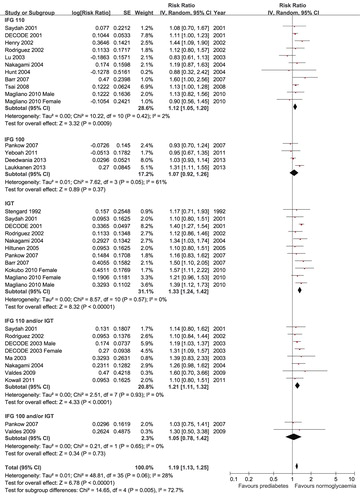
For all-cause mortality, 4 studies (Citation6–8,Citation11) reported data for IFG 100, 10 studies (Citation13–16,Citation24,Citation26,Citation28,Citation29,Citation32,Citation35) reported data for IFG 110, 10 studies (Citation8,Citation13–16,Citation22,Citation29,Citation30,Citation34,Citation35) reported data for IGT, 2 studies (Citation8,Citation33) combined IFG 100 and/or IGT, and 7 studies (Citation15,Citation25,Citation27,Citation29,Citation33,Citation36,Citation37) combined IFG 110 and/or IGT. The data sets did not show significant heterogeneity except for IFG 100 (P = 0.05, I2 = 61%). Therefore, the random-effects models were used for the analyses. Prediabetes was associated with increased risk of all-cause mortality after multivariate adjustment when it was defined as IFG 110 (RR 1.12, 95% CI 1.05–1.20), IGT (RR 1.33, 95% CI 1.24–1.42), or combined IFG 110 and/or IGT (RR 1.21, 95% CI 1.11–1.32). However, prediabetes was not associated with all-cause mortality when it was defined as IFG 100 (RR 1.07, 95% CI 0.92–1.26) or combined IFG 100 and/or IGT (RR 1.05, 95% CI 0.78–1.42) (). When we compared the risk of all-cause mortality between the different definitions, the risk was greater for IGT than for IFG 110 (P < 0.001) or IFG 100 (P = 0.02).
Association between prediabetes and cardiovascular mortality
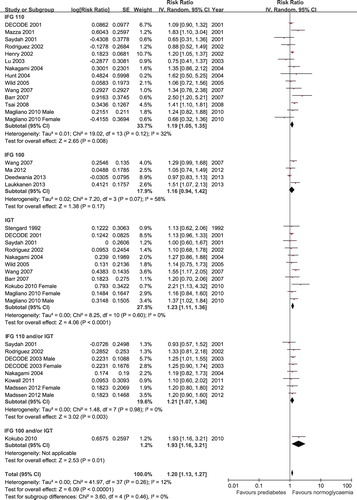
For cardiovascular mortality, 4 studies (Citation7,Citation9–11) reported data for IFG 100, 13 studies (Citation9,Citation13–16,Citation23,Citation24,Citation26,Citation28,Citation29,Citation31,Citation32,Citation35) reported data for IFG 110, 10 studies (Citation9,Citation13–16,Citation22,Citation29,Citation31,Citation34,Citation35) reported data for IGT, and 6 studies (Citation14,Citation15,Citation25,Citation29,Citation36,Citation37) combined IFG 110 and/or IGT. Because the data for IFG 100 showed moderate heterogeneity (P = 0.07, I2 = 58%), we used the random-effects models for the analyses. Prediabetes was associated with increased cardiovascular mortality after multivariate adjustment when it was defined as IFG 110 (RR 1.19, 95% CI 1.05–1.35), IGT (RR 1.23, 95% CI 1.11–1.36), or combined IFG 110 and/or IGT (RR 1.21, 95% CI 1.07–1.36). However, prediabetes defined as IFG 100 was not associated with cardiovascular mortality (RR 1.16, 95% CI 0.94–1.42) (). Only one study conducted in Japan reported an association between IFG 100 and/or IGT with cardiovascular mortality (Citation34). That study showed that prediabetes defined as IFG 100 and/or IGT was associated with increased risk of cardiovascular mortality (RR 1.93, 95% CI 1.16–3.21). There were no significant differences in the risk of cardiovascular mortality among the different definitions of prediabetes (all P > 0.05).
Figure 3. Forest plot of the comparison: prediabetes versus normoglycaemia, outcome: CVD mortality. IFG 100 = impaired fasting glucose (fasting glucose 100–125 mg/dL); IFG 110 = impaired fasting glucose (fasting glucose 110–125 mg/dL); IGT = impaired glucose tolerance.
Visual inspection of funnel plots suggested that there was no evidence of publication bias for either all-cause mortality (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.955051) or cardiovascular mortality (Supplementary Figure 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.955051).
Subgroup analyses
The results of subgroup analyses for all-cause mortality and cardiovascular mortality are presented in and , respectively. IFG 100 was significantly associated with all-cause mortality and cardiovascular mortality in males and in participants with a mean age of < 55 years at study entry, but not in other subgroups. There were significant differences in the risk estimates among sexes (males versus females versus both sexes) or the participants’ mean age at study entry (< 55 years versus ≥ 55 years) for all-cause mortality (both P < 0.001). There were also trends towards differences in the risk of cardiovascular mortality among these subgroups (P = 0.09 for sex and P = 0.05 for mean age at study entry).
Table II. Subgroup analyses of the association between prediabetes and all-cause mortality.
Table III. Subgroup analyses of the association between prediabetes and cardiovascular mortality.
There were no significant differences among subgroups of participants with IFG 110, IGT, or IFG 110 and/or IGT. We did not perform subgroup analyses in participants with IFG 100 and/or IGT because of the limited number of studies.
Sensitivity analyses
Sensitivity analyses were conducted using several methods, and these analyses confirmed that the primary results were not influenced by the use of fixed-effects models compared with random-effects models, odds ratios compared with RRs, or recalculating the RRs by omitting one study at a time.
Discussion
In this meta-analysis, we found that, after controlling for multiple cardiovascular risk factors, the presence of prediabetes at baseline, defined as IFG 110, IGT, or combined IFG 110 and/or IGT, was associated with increased risk of all-cause and cardiovascular mortality. However, the current ADA definition of IFG, with FPG of 100–125 mg/dL, was not associated with increased risk of all-cause or cardiovascular mortality in the total study population.
The 2003 ADA definition of IFG resulted in a 2- to 5-fold increase in the prevalence of IFG in most populations. For example, the lowered threshold would increase the prevalence of IFG from 6.7% to 24.1% in the United States, and 11.8% to 37.6%, 15.9% to 45.2%, and 11.2% to 26.7% in Denmark, France, and urban China, respectively (Citation5). However, the clinical implication of this increase in prevalence of IFG is still under dispute. While the International Diabetes Federation (Citation38) and the American Heart Association (Citation39) have adopted this definition, the WHO Expert Group (Citation3) and other international guideline committees (Citation4,Citation5) have retained the original definition of 110–125 mg/dL. They argued that there was no justification for a change that would greatly increase the prevalence if there were no data to support the prognostic implications (Citation40). In this study, we had sufficient power to show that prediabetes defined as IFG 110, IGT, or combined IFG 110 and/or IGT was associated with increased all-cause and cardiovascular mortality. The results were consistent across ethnicities, age groups, study characteristics, follow-up duration, and adjustment for potential confounders. However, IFG 100 was not associated with all-cause or cardiovascular mortality in overall analysis. Although one study (Citation34) showed that combined IFG 100 and/or IGT was associated with cardiovascular mortality, the risk could have been driven by IGT.
It should be noted that, although IFG 100 was not associated with all-cause or cardiovascular mortality in the overall analysis, the risk was greater in young and middle-aged males according to subgroup analyses. Taking these results together, our data suggest that the risk of all-cause and cardiovascular mortality in people with IFG may be different in males and younger people compared to that in females and older people. Larger studies with a long-term follow-up are needed to clarify the different risk associated with IFG between sexes and age groups. However, unlike our study, prior meta-analyses showed that the risk of fatal coronary heart disease associated with T2DM was higher in females than in males (Citation41,Citation42). Possible causes of these inconsistent findings may include differences in exposure (prediabetes versus T2DM) and the type of events assessed, as well as the differences in cardiovascular risk profiles and possible disparities in treatment of T2DM that favoured males.
Considering the distinct pathophysiologies of IFG and IGT, they may have a different effect with clinical events (Citation1,Citation12). It has been suggested that IGT is a stronger predictor than IFG for macrovascular complications (Citation1). However, in this study, we found that the risk of all-cause mortality, but not cardiovascular mortality, was greater in IGT than in IFG, irrespective of the FPG cut-off value for IFG. Our results are supported by a prior meta-analysis, which showed that there was no significant difference in cardiovascular incidence between IGT and IFG (Citation43). The finding that IGT is a stronger predictor than IFG for all-cause mortality, but not for cardiovascular mortality, might be largely explained by significant association between IGT and non-cardiovascular mortality, especially cancer-related mortality. An Australian study showed that IGT may increase the risk of cancer mortality, because 59.3% of non-cardiovascular deaths were attributable to malignant neoplasms (Citation16). Similarly, a study in Mauritius showed that the risk of cancer-related mortality was greater for IGT than for IFG (Citation44).
These findings have important clinical and public health implications. It has been estimated that, by the year of 2025, the number of people with prediabetes defined as IFG 110 and/or IGT will be 472 million (Citation45). Considering the great incidence and the significant association between IFG 110 and/or IGT and mortality demonstrated in our study, successful interventions in this large population could have a major public health impact. The ADA suggested that lifestyle intervention is the mainstay treatment for prediabetes in the general population. Moreover, if individuals have combined IGT and IFG, or if they are < 60 years old, or have any related risk factors (such as body mass index ≥ 35 kg/m2, dyslipidemia, hypertension, family history of diabetes, and haemoglobin A1c > 6%), metformin could be used concomitantly (Citation46). However, a prior meta-analysis showed that, although lifestyle changes and drug-based interventions were successful in delaying progression to overt T2DM, the risks of all-cause or cardiovascular mortality were not reduced, possibly because of the small sample size, inadequate follow-up duration, and the low cardiovascular risk of the participants in the individual studies (Citation47). Accordingly, long-term, large-scale studies of high-risk individuals, especially those with IGT or a combination of IGT and IFG, are urgently needed to explore the effects of drug-based interventions on all-cause and cardiovascular mortality in people with prediabetes.
The main strengths of this meta-analysis are the very large sample size, with more than 280,000 participants. Furthermore, we only included prospective observational cohort studies that reported multivariate-adjusted RRs. Prediabetes is associated with other risk factors, including obesity, metabolic syndrome, dyslipidemia, and hypertension (Citation1), which are also acknowledged as risk factors for cardiovascular disease and all-cause mortality. Most of the studies included in our meta-analysis adequately adjusted for these risk factors, and consistent results were found in subgroup analysis according to adjustment for risk factors. Adjustment for these factors reduces the possibility that confounding factors influence the association between prediabetes and the risk of mortality observed here.
This meta-analysis has some limitations. First, although the associations between IFG 100 and all-cause mortality and cardiovascular mortality were significant in men and participants with a mean age of < 55 years in the subgroup analyses, these results should be interpreted with caution because of the small numbers of studies included in these analyses. Second, the blood glucose levels reported in most of the studies were based on a single measurement; this may misclassify categories of blood glucose. Furthermore, individuals with prediabetes are more likely to progress to T2DM than those with normoglycaemia, but most of the included studies did not adjust for subsequent blood glucose levels or interventions. Nevertheless, the results indicate that, on the basis of a ‘snapshot’ blood glucose measurement, prediabetes defined as IFG 110 and/or IGT is associated with increased risk of all-cause and cardiovascular mortality. Third, the ADA recently updated its screening recommendation for prediabetes to include haemoglobin A1c of 5.7%–6.4% as another diagnostic marker (Citation48). We did not include haemoglobin A1c criteria in our study because few of the retrieved articles reported it. Future prospective cohort studies that include testing of haemoglobin A1c may provide more information on the association between prediabetes and mortality.
In conclusion, this meta-analysis showed that prediabetes, defined as IFG 110, IGT, or combined IFG 110 and/or IGT were associated with increased risk of all-cause and cardiovascular mortality. However, the current ADA definition of IFG of 100–125 mg/dL was not associated with increased risk of all-cause or cardiovascular mortality. Unlike in people with T2DM, men with IFG may have a higher risk of all-cause and cardiovascular mortality than women. Finally, the risk of all-cause mortality, but not cardiovascular mortality, was greater for IGT than for IFG. These results reaffirmed the importance of screening for prediabetes using the WHO criteria, as well as the inhomogeneity of prediabetes subcategories. This information is important to health professionals and those engaged in the primary prevention of T2DM.
PRISMA 2009 Checklist 2.1.1
Download PDF (257 KB)Acknowledgements
The authors thank Dr Kokubo Y, from National Cardiovascular Centre, Suita Osaka, Japan, for clarification of data from the Suita Study.
Yi Huang and Xiaoyan Cai contributed equally to this work.
Declaration of interest: The authors report no conflicts of interest. No funding and economic support has been received for this study.
References
- DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108:3B–24B.
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20.
- World Health Organization (WHO) Consultation. Definition and diagnosis of diabetes and intermediate hyperglycaemia. 2006. Available at: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf.
- Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34: 3035–87.
- Forouhi NG, Balkau B, Borch-Johnsen K, Dekker J, Glumer C, Qiao Q, et al. The threshold for diagnosing impaired fasting glucose: a position statement by the European Diabetes Epidemiology Group. Diabetologia. 2006;49:822–7.
- Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;58:140–6.
- Laukkanen JA, Makikallio TH, Ronkainen K, Karppi J, Kurl S. Impaired fasting plasma glucose and type 2 diabetes are related to the risk of out-of-hospital sudden cardiac death and all-cause mortality. Diabetes Care. 2013;36:1166–71.
- Pankow JS, Kwan DK, Duncan BB, Schmidt MI, Couper DJ, Golden S, et al. Cardiometabolic risk in impaired fasting glucose and impaired glucose tolerance: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30:325–31.
- Wang J, Ruotsalainen S, Moilanen L, Lepisto P, Laakso M, Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: a 13- year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28:857–64.
- Ma SH, Park BY, Yang JJ, Jung EJ, Yeo Y, Whang Y, et al. Interaction of body mass index and diabetes as modifiers of cardiovascular mortality in a cohort study. J Prev Med Public Health. 2012;45:394–401.
- Deedwania P, Patel K, Fonarow GC, Desai RV, Zhang Y, Feller MA, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol. 2013;168:3616–22.
- Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52:1714–23.
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405.
- Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 2001;24:1397–402.
- Rodriguez BL, Abbott RD, Fujimoto W, Waitzfelder B, Chen R, Masaki K, et al. The American Diabetes Association and World Health Organization classifications for diabetes: their impact on diabetes prevalence and total and cardiovascular disease mortality in elderly Japanese-American men. Diabetes Care. 2002;25:951–5.
- Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151–7.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97.
- Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344:e3564.
- Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Stengard JH, Tuomilehto J, Pekkanen J, Kivinen P, Kaarsalo E, Nissinen A, et al. Diabetes mellitus, impaired glucose tolerance and mortality among elderly men: the Finnish cohorts of the Seven Countries Study. Diabetologia. 1992;35:760–5.
- Mazza A, Pessina AC, Pavei A, Scarpa R, Tikhonoff V, Casiglia E. Predictors of stroke mortality in elderly people from the general population. The CArdiovascular STudy in the ELderly. Eur J Epidemiol. 2001;17:1097–104.
- Henry P, Thomas F, Benetos A, Guize L. Impaired fasting glucose, blood pressure and cardiovascular disease mortality. Hypertension. 2002;40:458–63.
- Hu G. Gender difference in all-cause and cardiovascular mortality related to hyperglycaemia and newly-diagnosed diabetes. Diabetologia. 2003;46:608–17.
- Lu W, Resnick HE, Jain AK, Adams-Campbell LL, Jablonski KA, Gottlieb AM, et al. Effects of isolated post-challenge hyperglycemia on mortality in American Indians: the Strong Heart Study. Ann Epidemiol. 2003;13:182–8.
- Ma S, Cutter J, Tan CE, Chew SK, Tai ES. Associations of diabetes mellitus and ethnicity with mortality in a multiethnic Asian population: data from the 1992 Singapore National Health Survey. Am J Epidemiol. 2003;158:543–52.
- Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–7.
- Nakagami T. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47:385–94.
- Hiltunen L. Ten-year mortality and glucose tolerance status in an elderly Finnish population. Diabetes Res Clin Pract. 2005;69:81–7.
- Wild SH, Smith FB, Lee AJ, Fowkes FG. Criteria for previously undiagnosed diabetes and risk of mortality: 15-year follow-up of the Edinburgh Artery Study cohort. Diabet Med. 2005;22:490–6.
- Tsai SP, Wen CP, Chan HT, Chiang PH, Tsai MK, Cheng TY. The effects of pre-disease risk factors within metabolic syndrome on all-cause and cardiovascular disease mortality. Diabetes Res Clin Pract. 2008;82: 148–56.
- Valdes S, Botas P, Delgado E, Diaz CF. Mortality risk in Spanish adults with diagnosed diabetes, undiagnosed diabetes or pre-diabetes. The Asturias study 1998–2004. Rev Esp Cardiol. 2009;62:528–34.
- Kokubo Y, Makino H, Okamura T, Miyamoto Y, Watanabe M, Higashiyama A, et al. The relationship of oral glucose tolerance test with all-cause and stroke mortality in a general Urban Japanese cohort: The Suita Study. Stroke. 2010;41:e241.
- Magliano DJ, Soderberg S, Zimmet PZ, Cartensen B, Balkau B, Pauvaday V, et al. Mortality, all-cause and cardiovascular disease, over 15 years in multiethnic Mauritius: impact of diabetes and intermediate forms of glucose tolerance. Diabetes Care. 2010;33:1983–9.
- Kowall B, Rathmann W, Heier M, Giani G, Peters A, Thorand B, et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol. 2011;26:637–45.
- Madssen E, Vatten L, Nilsen TI, Midthjell K, Wiseth R, Dale AC. Abnormal glucose regulation and gender-specific risk of fatal coronary artery disease in the HUNT 1 study. Scand Cardiovasc J. 2012;46:219–25.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
- De Caterina R, Madonna R. Impaired fasting plasma glucose and long-term cardiovascular risk: still a foggy relationship. Eur Heart J. 2010;31:1159–62.
- Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23:962–8.
- Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8.
- Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–17.
- Harding JL, Soderberg S, Shaw JE, Zimmet PZ, Pauvaday V, Kowlessur S, et al. All-cause cancer mortality over 15 years in multi-ethnic Mauritius: the impact of diabetes and intermediate forms of glucose tolerance. Int J Cancer. 2012;131:2385–93.
- Magalhaes ME, Cavalcanti BA, Cavalcanti S. Could pre-diabetes be considered a clinical condition? Opinions from an endocrinologist and a cardiologist. Diabetol Metab Syndr. 2010;2:2.
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9.
- Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil. 2011;18:813–23.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36:S67–74.