Abstract
MicroRNAs are small non-coding RNAs that bind to multiple target mRNAs to control gene expression post-transcriptionally by inhibiting translation. In mammalian cells, microRNAs play important roles in a diverse array of cellular processes (e.g. cell proliferation and differentiation). However, alterations in their levels may compromise cellular function, predisposing to disease. In this review, we discuss microRNAs that have been linked with pathogenesis of asthma and propose functional roles in the regulation of disease. MicroRNAs have the potential to be biomarkers for asthma and provide the platform for the development of new classes of therapeutic compounds.
Key words::
Key messages
MicroRNAs levels are altered in asthmatic tissue.
MicroRNAs regulate inflammation in asthma.
Allergic asthma is an inflammatory disorder of the airways characterized by airway hyper-responsiveness (AHR), mucus hypersecretion, eosinophil recruitment, and remodeling of the airways, including smooth muscle hypertrophy/hyperplasia and thickening of the reticular basement membrane. CD4+ T-helper type 2 lymphocytes (Th2 cells) are thought to play a central role in regulating disease in allergic asthma. Levels of the Th2 cytokines interleukin (IL)-4, IL-5, and IL-13 are elevated in respiratory tissues and secretions. However, typical allergic asthma represents one form of what is now recognized as a heterogeneous disease, and the inflammatory response may differ in other clinical variants (Citation1). Currently, the different patterns of disease, so-called endotypes, are characterized primarily on the basis of the percentage of different populations of inflammatory cells in sputum, with distinct molecular biomarkers yet to be discovered (Citation2). The inflammatory cell profiles of sputum allow classification of asthma as eosinophilic, neutrophilic, granulocytic (mixed eosinophilic and neutrophilic), or paucigranulocytic (an absence of granulocytes in the sputum) (Citation3). It is likely that multiple mechanisms underlie these phenotypes. In severe asthma, inflammation of the airways and clinical manifestations persist in spite of treatment (Citation4).
MicroRNAs (miRNAs) are small non-coding RNAs that are approximately 22 nucleotides in length. MiRNAs control cellular function by inhibiting translation, either by destabilizing mRNA transcripts or repressing their translation. The mechanisms of action of miRNAs are reviewed elsewhere (Citation5). In immune cells, miRNAs control differentiation and function, and thus can regulate inflammation. In other settings, detecting or assessing miRNA expression levels can have diagnostic and/or prognostic value, especially for malignant disease (Citation6). This suggests that miRNAs may be useful biomarkers for phenotyping respiratory diseases such as asthma, or for assessing underlying disease activity.
MiRNAs as potential biomarkers for asthma
It has been suggested repeatedly that miRNAs may be useful as biomarkers of human diseases including asthma. MiRNAs have been detected in easily accessible body fluids such as saliva, plasma, serum, and even in exhaled breath condensate (Citation7–9). Within these samples, miRNAs are most commonly associated with argonaute-2, a key protein in the RNA-induced silencing complex that also acts to protect the miRNAs from degradation by RNAses (Citation10–12). Further protection is often conveyed by enclosure in exosomes, which are 30–100 nm nanovesicles that surround the complex in a phospholipid bilayer (Citation13–15).
Although initial studies failed to find significant differences between miRNA expression in bronchial biopsies from asthmatics and healthy controls, the numbers of patients were small (Citation16). However, more recent studies have identified distinct miRNA profiles in brushings of bronchial epithelial cells (BECs) (Citation17,Citation18).
Exosomes isolated from bronchoalveolar lavage (BAL) samples have distinct miRNA profiles in asthmatics compared to healthy subjects. Significant differences were reported for a number of miRNAs, predominantly of the let-7 and miR-200 families, with let-7a, miR-21, miR-24, miR-26a, miR-99a, and miR-200c being decreased, while miR-658 and miR-1268 were increased in asthmatic patients (Citation19).
Interestingly, all of these miRNAs are likely to be biologically relevant. The let-7 family of miRNAs has previously been shown to regulate IL-13 production in human T cells, while in a murine model of asthma, intranasal delivery of let-7 reduced IL-13 levels in the lungs and alleviated both AHR and airway inflammation (Citation20). Thus decreased levels of let-7 could potentially increase the severity of asthma. Similarly, miR-21 is a negative regulator of toll-like receptor 4 (TLR4) signaling and of the activation of nuclear factor kappa B (Citation21). The miR-200 family of miRNAs regulates epithelial to mesenchymal transition and decreases expression of E-cadherin (Citation22). This adhesion molecule is diminished in asthmatic airways (Citation23), and in vitro studies have shown that reduced levels of E-cadherin are associated with increases in chemokine (C-C motif) ligand 17, a Th2 cell chemoattractant (Citation24).
MiRNA profiles in the serum also have the potential to be informative in asthma. The reported profile of differentially expressed miRNAs in serum also identified the let-7 family, with let-7a, let-7d, and miR-26a being decreased and miR-1248 being increased in asthmatics (Citation25). These differences were related to potential effects on the expression of IL-5, a cytokine strongly linked to the pathogenesis of allergic asthma.
What remains unclear is whether distinct asthma endotypes can be characterized on the basis of miRNAs profiles from e.g. blood, sputum, or exhaled breath condensate. If this proves to be the case, which is plausible given that miRNA expression differs between various populations of inflammatory cells, such information could help to predict responsiveness to treatment.
MiRNAs in bronchial epithelial cells
Airway epithelium is the first line of cellular defense against pathogens and forms an important interface between host and environment. BECs express pattern recognition receptors and release cytokines, chemokines, and antimicrobial peptides upon exposure to various external stimuli (e.g. infectious agent, allergens, and airborne particulate matter) (Citation26).
MiRNAs are differentially expressed in BECs of asthmatics, with evidence that the miR-34/449 family of miRNAs is down-regulated (Citation18). Stimulation of human bronchial BECs with IL-13 also decreases miR-34/449 family members, in a time- and dose-dependent manner, suggesting that their expression is controlled by a Th2 immune response (Citation18). In asthmatic BECs, IL-13 plays an important role in proliferation and repair, a key event for airway remodeling. Further, members of the miR-34/449 family have previously been implicated in regulating proliferation of BECs (Citation27,Citation28). In another study, induction of miR-146a expression following cytokine stimulation protected human bronchial BECs from cytokine-induced apoptosis and promoted cell proliferation (Citation29). Thus it is possible that miRNAs may regulate epithelial changes that contribute to airway remodeling in asthma. It is noteworthy that increased epithelial thickness correlates with decreased lung function and disease severity in asthmatics (Citation30). However, the functional roles of miR-146a and the miR-34/449 family in established asthma remain to be elucidated.
Altered miRNAs expression in airway epithelium may also be important in the initial induction of asthma. For example, in a murine model of the onset of allergic asthma in childhood, we have shown that exposure to ambient particulate matter contributed to sensitization via the airways and thus to the pathogenesis of disease (Citation31). Exposure of bronchial BECs to ambient particulate matter induces proinflammatory mediators such as IL-1β, IL-6, leukemia inhibitory factor, and granulocyte-macrophage colony-stimulating factor (Citation32). Both ambient particulate matter and diesel exhaust particles also up-regulate miR-375 in human BECs (Citation33). Interestingly, overexpression of miR-375 with a synthetic mimic increases the expression of thymic stromal lymphopoietin (TSLP). A polymorphism in the TSLP promoter leads to its increased expression and increases asthma susceptibility in both children and adults (Citation34).
MiRNAs in human bronchial airway smooth muscle cells
One of the major characteristics of asthma is AHR. This excessive narrowing of airways in response to non-specific challenge is related to airway remodeling and primarily to the response of bronchial smooth muscle. Airway smooth muscle (ASM) cells obtained from asthmatics exhibit increased proliferation in culture (Citation35).
Treatment of human bronchial ASM cells with IL-13 decreased miR-133a expression and increased its target protein Rho kinase A (RhoA) (Citation36). RhoA regulates ASM contraction and proliferation, and targeting RhoA using Rho-kinase inhibitors blocks smooth muscle contraction and reduces AHR following antigen challenges (Citation37). Thus it is possible that miR-133a may be modulated to suppress the function of RhoA in bronchial ASM to control AHR.
Exposure of ASM cells to a miR-221 mimic increased proliferation and IL-6 release, with more marked effects in ASM cultured from bronchial biopsies of severe asthmatics as compared to non-severe asthmatics (Citation38). Interestingly, miR-221 was also increased in mast cells after activation with stem cell factor. Overexpression of miR-221 in mast cells led to an increase in cytokine production and degranulation in response to stimulation with IgE-antigen complexes (Citation39). In this context, it is of interest that miR-221 is up-regulated both in asthmatic children and in mouse models of asthma (Citation40). Collectively, these reports indicate that overexpression of miR-221 in ASM could potentially contribute to the pathogenesis of asthma.
MiRNAs in animal studies of allergic airways disease
A potential role for miRNAs in the regulation of allergic asthma is also supported by studies employing various mouse models of disease. House dust mite (HDM) is a common allergen to which asthmatics can be sensitized, and it is widely employed to model disease in mice by directly instilling HDM extract into the lungs. This induces the differentiation of allergen-specific Th2 cells, leading to the development of allergic airways disease (Citation41). The presence of TLR ligands in HDM, in particular those activating TLR4, contributes to the development of disease through activation of the TLR4-MyD88 (myeloid differentiation primary response 88) signaling pathway (Citation41).
We showed that exposure of wild-type mice to HDM resulted in increased expression of a number of miRNAs in the airway wall (Citation42). Up-regulated expression of one candidate miRNA, miR-126, was dependent on both TLR4 and MyD88, linking expression with activation of this innate immune pathway and the induction of allergic disease. The function of miRNAs can be inhibited using antagomirs, single-stranded RNA molecules complementary to their corresponding mature miRNAs (described in detail in the following section). Antagomir-mediated inhibition of miR-126 in the lung abolished HDM-induced AHR, attenuated recruitment of both eosinophils and neutrophils, and reduced mucus hypersecretion (Citation42). Both IL-5 and IL-13 were suppressed with antagomir treatment, suggesting suppression of the effector function of Th2 cells (Citation42). MiR-126 has been shown to target a variety of other mRNAs (Citation43–45). Therefore, further studies are necessary to elucidate the contribution of both known and as yet unknown targets in allergic inflammation. Importantly, this study demonstrated that inhibition of a specific miRNA could attenuate the development of allergic disease. This highlights the potential of miRNAs as novel targets for the treatment of asthma.
We have further shown that let-7b, miR-21, and miR-145 are also significantly increased in the airway wall following exposure to HDM. Using antagomirs, we showed that miR-145 played a proinflammatory role in the onset of allergic airways disease (Citation46). Inhibition of let-7b and miR-21 had no discernible effects in this mouse model of disease (Citation46). Similar to miR-126, antagomir inhibition of miR-145 could attenuate the production of IL-5 and IL-13 by Th2 cells, eosinophil recruitment, mucus hypersecretion, and the development of AHR (Citation46). To highlight the potential of antagomir treatment as a novel therapy, we compared antagomir treatment with systemic glucocorticoid therapy in our HDM-induced model of allergy, demonstrating a comparable therapeutic effect (Citation46). MiR-145 has also been shown to target a variety of mRNA transcripts, suggesting that, like miR-126, miR-145 inhibits allergic inflammation by affecting multiple pathways (Citation47–51).
The consequences of blocking miR-126 function were less dramatic in a mouse model of chronic asthma (Citation52). This suggests that the regulation of allergic airways disease by miRNAs may be dependent on the stage of the disease process and the temporal expression of specific miRNAs (Citation52). Antagomir treatment inhibited eosinophil recruitment, but did not alter the accumulation of other chronic inflammatory cells or remodeling of the airway wall. Thus miR-126 is perhaps more able to regulate acute inflammatory events but has limited effects on chronic aspects of disease. However, it is worth pointing out that this last-mentioned study involved a different TLR4-independent antigen, ovalbumin (OVA), and a different mode of sensitization, with the protein initially being administered systemically with an adjuvant and only subsequently via the airways. This was in contrast to the acute model, in which HDM was directly introduced into the lungs and activated TLR4-dependent innate immune pathways to induce sensitization and allergic disease (Citation41).
We have recently identified a limited number of miRNAs that are markedly up- or down-regulated in a mouse model of childhood allergic asthma (Citation53). Many miRNAs that were altered following early-life viral infection and allergen sensitization were further changed following chronic challenge with the sensitizing antigen (Citation53). Targets of these miRNAs included genes involved in immune or inflammatory responses (e.g. GATA binding protein 3 and kit ligand) and in tissue remodeling (e.g. insulin-like growth factor 1, transforming growth factor beta receptor 1), as well as genes for various transcription factors and signaling proteins (Citation53). Notably, the identified changes in miRNA expression were mostly different to those observed in acute models of allergic asthma, supporting the idea of temporal and stage-specific expression of miRNAs.
Other studies have also identified miRNAs that may regulate allergic airways inflammation. Lu et al. showed an important role for miR-21 in the development of asthma (Citation54). Amongst 21 differentially expressed miRNAs that were identified in three different models of allergic airways inflammation, miR-21 was increased and miR-1 was decreased across all three models (Citation54). Target prediction analysis suggested that miR-21 may directly regulate a number of IL-13-regulated transcripts, most notably IL-12p35 (Citation54). In a subsequent study, the authors showed that miR-21-deficient (gene-targeted) mice had reduced eosinophilic inflammation in the lungs and increased levels of interferon (IFN)-γ (Citation55). Collectively, the data suggested that miR-21 regulates IL-12 production in antigen-presenting cells, in particular dendritic cells, which in turn alters the degree of polarization of the Th cells in allergic airways disease. In the absence of miR-21, Th cell responses are skewed towards IFN-γ-producing Th1 cells with a reduction in IL-4-producing Th2 cells (Citation55). It should be noted, however, that the above models were independent of proinflammatory TLR4 signaling, a contributing pathway in some forms of allergic asthma. Furthermore, TLR4 has since been shown to be inhibited by miR-21 directly (Citation21). Indeed, silencing of miR-21 in a TLR4-dependent, HDM-induced model of allergic airways disease showed no difference in disease state (Citation45). This implies a degree of balance between pro- and anti- inflammatory miR-21 signaling in a complex allergic milieu. Together these studies identify miR-21 as a key miRNA in the regulation of multiple aspects of allergic responses. Further investigations into the ability of miR-21 to target both pro- and anti-inflammatory signaling pathways are required to understand fully the role of miR-21 in asthma.
Recently, a role for miR-1 in asthma was further defined in an animal model of allergic inflammation (Citation56). This study demonstrated that vascular endothelial growth factor A (VEGF) contributes to Th2 inflammation by down-regulating miR-1 in the endothelium, thereby increasing the expression of myeloproliferative leukemia virus oncogene and its downstream target, P-selectin (Citation56). This in turn promoted recruitment of activated T cells and subsequent eosinophilic inflammation, together with Th2 cytokine production, mucus metaplasia, and AHR (Citation56). Similarly, miR-155 has been shown to regulate inflammation in a mouse model of allergic airways disease by modulating Th2 responses. Mice deficient in miR-155 had reduced eosinophil numbers, mucus secretion, and Th2 cell numbers and cytokine levels in the lungs following OVA allergen exposure (Citation57). Interestingly, miR-155 also plays a central role in determining human macrophage phenotypes by targeting IL-13Rα1 protein (Citation58).
Several additional studies have profiled miRNA expression in murine models of asthma, identifying various miRNAs, such as miR-29b, -29c, -146b, -223, -483, -574-5p, -672, and -690, to be altered in disease (Citation59,Citation60). Other noteworthy miRNAs that have been shown to have a regulatory role in murine models of allergic airways disease include miR-106a and miR-221 (Citation61–63).
Methods and therapeutic potential of modulating miRNA molecules
While miRNAs are likely to play important roles in the regulation of the immune system and thus potentially in allergic diseases such as asthma, the manipulation of these RNA molecules for therapeutic purposes remains a challenge. Their small size and changing expression patterns during the course of disease may make it difficult to direct specific treatments to the tissues of interest. This is critical, because systemic treatment risks complications due to off-target effects. Despite this challenge, considerable effort has been invested in developing novel techniques and methods of delivery to achieve specific targeting of miRNAs function. The unique ability of these molecules to act as master regulators of disease pathways, through targeting multiple mRNAs within a network, potentially makes them ideal candidates for globally suppressing aberrant responses.
Cellular levels of miRNAs can either be decreased using specific inhibitors, or increased by employing miRNA mimetics (). The in vivo delivery of these miRNA therapeutics is summarized in .
Figure 1. Targeting miRNAs with therapeutic molecules. (A) Chemically modified AONs with a sequence complementary to the miRNA of interest. Antagomirs are 3’ cholesterol conjugated, 2’-O-Me-modified oligonucleotides with terminal phosphorothioate linkages for increased cellular uptake and stability in vivo. LNA/DNA mixmers are AONs that inhibit miRNA function by targeting the 5’ region of the miRNA. (B) Liposomes are vesicles with a lipid bilayer that are used to deliver miRNA mimics in vivo. Liposomes can be coated with polyethylene glycol (PEG) to reduce recognition by macrophages. Monoclonal antibodies can be covalently coupled to liposomes for targeted delivery.
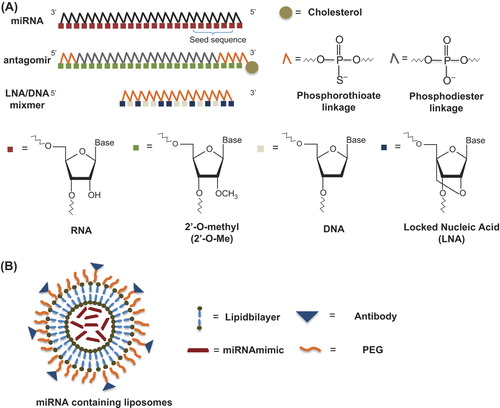
Table I. In vivo administration of miRNA therapeutics.
One common method to inhibit miRNA function in vivo is the delivery of oligonucleotides with a sequence complementary to the miRNAs of interest. These antisense oligonucleotides (AONs) commonly have stabilizing modifications that increase their half-life (e.g. 2’-O-methyl-modified oligo-ribonucleotides) (Citation64,Citation65) and can also contain cholesterol tags to facilitate uptake into target cells (Citation66). These inhibitors are often called antagomirs and have been widely used to effectively block miRNA function in mice (Citation42,Citation46,Citation67,Citation68). Antagomir-mediated miRNA-silencing is dose-dependent and can last for several weeks, making antagomirs powerful tools specifically to target miRNA function in vivo (Citation64). A particularly relevant example of this is the therapeutic silencing of miR-122 in primates infected with hepatitis C virus (HCV) where viremia was effectively reduced following treatment with a locked nucleic acid (LNA)-modified complementary inhibitor to miR-122 (also known as SPC3649 or miravirsen) (Citation69). Importantly, the effect was long-lasting and showed no indication of viral resistance or side-effects in treated chimpanzees (Citation69). This miRNA is currently in phase II clinical trials and miR-122 may thus be the first miRNA that serves as a therapeutic target in human disease.
‘MiRNA sponges’ have also been used efficiently to achieve stable knock-down of miRNAs in vivo (Citation70). This technology requires the delivery of a ‘miRNA sponge’-encoding transgene into the cell (Citation71). The expressed ‘miRNA sponge’ is an engineered RNA molecule that contains multiple repeats of a miRNA target site and generates competing miRNA binding sites, thus abrogating inhibition of endogenous targets (Citation71). Conversely, the overexpression of miRNAs in vivo has been achieved by intravenous injection of liposome- encapsulated synthetic miRNA mimics (Citation72). Liposomes are vesicles with a lipid bilayer that can encapsulate hydrophilic drugs such as miRNA mimics in the central aqueous cavity. Liposome surfaces can be coated with biocompatible polymers such as PEG to reduce recognition by macrophages and prolong the circulation time of the liposomes (Citation73). For targeted delivery, monoclonal antibody can be attached to PEGylated liposomes to increase specificity and effectiveness of the drug delivery to target cells (Citation74).
Clinical application of miRNAs
In the last decade, increasing evidence suggests that miRNAs are dysregulated during the progression of a variety of diseases. Currently, several pharmaceutical companies are investigating opportunities to develop new diagnostic, prognostic, and therapeutic applications involving miRNAs. The tested miRNA-related therapies aim to treat various diseases such as HCV, cancer, heart failure, fibrosis, and atherosclerosis ().
Table II. Companies developing miRNA-related therapies.
Considerable interest and effort is put into the development of miRNA-related therapeutics, in particular AONs (Citation81). However, our knowledge on the uptake, pharmacokinetic properties, and safety of AONs is limited, and to date only a few AONs, such as VitraveneTM (Isis pharmaceuticals, San Diego, USA), an AON that inhibits human cytomegalovirus replication by targeting coding region of the major immediate-early gene of the virus, or KynamroTM (Genzyme, Boston, USA), an AON that reduces low-density lipoproteins level by targeting the mRNA for apolipoprotein B, have been granted FDA approval (Citation82,Citation83). Whilst this is encouraging, a miRNA- targeting AON is yet to be approved for clinical use. The best-studied example of a miRNA-related therapeutic to date is miravirsen, a small LNA-based inhibitor of miR-122 (Citation69). Intravenously administered miravirsen could specifically reduce the levels of free miR-122 over 300-fold in the liver of primates and was effective up to 8 weeks after the last treatment (Citation69). The study further determined a terminal plasma half-life of 20 days, good pharmacokinetic properties, and importantly failed to detect adverse side-effects over prolonged in vivo administration (Citation69). This suggests that miravirsen may serve as a novel treatment for miR-122-related liver diseases such as HCV (Citation84). To date, phase I and II clinical trials have shown promising results using miravirsen, but there are indications for potential nephrotoxicity (Citation84–86). Therefore, further clinical trials are needed to determine its effectiveness and safety for use in patients. This is highlighted by recent findings that miravirsen may not only target mature miR-122 but can also bind to pri- and pre-mir-122 (Citation87).
How might targeting relevant miRNAs contribute to the treatment of asthma? Current therapy for mild or moderate asthma is based on inhaled corticosteroids in combination with inhaled long-acting beta-agonists and is generally satisfactory. However, a minority of patients has persistent uncontrolled disease and numerous exacerbations, despite optimal therapy (Citation4). These individuals account for most of the health care burden of asthma (Citation88). Could miRNAs-directed therapy be useful for these patients e.g. by reducing background inflammation and thus the likelihood of developing exacerbations? Or, given the role of miRNAs in remodeling, could such therapy be useful in preventing the long-term decline in lung function that is related to structural remodeling of the airways? Because current treatments have limited value in these situations, new approaches are clearly warranted, and further investigation of how miRNAs regulate underlying processes in asthma may lead to the development of new classes of therapeutic compounds.
Acknowledgements
Hock L. Tay, Maximilian Plank, and Adam Collison contributed equally to this work.
Declaration of interest: The authors report no conflicts of interest.
References
- Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127:355–60.
- Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–72.
- Nair P, Dasgupta A, Brightling CE, Chung KF. How to diagnose and phenotype asthma. Clin Chest Med. 2012;33:445–57.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Resp J. 2014;43:343–73.
- Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13: 271–82.
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–801.
- Sinha A, Yadav A, Charkraborty S, Kabra S, Lodha R, Kumar M, et al. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. J Allergy Clin Immunol. 2013;132:219–22.
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
- Zen K, Zhang C-Y. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32: 326–48.
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8.
- Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39: 7223–33.
- Starczynowski DT, Li L, Zhu D, Huang L, Zhang J, Bian Z, et al. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One. 2012;7:e46957.
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9:654–9.
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33.
- Lu TX, Rothenberg ME. Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013;132:3–13.
- Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, et al. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One. 2009;4:e5889.
- Jardim MJ, Dailey L, Silbajoris R, Diaz-Sanchez D. Distinct microRNA expression in human airway cells of asthmatic donors identifies a novel asthma-associated gene. Am J Respir Cell Mol Biol. 2012;47:536–42.
- Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–74.
- Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013; 131:894–903.
- Kumar M, Ahmad T, Sharma A, Mabalirajan U, Kulshreshtha A, Agrawal A, et al. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol. 2011;128: 1077–85 e1–10.
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–7.
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10: 593–601.
- de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105–12.
- Heijink IH, Kies PM, Kauffman HF, Postma DS, van Oosterhout AJ, Vellenga E. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol. 2007;178:7678–85.
- Panganiban RP, Pinkerton MH, Maru SY, Jefferson SJ, Roff AN, Ishmael FT. Differential microRNA expression in asthma and the role of miR-1248 in regulation of IL-5. Am J Clin Exp Immunol. 2012; 1:154–65.
- Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92.
- Kasinski AL, Slack FJ. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 2012;72:5576–87.
- Semlali A, Jacques E, Koussih L, Gounni AS, Chakir J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol. 2010;125:844–50.
- Liu X, Nelson A, Wang X, Kanaji N, Kim M, Sato T, et al. MicroRNA-146a modulates human bronchial epithelial cell survival in response to the cytokine-induced apoptosis. Biochem Biophys Res Commun. 2009;380:177–82.
- Cohen L, E X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, et al. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med. 2007;176:138–45.
- Herbert C, Siegle JS, Shadie AM, Nikolaysen S, Garthwaite L, Hansbro NG, et al. Development of asthmatic inflammation in mice following early-life exposure to ambient environmental particulates and chronic allergen challenge. Dis Model Mech. 2013;6:479–88.
- Fujii T, Hayashi S, Hogg JC, Vincent R, Van Eeden SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;25:265–71.
- Bleck B, Grunig G, Chiu A, Liu M, Gordon T, Kazeros A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol. 2013;190:3757–63.
- Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44:787–93.
- Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–7.
- Chiba Y, Misawa M. MicroRNAs and their therapeutic potential for human diseases: MiR-133a and bronchial smooth muscle hyperresponsiveness in asthma. J Pharmacol Sci. 2010;114:264–8.
- Kume H. RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem. 2008;15:2876–85.
- Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol. 2014;50:7–17.
- Mayoral RJ, Deho L, Rusca N, Bartonicek N, Saini HK, Enright AJ, et al. MiR-221 influences effector functions and actin cytoskeleton in mast cells. PLoS One. 2011;6:e26133.
- Liu F, Qin HB, Xu B, Zhou H, Zhao DY. Profiling of miRNAs in pediatric asthma: upregulation of miRNA-221 and miRNA-485-3p. Mol Med Rep. 2012;6:1178–82.
- Phipps S, Lam CE, Kaiko GE, Foo SY, Collison A, Mattes J, et al. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 responses. Am J Respir Crit Care Med. 2009;179: 883–93.
- Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;106:18704–9.
- Oglesby IK, Bray IM, Chotirmall SH, Stallings RL, O’Neill SJ, McElvaney NG, et al. miR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol. 2010; 184:1702–9.
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008; 15:272–84.
- Otsubo T, Akiyama Y, Hashimoto Y, Shimada S, Goto K, Yuasa Y. MicroRNA-126 inhibits SOX2 expression and contributes to gastric carcinogenesis. PLoS One. 2011;6:e16617.
- Collison A, Mattes J, Plank M, Foster PS. Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J Allergy Clin Immunol. 2011;128:160–7 e4.
- Riggi N, Suva ML, De Vito C, Provero P, Stehle JC, Baumer K, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–32.
- Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58.
- Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–87.
- Gotte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M, et al. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569–80.
- Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, de Angelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem. 2007;282:32582–90.
- Collison A, Herbert C, Siegle JS, Mattes J, Foster PS, Kumar RK. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm Med. 2011;11:29.
- Collison A, Siegle JS, Hansbro NG, Kwok CT, Herbert C, Mattes J, et al. Epigenetic changes associated with disease progression in a mouse model of childhood allergic asthma. Dis Model Mech. 2013;6:993–1000.
- Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182:4994–5002.
- Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–73.
- Takyar S, Vasavada H, Zhang JG, Ahangari F, Niu N, Liu Q, et al. VEGF controls lung Th2 inflammation via the miR-1-Mpl (myeloproliferative leukemia virus oncogene)-P-selectin axis. J Exp Med. 2013;210: 1993–2010.
- Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M, et al. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014;133:1429–38, 38 e1–7.
- Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem. 2011;286:1786–94.
- Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, et al. MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One. 2011; 6:e16509.
- Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C, et al. Proinflammatory role for let-7 microRNAS in experimental asthma. J Biol Chem. 2010;285:30139–49.
- Sharma A, Kumar M, Aich J, Hariharan M, Brahmachari SK, Agrawal A, et al. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc Natl Acad Sci U S A. 2009;106:5761–6.
- Sharma A, Kumar M, Ahmad T, Mabalirajan U, Aich J, Agrawal A, et al. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J Appl Physiol. 2012;113:459–64.
- Qin HB, Xu B, Mei JJ, Li D, Liu JJ, Zhao DY, et al. Inhibition of miRNA-221 suppresses the airway inflammation in asthma. Inflammation. 2012;35:1595–9.
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9.
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98.
- Ameres SL, Horwich MD, Hung JH, Xu J, Ghildiyal M, Weng Z, et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010;328:1534–9.
- Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–9.
- Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007; 129:147–61.
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201.
- Gentner B, Schira G, Giustacchini A, Amendola M, Brown BD, Ponzoni M, et al. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat Methods. 2009;6:63–6.
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6.
- Kelnar K, Peltier HJ, Leatherbury N, Stoudemire J, Bader AG. Quantification of therapeutic miRNA mimics in whole blood from nonhuman primates. Anal Chem. 2014;86:1534–42.
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–7.
- Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, et al. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74:95–113.
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21: 185–91.
- da Costa Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010;12:1220–7.
- Speranza MC, Frattini V, Pisati F, Kapetis D, Porrati P, Eoli M, et al. NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget. 2012;3:723–34.
- Garchow BG, Bartulos Encinas O, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, et al. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3: 605–15.
- Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr-/- mice—brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–7.
- Di Martino MT, Leone E, Amodio N, Foresta U, Lionetti M, Pitari MR, et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clin Cancer Res. 2012;18:6260–70.
- Sharma VK, Rungta P, Prasad AK. Nucleic acid therapeutics: basic concepts and recent developments. RSC Advances. 2014;4:16618–31.
- Mulamba GB, Hu A, Azad RF, Anderson KP, Coen DM. Human cytomegalovirus mutant with sequence-dependent resistance to the phosphorothioate oligonucleotide fomivirsen (ISIS 2922). Antimicrob Agents Chemother. 1998;42:971–3.
- McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One. 2012;7: e49006.
- Qiu Z, Dai Y. Roadmap of miR-122-related clinical application from bench to bedside. Expert Opin Investig Drugs. 2014;23:347–55.
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94.
- Sanchez-Nino MD, Ortiz A. HCV infection and miravirsen. N Engl J Med. 2013;369:877–8.
- Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609–21.
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24.

