Abstract
Several studies reported on the association between antiphospholipid syndrome (APS) and venous thrombosis. In contrast, little is known about cardiovascular (CV) risk in APS. We performed a meta-analysis on the impact of APS on major markers of CV risk.
Studies on the relationship between APS and common carotid artery intima-media thickness (CCA-IMT), internal carotid artery IMT (ICA-IMT), carotid bifurcation IMT (BIF-IMT), prevalence of carotid plaques, flow-mediated dilation (FMD), nitrate-mediated dilation (NMD), and ankle-brachial index (ABI) were systematically searched in PubMed, Web of Science, Scopus, and EMBASE databases. Twenty case-control studies (668 cases, 678 controls) were included. Compared to controls, APS patients showed a higher CCA-IMT (mean difference [MD] 0.11 mm; 95% CI 0.07, 0.14), ICA-IMT (MD 0.08 mm; 95% CI 0.05, 0.11), BIF-IMT (MD 0.09 mm; 95% CI 0.06, 0.12) and a higher frequency of carotid plaques (OR 3.87; 95% CI 1.61, 9.31). Moreover, a lower FMD was found in APS subjects than in controls (MD –4.49%; 95% CI –6.20, –2.78), with no differences in NMD (MD –1.80%; 95% CI –4.01, 0.42). Finally, an increased prevalence of pathological ABI was found in APS patients compared to controls (OR 7.26; 95% CI 1.77, 29.71).
Despite heterogeneity among studies, APS appears significantly associated with markers of subclinical atherosclerosis and CV risk. These findings can be useful to plan adequate prevention strategies and therapeutic approaches.
Key messages
Antiphospholipid syndrome (APS) is recognized as a major acquired thrombophilic condition associated with an increased risk of both venous and arterial events.
The association of APS with markers of cardiovascular risk is widely discussed.
We found that APS is associated with an increased subclinical atherosclerosis and with impaired endothelial function, both being recognized as markers of cardiovascular risk.
Introduction
The antiphospholipid syndrome (APS) is an acquired autoimmune disease characterized by the persistent presence of antiphospholipid antibodies (aPL) in patients with history of venous or arterial thrombosis and/or recurrent miscarriage (Citation1,Citation2). Antiphospholipid antibodies include lupus anticoagulant (LA), anticardiolipin (aCL), and anti-β2 glycoprotein-I (β2GPI) antibodies (Citation3). With a prevalence of 40–50 cases per 100,000 persons (Citation4), APS can occur in patients with systemic lupus erythematosus (SLE) or other autoimmune rheumatic diseases (secondary APS) or in patients without any concomitant clinical condition (primary APS) (Citation5).
Overall, APS has been recognized as the most common acquired thrombophilic condition and, although associated with an increased risk of both venous and arterial events (Citation6), some data suggest that venous thromboembolism represent > 60% of vascular events secondary to APS (Citation7), arterial thrombosis being less common in APS subjects (Citation8,Citation9).
Mechanisms leading to arterial thrombosis in APS are largely unknown (Citation10), and the association between APS and subclinical atherosclerosis, known as a major marker of cardiovascular (CV) disease (Citation11), is still controversial.
Carotid intima-media thickness (IMT) assessment is a non-invasive imaging test for subclinical atherosclerosis (Citation12,Citation13) and has been widely accepted as one of the strongest predictors of CV events (Citation14,Citation15). Similarly, flow-mediated dilation (FMD) and nitrate-mediated dilation (NMD), pulse-wave velocity (PWV), augmentation index (AIx), and ankle-brachial index (ABI) are considered surrogate markers of subclinical atherosclerosis and independent predictors of CV events (Citation13,Citation16,Citation17), thus providing important prognostic data over and above traditional CV risk factors.
During recent years, there has been growing interest in the relationship between these markers of CV risk and APS. In particular, some case-control studies reported accelerated atherosclerosis (Citation18,Citation19), impaired endothelial function (Citation20), and increased arterial stiffness (Citation21) in patients with primary or secondary APS. However, these data have been challenged in other studies (Citation22,Citation23), and no meta-analytical data providing an overall information about this issue are currently available.
The aim of the present study is to perform a systematic review and meta-analysis of all studies evaluating the impact of APS on the major markers of CV risk.
Methods
A protocol for this review was prospectively developed, detailing the specific objectives, the criteria for study selection, the approach to assess study quality, the outcomes, and the statistical methods.
Search strategy
To identify all available studies, a detailed search pertaining to APS and the markers of CV risk (i.e. IMT, FMD, NMD, PWV, AIx, and ABI) was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Citation24). A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, EMBASE), using the following search terms in all possible combinations: intima-media thickness, carotid plaques, flow-mediated dilation, nitrate-mediated dilation, endothelium-dependent dilation, endothelium-independent dilation, pulse wave velocity, tonometry, augmentation index, atherosclerosis, ankle-brachial index, antiphospholipid syndrome. The last search was performed on 10 March 2014. The search strategy was developed without any language restriction.
In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study authors were contacted by e-mail to try to retrieve original data. Two independent authors (P.A. and R.L.) analyzed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (M.N.D.D.M.). Discrepancies were resolved by consensus. Selection results have been reported according to PRISMA flowchart (Supplementary Appendix 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.959559).
Data extraction and quality assessment
According to the pre-specified protocol, all studies evaluating the impact of APS on the markers of CV risk were included. Case reports, case series without a control group, reviews, and animal studies were excluded. Only APS, both primary and secondary, was considered in this meta-analysis, while positivity of aPL without thrombotic history was not taken into account. To be included in the analysis, a study had to provide values (means with standard deviation) of at least one variable among the following: common carotid artery IMT (CCA-IMT), internal carotid artery IMT (ICA-IMT), carotid bifurcation IMT (BIF-IMT), brachial artery FMD or NMD, aortic PWV or AIx, and ABI. Studies reporting the prevalence of carotid plaques were also included.
In each study, data regarding sample size, major clinical and demographic variables, values of IMT, FMD, NMD, PWV, AIx, and ABI, and prevalence of carotid plaques in APS patients and healthy controls were extracted.
Given the characteristics of the included studies, the evaluation of methodological quality of each study was performed with the Newcastle–Ottawa Scale (NOS), which is specifically developed to assess quality of non-randomized observational studies (Citation25). The scoring system encompasses three major domains (selection, comparability, exposure) and a resulting score range between 0 and 8, a higher score representing a better methodological quality. Results of the NOS quality assessment are reported in Supplementary Appendix 2 (to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.959559).
Statistical analysis and risk of bias assessment
Statistical analysis was carried out using Review Manager (Version 5.2, The Cochrane Collaboration, Copenhagen, Denmark) provided by The Cochrane Collaboration.
Differences among cases and controls were expressed as mean difference (MD) with pertinent 95% confidence intervals (95% CI) for continuous variables, and as odds ratio (OR) with pertinent 95% CI for dichotomous variables.
IMT has been expressed in millimeters (mm), FMD, NMD, and AIx as percentage (%), PWV as mm per second (mm/s), and ABI as absolute number.
The overall effect was tested using Z scores, and significance was set at P < 0.05. Statistical heterogeneity between studies was assessed with chi-square, Cochran's Q test, and with I2 statistic, which measures the inconsistency across study results and describes the proportion of total variation in study estimates that is due to heterogeneity rather than sampling error. In detail, I2 values of 0% indicate no heterogeneity, 25% low, 25%–50% moderate, and 50% high heterogeneity (Citation26).
Publication bias was represented graphically by funnel plots of the standard difference in means versus the standard error. Visual inspection of funnel plot asymmetry was performed to address for possible small-study effect (Citation27).
In order to be as conservative as possible, the random-effect method was used to take into account the variability among included studies.
Sensitivity analyses
We repeated sensitivity analyses by including only the studies judged as ‘high quality’ according to NOS (i.e. NOS ≥ the median value found among included studies).
In order to avoid the risk of data overlap, a further analysis was performed after excluding studies involving same recruitment centers and enrolling patients in the same period time as other included studies.
Subgroup analyses
Given the potential influence of underlying clinical conditions on the outcomes, we planned to perform a separate subgroup analysis only including studies reporting on primary APS.
Meta-regression analyses
We hypothesized that changes in IMT, FMD, NMD, PWV, AIx, and ABI values, and number of carotid plaques may be affected by differences in baseline characteristics of patients included in different studies (mean age, percentage of male patients) or by the coexistence of other CV risk factors (hypertension, smoking, obesity, diabetes mellitus, hypercholesterolemia, hypertriglyceridemia). To assess the possible effect of such variables in explaining the different results observed across studies, we planned to perform a meta-regression analysis after implementing a regression model with changes in IMT, FMD, NMD, PWV, AIx, and ABI values, or presence of carotid plaques as dependent variable (y) and the above-mentioned variables as independent variables (x). This analysis was performed with STATA 11.1 (Stata Corp, Austin, TX, USA).
Results
After excluding duplicate results, the search retrieved 1071 articles. Of these studies, 683 were excluded because they were off the topic after scanning the title and/or the abstract, 358 because they were reviews/comments/case reports or they lacked of data of interest. For 1 study the online full-length version was not available, and another 9 studies were excluded after full-length paper evaluation.
Thus, 20 articles (on 668 APS patients and 678 healthy controls) were included in the final analysis (Supplementary Appendix 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.959559). In detail, we included 14 studies with data on CCA-IMT (19 data sets on 492 cases and 645 controls), 5 on ICA-IMT and BIF-IMT (6 data sets on 136 cases and 126 controls), 4 studies reporting on the prevalence of carotid plaques (6 data sets on 142 cases and 239 controls), 8 studies on FMD (10 data sets on 271 patients and 398 controls), and 4 on NMD (5 data sets on 154 cases and 257 controls).
The ABI has been tested in only 2 studies (73 cases and 79 controls) (Citation19,Citation28). No mean ABI values were reported for cases and controls, but only the prevalence of pathological ABI, defined as ABI < 1. No study on PWV and AIx could be included in the final analysis.
Study characteristics
All included studies had a case-control design, and major characteristics are shown in .
Table I. Characteristics of included studies.
The number of patients varied from 10 to 58, the mean age from 29.1 to 52.6 years, and the prevalence of male gender from 0% to 65%.
The presence of hypertension was reported by 0%–36% of patients, smoking habit by 4%–48%, diabetes mellitus by 0%–5%, obesity by 0%–54%, hypercholesterolemia by 0%–55%, and hypertriglyceridemia by 0%–25%.
Mean body mass index (BMI) varied from 22.8 kg/m2 to 26 kg/m2. Mean values of total cholesterol (TC) ranged from 175 to 211 mg/dL, of LDL-cholesterol (LDLc) from 97 to 132 mg/dL, of HDL-cholesterol (HDLc) from 37 to 66 mg/dL, and of triglycerides (TGs) from 89 to 137 mg/dL.
One study (Citation29) provided separate data for three different age groups (< 30 years, 30–40 years, > 40 years). The first age group was excluded from our meta-analysis to avoid a potential source of bias secondary to the young age of patients as compared to the mean age reported in the other included studies. The remaining two groups were included as two different data sets. In addition, separate data for primary and secondary APS were reported by 3 studies for CCA-IMT (Citation30–32), by 1 study for FMD (Citation31), and by 2 studies (Citation5,Citation32) for the prevalence of carotid plaques. Finally, 1 study (Citation21) provided separate data on CCA-IMT, FMD, and NMD for APS patients with pregnancy loss and those with venous thromboembolism. In all these cases, data were split into different data sets.
The NOS for quality assessment of included studies showed a median value of 6.
Intima-media thickness (IMT) ()
Figure 1. Intima-media thickness in antiphospholipid syndrome (APS) patients and controls. A: Common carotid artery intima-media thickness (CCA-IMT). B: Internal carotid artery IMT (ICA-IMT). C: Carotid bifurcation IMT (BIF-IMT). D: Prevalence of carotid plaques. (Abort = recurrent abortions; pAPS = primary antiphospholipid syndrome; sAPS = secondary antiphospholipid syndrome; VTE = venous thromboembolism; y = years of age).
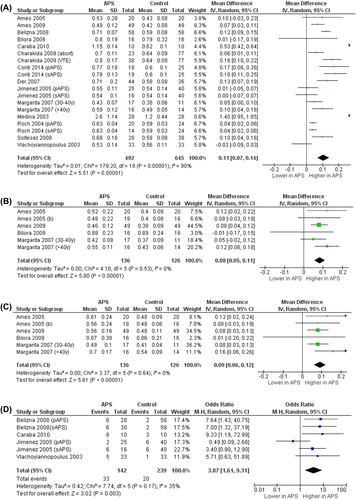
In 14 studies (19 data sets) (Citation5,Citation18,Citation21,Citation22,Citation29–38), we found that the 492 APS patients showed significantly higher CCA-IMT than the 645 controls (MD 0.11 mm; 95% CI 0.07, 0.14; P < 0.00001). Heterogeneity among these studies was statistically significant (I2 = 90%; P < 0.00001), and no reduction in the overall heterogeneity was found after excluding one study at time. Five studies (6 data sets) (Citation18,Citation29,Citation34,Citation35,Citation39), evaluating a total of 136 cases and 126 controls, showed significantly higher ICA-IMT (MD 0.08 mm; 95% CI 0.05, 0.11; P < 0.00001) and BIF-IMT (MD 0.09 mm; 95% CI 0.06, 0.12; P < 0.00001) in APS subjects than in controls, without heterogeneity among studies (I2 = 0%; P = 0.53 and I2 = 0%; P = 0.64, respectively). Finally, in 4 studies (6 data sets) (Citation5,Citation22,Citation32,Citation38) 142 APS patients showed an increased prevalence of carotid plaques as compared to 239 controls (23.2% versus 8.37%), with a corresponding OR of 3.87 (95% CI 1.61, 9.31; P = 0.0003) and without a significant heterogeneity among studies (I2 = 35%; P = 0.17).
Flow-mediated dilation (FMD) and nitrate-mediated dilation (NMD) ()
Figure 2. Flow-mediated dilation and nitrate-mediated dilation in antiphospholipid syndrome (APS) patients and controls. A: Flow-mediated dilation (FMD). B: Nitrate-mediated dilation (NMD). (Abort = recurrent abortions; pAPS = primary antiphospholipid syndrome; sAPS = secondary antiphospholipid syndrome; VTE = venous thromboembolism).
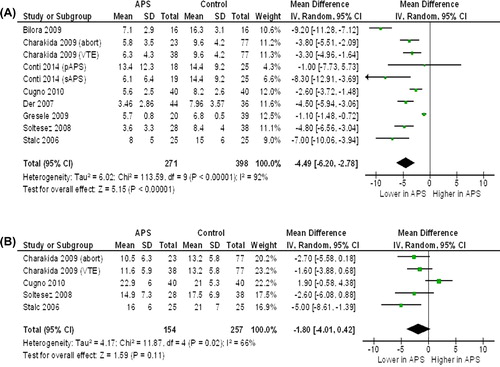
Eight studies (10 data sets) (Citation20,Citation21,Citation31,Citation33,Citation35,Citation37,Citation40,Citation41), evaluating a total of 271 cases and 398 controls, showed a significantly lower FMD in APS subjects as compared to controls (MD − 4.49%; 95% CI − 6.20, − 2.78; P < 0.00001). Significant heterogeneity among studies was found (I2 = 92%; P < 0.00001), and it was not reduced by excluding one study at time.
Four studies (5 data sets) (Citation20,Citation21,Citation33,Citation41), evaluating a total of 154 APS subjects and 257 controls, showed a trend towards a lower NMD in APS patients than in controls (MD − 1.80%; 95% CI − 4.01, 0.42, P = 0.11). Although the difference was not significant and a high heterogeneity among studies was found (I2 = 66%; P = 0.02), after excluding one study (Citation41), significantly lower values of NMD were found in APS subjects as compared to controls (MD − 2.61%; 95% CI − 4.06, − 1.15; P = 0.0004) without heterogeneity among studies (I2 = 0%; P = 0.49).
Ankle-brachial index (ABI) ()
Figure 3. Prevalence of pathological ankle-brachial index in antiphospholipid syndrome (APS) patients and controls.
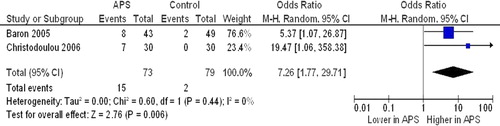
Two studies (73 cases and 79 controls) (Citation19,Citation28) reported on the impact of APS on ABI. A pathological ABI (< 1) was found to be more frequent in APS subjects compared to controls (20.5% versus 2.5%), with a corresponding OR of 7.26 (95% CI 1.77, 29.71; P = 0.006), without heterogeneity among studies (I2 = 0%; P = 0.44).
Publication bias
Because it is recognized that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using funnel plots analysis.
Funnel plots of effect size versus standard error for studies evaluating CCA-IMT and FMD were rather symmetrical, suggesting the absence of publication bias and of small-study effect (Supplementary Appendix 3, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.959559).
In contrast, the small sample size and the low number of studies makes publication bias assessment unlikely to be performed for ICA-IMT, BIF-IMT, carotid plaques, NMD, and ABI.
Sensitivity analysis
The median value of NOS quality assessment was 6, and the analyses were repeated by including only the studies classified as ‘high quality’ (NOS ≥ 6) (Supplementary Appendix 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.959559) (Citation5,Citation18,Citation21,Citation22,Citation29,Citation31–35,Citation38–41). For 1 study (Citation37) the data of interest were extracted by the abstract, thus no quality assessment could be performed.
Of interest, after excluding studies classified as ‘low quality’ (Citation19,Citation20,Citation28,Citation30,Citation36) and the one with only abstract available (Citation37), all results were confirmed for IMT, FMD, and NMD (). Both studies reporting on ABI (Citation19,Citation28) were classified as ‘low quality’, thus no data on sensitivity analysis are available for this outcome.
Table II. Sensitivity analysis including ‘high-quality’ studies (i.e. Newcastle–Ottawa Scale ≥ 6).
Similarly, results on IMT, FMD, NMD, and ABI were confirmed also after excluding studies (Citation18,Citation19,Citation29,Citation33,Citation39) potentially reporting on the same population as other included studies (Citation28,Citation34,Citation37) ().
Table III. Sensitivity analysis: exclusion of studies potentially evaluating the same population as other included studies.
Subgroup analysis
With the exception of 2 studies (Citation19,Citation22), all studies reported on primary APS, and other 4 studies (Citation5,Citation30–32) provided separate data for primary and secondary APS for at least one outcome. When the analyses were repeated by including only data on primary APS (Citation5,Citation18,Citation20,Citation21,Citation28–41), similar results were confirmed for all outcomes, with the only exception of prevalence of carotid plaques. This outcome has been reported by only 3 studies on primary APS (Citation5,Citation32,Citation38), and the difference among APS subjects and controls was no longer significant ().
Table IV. Subgroup analysis: studies on primary antiphospholipid syndrome.
Meta-regression analyses
Regression models showed that none of the clinical and demographic variables influenced the association between APS and CCA-IMT. In contrast, increasing age (P = 0.012) and the presence of diabetes mellitus (P = 0.011) significantly impacted on FMD impairment.
According to Cochrane Collaboration guidelines (Citation42), given the low number of studies, no meta-regression analysis was performed for ICA-IMT, BIF-IMT, prevalence of carotid plaques, NMD, and ABI.
Discussion
Results of the present meta-analysis show that APS is associated with subclinical atherosclerosis and endothelial dysfunction. In detail, we reported an increased carotid IMT, accompanied by impaired FMD, and increased prevalence of carotid plaques and of pathological ABI in APS subjects. In line with these findings, NMD was found to be lower in APS, but the difference did not reach statistical significance. Our findings are strengthened by the results of the subgroup and sensitivity analyses. Indeed, most results were confirmed after excluding the potential confounding effect due to other underlying medical conditions (i.e. systemic lupus erythematosus) and specifically evaluating data on patients with primary APS. Moreover, regression models have been performed to further assess any potential influence of clinical and demographic characteristics on evaluated outcomes
Overall, these data clearly show an increased CV risk in patients with APS and suggest a strict monitoring of cardiovascular risk factors and of subclinical signs of atherosclerosis in APS patients. The relevance of subclinical atherosclerosis lies in its ability to predict the CV risk and to contribute further to the morbidity of affected patients. This is in line with previously published studies reporting an increased CV risk in patients with other autoimmune and rheumatic diseases (Citation43,Citation44).
Many inherited and acquired risk factors are thought to have a causal role in the atherosclerotic process. However, the relationship between subclinical atherosclerosis and APS is complex, and the traditional CV risk factors do not seem to be the only parameters responsible for accelerated atherosclerosis in APS. Thus, inflammatory and immunological mechanisms have been proposed to explain the relationship between APS and atherosclerosis (Citation22). Endothelial dysfunction, oxidative stress, increased expression of cell adhesion molecules, and platelet activation are common findings in this clinical setting (Citation45). Moreover, growing evidence suggests that aPL are not only a marker of thrombophilia but play a direct pathogenic role (Citation46–50). In keeping with this, some studies hypothesized a pro-atherogenic role of aPL and, in particular, of anti-β2GPI antibodies (Citation51,Citation52). β2GPI reduces the intake of oxidized LDL (oxLDL) by macrophages in the vessel wall (Citation53), but this effect is blocked when anti-β2GPI antibodies are present. Thus, macrophage uptake of oxLDL is increased, leading to accelerated atherosclerosis (Citation28). In keeping with this, anti-β2GPI antibodies have been found in atherosclerotic plaques obtained from human carotid endarterectomies (Citation54). Other studies reported that aCL can cross-react with anti-oxLDL antibodies (Citation55). Thus, aCL may have the same pro-atherogenic effect as anti-oxLDL antibodies (Citation28).
Positivity to aPL has been associated also with reduced activity of paraoxonase, a HDL-related antioxidant enzyme (Citation21). Animal models suggest that paraoxonase deficiency can lead to premature atherosclerosis (Citation56).
Recently, an association between subclinical atherosclerosis and venous thrombosis has been reported, suggesting that the two conditions may share common risk factors (Citation6,Citation57).
Our findings are in line with this experimental and clinical evidence, supporting the hypothesis that, besides venous thrombosis (Citation7), also premature atherosclerosis may be one of the main features of APS. The clinical relevance of our results can be better understood when we consider that the risk of myocardial infarction increases 43% with every 0.163 mm increase in carotid IMT (Citation58). Moreover, each 1% decrease in FMD has been associated with a 12% increase of cardiovascular events (Citation59). In order to provide a comprehensive overview of the relationship between APS and subclinical atherosclerosis, all the major recognized markers of CV risk were taken into account in the current meta-analysis. Although no meta-analytical evaluation was possible for arterial stiffness parameters (PWV and AIx), it is relevant that, in line with our findings, higher values of PWV have been documented in women with primary APS (Citation21).
It is noteworthy that some data suggested that an unsolved issue is which of the carotid segments (CCA, ICA, or bifurcation) best predicts CV risk (Citation60). Interestingly, in our meta-analysis, we found a wide agreement among different segments, consistently confirming an increased IMT in APS patients.
Some potential limitations of our study need to be discussed. First, studies included in our meta-analysis have different inclusion and exclusion criteria, and most of the patients included in the analysis had concomitant CV risk factors (hypertension, smoking, obesity, diabetes mellitus, dyslipidemia). Since meta-analysis is performed on aggregate data and some information is missing in each study, the multivariate approach allowed for the adjustment for some (but not all) potential confounders. Moreover, based on Cochrane Collaboration guidelines, a meta-regression approach is effective when a covariate is reported by at least 10 studies (Citation42). Thus, because of the limited number of studies, a meta-regression analysis has been performed only for CCA-IMT and FMD, and we cannot exclude the influence of CV risk factors on the other outcomes. However, this issue cannot be fully addressed in a meta-analysis of aggregate data, making necessary an individual patient-level analysis.
As a further potential source of bias, we have to consider that some included studies presented data without making a distinction between patients with primary or secondary APS. Thus, we cannot exclude that the concomitant presence of systemic erythematosus lupus or other immune-mediated disorders might impact on our results. However, the large majority of studies reported separate data for primary APS, and the subgroup analysis confirmed all results. Thus, we are confident that the risk of bias due to the confounding effect of concomitant diseases could be low.
Finally, caution is necessary in the interpretation of the results on FMD and NMD. While IMT is a somewhat reproducible parameter, FMD and NMD measurements may be influenced by many confounding factors (Citation61), significantly limiting reproducibility of FMD and NMD assessment and, in turn, the relevance of results. However, it is interesting to highlight that the effect of APS was consistently confirmed for all evaluated outcomes, strongly suggesting an increased CV risk in these patients.
Conclusions
In conclusion, in our meta-analysis APS appeared significantly associated with subclinical atherosclerosis, endothelial dysfunction, and, in turn, with an increased CV risk. Thus, patients with APS may benefit from a more meticulous screening for CV risk factors and more specific CV prevention strategy. However, additional and specifically designed studies are needed in order to establish the optimal management of these patients.
Supplementary MOOSE checklist
Download PDF (455.2 KB)Acknowledgements
Authors want to thank all the members of the CaRRDs Study group for the help provided in the conduction of the present study (Supplementary Appendix 4 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.959559).
Funding: No funding and economic support has been received for this study.
Declaration of interest: All the authors have nothing to declare.
References
- Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–11.
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306.
- Mullen MT, Messé SR, Kasner SE, Sansing L, Husain MR, Norman GL, et al. Anti-phosphatidylserine-prothrombin antibodies are associated with outcome in a TIA cohort. Front Neurol. 2012;3:137.
- Mehrania T, Petri M. Epidemiology of the antiphospholipid syndrome. Amsterdam: Elsevier; 2009.
- Belizna CC, Richard V, Primard E, Kerleau JM, Cailleux N, Louvel JP, et al. Early atheroma in primary and secondary antiphospholipid syndrome: an intrinsic finding. Semin Arthritis Rheum. 2008;37:373–80.
- Di Minno MN, Tufano A, Ageno W, Prandoni P, Di Minno G. Identifying high-risk individuals for cardiovascular disease: similarities between venous and arterial thrombosis in perspective. A 2011 update. Intern Emerg Med. 2012;7:9–13.
- Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–27.
- Martini A, Ravelli A. The clinical significance of antiphospholipid antibodies. Ann Med. 1997;29:159–63.
- Tufano A, Guida A, Di Minno MN, De Gregorio AM, Cerbone AM, Di Minno G. Cardiovascular events in patients with antiphospholipid antibodies: strategies of prevention. Nutr Metab Cardiovasc Dis. 2010; 20:217–23.
- Koike T. Antiphospholipid antibodies in arterial thrombosis. Ann Med. 2000;32:27–31.
- Simon A, Chironi G, Levenson J. The performance of subclinical arterial disease detection as screening test for coronary heart disease. Hypertension. 2006;48:392–6.
- Bots ML, Grobbee DE. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther. 2002;16: 341–51.
- de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–8.
- O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340: 14–22.
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–94.
- Calabia J, Torguet P, Garcia M, Garcia I, Martin N, Guasch B, et al. Doppler ultrasound in the measurement of pulse wave velocity: agreement with the Complior method. Cardiovasc Ultrasound. 2011;9:13.
- Khan TH, Falahat AF, Niazi K. Critical review of the ankle brachial index. Curr Cardiol Rev. 2008;4:101–6.
- Ames PRJ, Margarita A, Sokoll KB, Weston M, Brancaccio V. Premature atherosclerosis in primary antiphospholipid syndrome: preliminary data. Ann Rheum Dis. 2005;64:315–17.
- Christodoulou C, Zain M, Bertolaccini ML, Sangle S, Khamashta MA, Hughes GRV, et al. Prevalence of an abnormal ankle-brachial index in patients with antiphospholipid syndrome with pregnancy loss but without thrombosis: a controlled study. Ann Rheum Dis. 2006; 65:683–4.
- Stalc M, Poredos P, Peternel P, Tomsic M, Sebestjen M, Kveder T. Endothelial function is impaired in patients with primary antiphospholipid syndrome. Thromb Res. 2006;118:455–61.
- Charakida M, Besler C, Batuca JR, Sangle S, Marques S, Sousa M, et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA. 2009;302:1210–17.
- Vlachoyiannopoulos PG, Kanellopoulos PG, Ioannidis JP, Tektonidou MG, Mastorakou I, Moutsopoulos HM. Atherosclerosis in premenopausal women with antiphospholipid syndrome and systemic lupus erythematosus: a controlled study. Rheumatology (Oxford). 2003; 42:645–51.
- Bilora F, Boccioletti V, Girolami B, Zanon E, Armani M, Petrobelli F, et al. Are antiphospholipid antibodies an independent risk factor for atherosclerosis? Clin Appl Thromb Hemost. 2002;8:103–13.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed on 18th September 2014.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5.
- Barón MA, Khamashta MA, Hughes GR, D’Cruz DP. Prevalence of an abnormal ankle-brachial index in patients with primary antiphospholipid syndrome: preliminary data. Ann Rheum Dis. 2005;64:144–6.
- Margarita A, Batuca J, Scenna G, Alves JD, Lopez L, Iannaccone L, et al. Subclinical atherosclerosis in primary antiphospholipid syndrome. Ann N Y Acad Sci. 2007;1108:475–80.
- Roch B, Kopprasch S, Pietzsch J, Schröder HE. Oxidatively modified lipoproteins and their antibodies in patients with antiphospholipid syndrome and systemic lupus erythematosus. Z Rheumatol. 2004; 63:331–7.
- Conti F, Spinelli FR, Alessandri C, Pacelli M, Ceccarelli F, Marocchi E, et al. Subclinical atherosclerosis in systemic lupus erythematosus and antiphospholipid syndrome: focus on β2GPI-specific T cell response. Arterioscler Thromb Vasc Biol. 2014;34:661–8.
- Jiménez S, García-Criado MA, Tàssies D, Reverter JC, Cervera R, Gilabert MR, et al. Preclinical vascular disease in systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology (Oxford). 2005;44:756–61.
- Soltesz P, Der H, Veres K, Laczik R, Sipka S, Szegedi G, et al. Immunological features of primary anti-phospholipid syndrome in connection with endothelial dysfunction. Rheumatology (Oxford). 2008;47: 1628–34.
- Ames PR, Antinolfi I, Scenna G, Gaeta G, Margaglione M, Margarita A. Atherosclerosis in thrombotic primary antiphospholipid syndrome. J Thromb Haemost. 2009;7:537–42.
- Bilora F, Sartori MT, Zanon E, Campagnolo E, Arzenton M, Rossato A. Flow-mediated arterial dilation in primary antiphospholipid syndrome. Angiology. 2009;60:104–7.
- Medina G, Casaos D, Jara LJ, Vera-Lastra O, Fuentes M, Barile L, et al. Increased carotid artery intima-media thickness may be associated with stroke in primary antiphospholipid syndrome. Ann Rheum Dis. 2003;62:607–10.
- Der H, Kerekes G, Veres K, Szodoray P, Toth J, Lakos G, et al. Impaired endothelial function and increased carotid intima-media thickness in association with elevated von Willebrand antigen level in primary antiphospholipid syndrome. Lupus. 2007;16:497–503.
- Caraba A, Şerban C, Mihǎescu R, Romoşan I. Subclinical atherosclerosis in primary antiphospholipid syndrome. Annals of the Romanian Society for Cell Biology. 2010;15:293–8.
- Ames PR, Tommasino C, Fossati G, Matsuura E, Margarita A, Saulino A, et al. Lymphocyte subpopulations and intima media thickness in primary antiphospholipd syndrome. Lupus. 2005;14:809–13.
- Gresele P, Migliacci R, Vedovati MC, Ruffatti A, Becattini C, Facco M, et al. Patients with primary antiphospholipid antibody syndrome and without associated vascular risk factors present a normal endothelial function. Thromb Res. 2009;123:444–51.
- Cugno M, Borghi MO, Lonati LM, Ghiadoni L, Gerosa M, Grossi C, et al. Patients with antiphospholipid syndrome display endothelial perturbation. J Autoimmun. 2010;34:105–10.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from: www.cochrane-handbook.org. Accessed on 18th September 2014.
- Di Minno MN, Iervolino S, Lupoli R, Russolillo A, Coppola A, Peluso R, et al. Cardiovascular risk in rheumatic patients: the link between inflammation and atherothrombosis. Semin Thromb Hemost. 2012;38:497–505.
- Tyrrell PN, Beyene J, Feldman BM, McCrindle BW, Silverman ED, Bradley TJ. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2010;30:1014–26.
- Jara LJ, Medina G, Vera-Lastra O. Systemic antiphospholipid syndrome and atherosclerosis. Clin Rev Allergy Immunol. 2007;32:172–7.
- Farmer-Boatwright MK, Roubey RA. Venous thrombosis in the antiphospholipid syndrome. Arterioscler Thromb Vasc Biol. 2009;29:321–5.
- Pengo V, Biasiolo A, Gresele P, Marongiu F, Erba N, Veschi F, et al. A comparison of lupus anticoagulant-positive patients with clinical picture of antiphospholipid syndrome and those without. Arterioscler Thromb Vasc Biol. 2007;27:e309–10.
- Galve-de Rochemonteix B, Kobayashi T, Rosnoblet C, Lindsay M, Parton RG, Reber G, et al. Interaction of anti-phospholipid antibodies with late endosomes of human endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:563–74.
- Reverter JC, Tàssies D, Font J, Monteagudo J, Escolar G, Ingelmo M, et al. Hypercoagulable state in patients with antiphospholipid syndrome is related to high induced tissue factor expression on monocytes and to low free proteins. Arterioscler Thromb Vasc Biol. 1996;16:1319–26.
- Valesini G, Pittoni V. Treatment of thrombosis associated with immunological risk factors. Ann Med. 2000;32:41–5.
- George J, Afek A, Gilburd B, Blank M, Levy Y, Aron-Maor A, et al. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with beta2- glycoprotein I. Circulation. 1998;98:1108–15.
- de Groot PG, Bouma B, Lutters BC, Derksen RH. Lupus anticoagulant in cardiovascular diseases: the role of beta2-glycoprotein I. Ann Med. 2000;32:32–6.
- Matsuura E, Koike T. Accelerated atheroma and anti-b2-glycoprotein I antibodies. Lupus. 2000;9:210–16.
- George J, Harats D, Gilburd B, Afek A, Levy Y, Schneiderman J, et al. Immunolocalization of beta2-glycoprotein I (apolipoprotein H) to human atherosclerotic plaques: potential implications for lesion progression. Circulation. 1999;99:2227–30.
- Hörkkö S, Olee T, Mo L, Branch DW, Woods VL Jr, Palinski W, et al. Anticardiolipin antibodies from patients with the antiphospholipid antibody syndrome recognize epitopes in both beta2- glycoprotein i and oxidized lowdensity lipoprotein. Circulation. 2001;103:941–6.
- Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–7.
- Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–41.
- van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: The Rotterdam Study. Circulation. 2004;109:1089–94.
- Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192:197–203.
- Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, et al. Progression of carotid intima-media thickness as predictor of vascular events: results from the IMPROVE study. Arterioscler Thromb Vasc Biol. 2013;33:2273–9.
- Soltész P, Kerekes G, Dér H, Szücs G, Szántó S, Kiss E, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev. 2011; 10:416–25.

