Abstract
Aims. A hypothetical benefit of statins after an ischemic stroke could be provided by their pleiotropic effects. Our aim is to test if statins are able to avoid mortality and readmissions of patients with ischemic stroke, by lowering their levels of not only LDL-cholesterol but also CRP.
Methods. A prospective cohort study was performed. Pre-stroke and post-stroke medications were recorded. Cholesterol and hsCRP levels were measured at admission and 90 days post-stroke. Rankin score and fatality or readmissions were assessed at 90 days and 1 year. We have used robust statistical methods.
Results. Of 359 stroke patients, statins were prescribed before stroke onset in 30.6% (110/359) and were begun during hospitalization in an additional 32.3% (116/359). In logistic regression analysis adjusted, statins therapy was independently associated with improved total mortality (OR 0.30; 95% CI 0.11–0.86; P < 0.02), improved cardiovascular mortality (OR 0.29; 95% CI 0.08–0.98; P < 0.04), and improved total mortality and readmission rates (OR 0.35; 95% CI 0.18–0.7; P < 0.003). In the final model, lowering the levels of hsCRP by 0.4mg/dL, a 30% of mortality or readmissions would be avoided.
Conclusions. Therapy with statins, either previous or early initiation, after an ischemic stroke, could improve the survival and readmission rates by lowering both cholesterol and hsCRP levels.
Key words::
The use of statins after an ischemic stroke improves the outcome, even in elderly people.
The improvement of patients after statins therapy is not only due to a lowering in cholesterol levels but a lowering in their inflammation, measured as C-reactive protein.
We have used robust statistical analysis to improve the quality of our results.
Introduction
Therapy with statins reduces the risk of stroke among patients with coronary heart disease and those at increased risk for cardiovascular disease (Citation1–3). However, data are conflicting regarding their relationship with the outcome after ischemic stroke. Some studies have reported improved survival and functional outcome with statins, but these findings have not been consistently replicated (Citation4–9). In the largest meta-analysis to date (Citation10), statin therapy at stroke onset was associated with improved outcome at 90 days from discharge, but not with reduction of fatality at 1 year. The role of statins in secondary prevention after an ischemic stroke is unclear. In contrast to its established role in the pathogenesis of coronary heart disease, plasma cholesterol is less well established as an important risk factor for cerebrovascular disease (Citation11); in fact it has been suggested that lowering cholesterol through dietary modification or non-statin drugs fails to reduce stroke mortality or morbidity (Citation12). Therefore, the hypothetical benefit of statins could be provided by their pleiotropic effects regarding their anti-inflammatory and anti-atherothrombotic effects. Actually, previous studies have suggested that the post-stroke inflammation may exacerbate the tissue damage after cerebral infarction and contribute to the poor prognosis (Citation13); a variety of circulating markers of inflammation, including C-reactive protein (CRP), serum amyloid A (SAA), interleukin-6 (IL-6), and a number of leukocyte adhesion molecules, have been shown to predict either the extent of atherosclerosis or the risk of vascular events. In several trials, statin therapy has been shown to decrease serum CRP (Citation14,Citation15), this effect possibly being responsible for improving the outcome after a stroke episode.
Our aim is to test if statins are able to improve the mortality and readmission rates in patients with ischemic stroke, not only by lowering the low-density lipoprotein cholesterol (LDL-cholesterol) but also by decreasing CRP as a pleiotropic effect.
Methods
We included all consecutive ischemic stroke patients, admitted within 24 hours after the episode, between 1 January 2005 to 31 December 2010, who signed the consent to participate in a registry under an ethics committee-approved protocol; of those some particular data have been previously published (Citation16–18). The study was performed conforming to the declaration of Helsinki.
The diagnosis of stroke was based on the clinical evaluation and computed tomographic scan or magnetic resonance imaging scan of the brain performed in all cases within 24 hours of the event. The subtypes of stroke were classified according to the TOAST classification (Citation19). We excluded patients with intracerebral hemorrhage, subarachnoid hemorrhage, cerebral venous sinus thrombosis, and late admission (> 24 hours after stroke onset). And to avoid confounding factors we also excluded patients with history of recent clinical infections; either severe kidney or hepatic or cancerous diseases; surgery or major trauma in the previous month; and observable signs and clinical evidence of acquired in-hospital infection. Previous infections were monitored with an exhaustive medical history focusing on signs and symptoms of potential clinical infection during the last 4 weeks before stroke, together with a review of patient's hospital access schedule.
All patients were treated with either antiaggregant or anticoagulant drugs, depending on their etiology, and with anti-hypertensive agents. Statins were continued when patients were previously under treatment, and when they were started it was between the second and the sixth day after the event. Simvastatin, atorvastatin, pravastatin, and fluvastatin were used.
All patients underwent several laboratory investigations and were asked for vascular risk factors or documented treatment with statins before the index event. CRP was measured, both in the first 24 hours and in the third month after the event using a high-sensitivity assay (BN100 nephelometer, Dade Behring, Marburg, Germany). Total-cholesterol and triglyceride determinations were made by using enzymatic methods with automatic analyzer (HItachi 704, Mannheim, Germany). LDL-cholesterol determination was made using the Friedewald formula.
The National Institute of Health stroke scale (NIHSS) (Citation20), Charlson co-morbidity index (CCI) (Citation21), and Rankin score (Citation22) assessed stroke severity, co-morbidity, and disability. Although patients were not in primary prevention we used the INDANA score (Citation23) at the beginning to value their cardiovascular risk.
Study protocol
The patients who were finally recruited were classified into three groups depending on the treatment, or not, with statins: group 1, patients who were under treatment with statins (both before and after stroke); group 2, patients who started treatment with statins only after index stroke; and group 3, patients untreated with statins after index event (patients who had taken statins neither before nor after the stroke and patients whose statin treatment was removed after the index event, 6 patients, 4.5%).
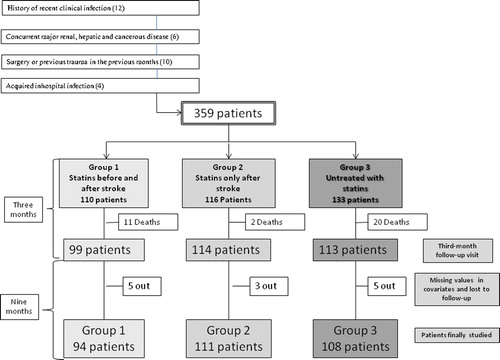
All patients were followed up regularly as outpatients for 1 year. Follow-up was obtained through a 3-month follow-up visit, when the patients underwent a clinical examination and a new blood sample to get new levels of CRP and cholesterol, and through other periodic follow-up visits, direct contact with the patient or the patient's family or physician, and a chart review. Patients who died or were lost between the beginning and the 3-month follow-up visit were not included. Likewise, patients who were lost during the follow-up for 1 year or without second blood sample were also excluded. A flow diagram of study profile is shown in .
The primary end-point considered was the combination of death from any cause or hospitalization. Secondary objectives were cardiovascular mortality which included sudden death, death resulting from acute coronary syndromes, congestive heart failure, systemic embolism, or decease as a consequence of a new fatal stroke in the absence of other intervening causes during the 1-year follow-up. A final end-point was the improvement of severity in functional status, achieved based on the Rankin score at 12 months in the outpatients who survived. Good functional outcome was defined as Rankin score ≤ 2.
Statistical analysis
We have used robust statistical methods which are not unduly affected by outliers or the small departures from model assumptions.
Qualitative and categorical data are expressed as absolute number (and percentage), and to compare them in univariate analysis we have used the chi-square test. Quantitative data are expressed as 20% trimmed mean (and median absolute deviation, MAD). To compare them in univariate analysis we have used ANOVA in case of normality and homoscedasticity, and the Rust and Fligner test in the rest of comparisons. To estimate differences in levels of cholesterol and hsCRP between the beginning and the 3-month follow-up, we used t test for paired data.
Multivariate analysis has been made by using the approach by Cantoni and Ronchetti (Citation24), based on robust quasi-deviance functions for estimation and variable selection. The models, based on the primary end-point and severity in functional status, were adjusted for potential confounders such as sex, age, smoking, hypertension, diabetes, hyperlipidemia, INDANA score, systolic and diastolic blood pressure, CCI, NIHSS, hsCRP, as well as total-, HDL-, and LDL-cholesterol, and the gradients between the start and 3 months of both hsCRP and LDL-cholesterol.
We used Kaplan–Meier survival and cumulative hazard curves to compare event-free survival between groups and compared curves with log-rank test. We built a multivariate Cox regression model to adjust for confounders.
Probability values of 0.05 were considered as significant. These analyses were completed with R (version 2.15.3, 2013-03-01).
Results
Patient characteristics
Overall, 391 patients were initially included (37 patients did not sign the consent). Of these, we excluded 32 (). Hence we initially studied 359 patients. Of these, 110 (30.6%) were using statin therapy before stroke onset, and 116 (32.3%) began to use statins at 2–6 days after the index stroke. The most commonly prescribed statins were simvastatin (42.5%, 96/226 patients) and atorvastatin (28.8%, 65/226 patients).
The patients who were under treatment with statins before index event (group 1), had better frequencies of vascular risk factors including hypertension (P = 0.001), diabetes mellitus (P = 0.0007), and hyperlipidemia (P = 0.0001). Compared to statin-untreated patients, those with statins were younger (P = 0.005), and they had better functional status measured by Rankin score (P = 0.01) and better stroke severity score by NIHSS (P = 0.0006).
After 3 months of follow-up, data were available for 313 patients (87.2%): 90% in group 1, 98.2% in group 2, and 84.9% in group 3 (); 33 patients died before the 3-month follow-up visit, and 13 more were lost to follow-up.
In the patients finally studied there was an increased cardiovascular risk among those treated with statins. However, age, co-morbidity, and neurologic severity or functional status were similar among the three groups. There were no significant differences in TOAST classification. Other characteristics are shown in .
Table I. Baseline epidemiologic, clinical, and laboratory characteristics by group. On the left, data of initial groups, on the right data of definitive groups. Values of qualitative variables are expressed as number (percentage). Values of quantitative variables are expressed as 20% trimmed mean (median absolute deviation, MAD).
Modification in cholesterol and hsCRP levels 3 months after the index stroke
The change in hsCRP and cholesterol between the index event and the 3-month follow-up is shown in and . The levels of total-cholesterol and LDL-cholesterol were higher in groups 1 and 2 (patients treated with statins) at the beginning of study (P = 0.0001), and the levels of hsCRP were similar among the three groups. In the sample obtained after 3 months, total cholesterol and LDL-cholesterol were higher in group 3. HsCRP was also higher in this group (P = 0.001).
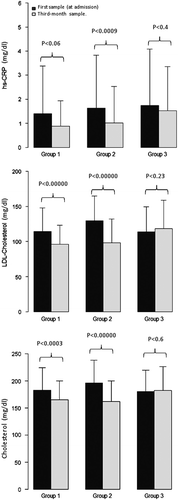
In group 1, the mean of change in serum hsCRP was 1.12 mg/dL (95% CI 0.99–1.27, P = 0.06), in group 2 there was a significant difference of 1.18 mg/dL (95% CI 1.07–1.31, P = 0.0009), and in group 3 there was a not significant difference (mean 0.9 mg/dL, 95% CI 0.87–1.05, P = 0.4).
Primary and secondary end-points
The mean of follow-up was 45.4 ± 14.5 weeks for 85.4% of patients in group 1, 95.7% in group 2, and 81.2% in group 3.
On univariate analysis regarding statins treatment (205 patients) and post-stroke mortality and admissions, the OR and 95% CI were consistently < 1 (), indicating a statin-associated benefit. The results were similar for total mortality, cardiovascular mortality, and cardiovascular events, either in univariate and multivariate adjusted analysis. However, a beneficial outcome regarding functionality was not significantly achieved by the patients who were treated with statins (, and Supplementary Table II to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1010227). We further conducted a subgroup analysis by sex because of the differences in gender through the groups (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1010227), where the OR and 95% CI for the primary end-point and total mortality were similar to those of the whole sample, as were the results regarding functionality.
Table II. Results of primary and secondary end-points according to treatment, or not, with statins.
On regression analysis () an independent association over 1 year of follow-up was found in the case of initial levels of hsCRP (P = 0.0005), difference in hsCRP (P = 0.0000), presence of hypertension (P = 0.008), and treatment with statins (P = 0.0007), after adjusting for age, sex, smoking, diabetes mellitus, hyperlipidemia, hypertension, systolic and diastolic blood pressure, total-, HDL-, and LDL-cholesterol, hsCRP, treatment with statins, and gradients of total-cholesterol, LDL-cholesterol, and hsCRP from the stroke event to the 3-month second sample. In the final model, by lowering by 0.5mg/dL the levels of hsCRP, a 30% of mortality or admissions would be avoided.
Table III. Robust multivariate logistic regression analyses of factors associated with the primary end-point.
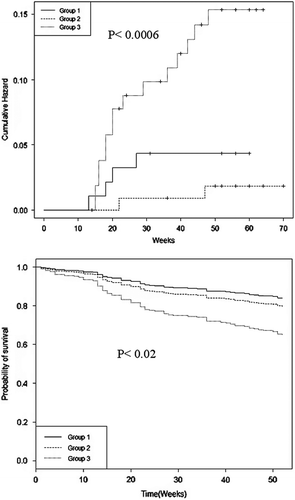
To analyze further the factors associated with death and readmissions over 1 year a multivariate Cox regression analysis was conducted for age, sex, history of either hypertension, smoking, hyperlipidemia, or diabetes mellitus, NIHSS at admission, CCI, total-, LDL-, and HDL-cholesterol, hsCRP, treatment with statins, and gradients of total-cholesterol, LDL-cholesterol, and hsCRP from the index stroke to the 3-month second sample. History of hypertension (OR 2.9, 95% CI 1.15–7.55, P = 0.023), baseline hsCRP levels (OR 1.19, 95% CI 1.07–1.33, P = 0.0008), NIHSS at admission (OR 1.05, 95% CI 1.01–1.11, P = 0.02), and gradient of hsCRP (OR 0.64, 95% CI 0.51–0.79, P = 0.00005) were significantly associated with death or admission by 1 year in our cohort of patients. Kaplan–Meier survival curves for total mortality and readmission and for cardiovascular mortality () show a higher risk for patients untreated with statins.
Discussion
This is a prospective cohort based on ordinary clinical practice in real-world patients followed up according to the treatment with statins after an ischemic stroke. The main findings were a consistent association between statins treatment (pre-stroke and new acute post-stroke) and improved survival or readmission rates after the event that were independent of the levels of lowering of both total- and LDL-cholesterol. However, a beneficial outcome regarding functionality was not achieved in these groups of patients. We further observed some evidence suggesting that hsCRP levels during the acute phase of ischemic stroke are significantly and independently able to predict the 1-year outcome (mortality and readmissions), and that serum hsCRP levels decreased after 3 months of statins therapy.
Several studies have reported improved clinical outcomes after a stroke when statins therapy was used. In the meta-analysis published by Ní Chróinín (Citation10) an association was shown between statin treatment at stroke onset and both functional independence and survival after stroke; however, the moment of the beginning of therapy with statins remains controversial because, on the one hand, some observational studies have reported either reduced mortality or better functional status in patients treated with statins before stroke onset and, on the other hand, other studies did not observe any benefit. Similarly, the findings from randomized controlled trials have been inconsistent (Citation25–27). In our cohort, the patients treated with statins before stroke were those who most benefited regarding mortality and admissions, a finding that has been described previously (Citation7). These patients (group 1) had lower levels of both cholesterol and hsCRP initially, compared with those that did not receive pre-stroke statin therapy, suggesting an underlying neuroprotective effect and not a treatment selection bias since stroke severity, age, and co-morbidity were the same through the groups. It has been shown that pre-stroke statin use is associated with a better collateralization during acute stroke. This collateralization effect could be fomented by both arteriogenesis and angiogenesis which help towards amelioration after an arterial occlusion (Citation28). The second group also showed improvement in mortality and admission rates. The timing of statins initiation in patients with acute ischemic stroke is controversial. This second group started treatment between the second and the sixth day after the event. In a recent meta-analysis (Citation10), improved functional outcome and survival were observed when statins therapy was commenced acutely, in observational studies but not randomized trials. The variability in the beginning of statins might be the reason for these dissimilarities in outcome. In a study that investigated the impact of statin therapy with different timings of initiation after acute ischemic stroke or transient ischemic attack (TIA) in patients without previous statin use, an in-hospital introduction of statins (as in our second group) was associated with better clinical results and mortality rates than the groups that started the treatment later (1 month and 1 year after the event) (Citation29). In our study, however, the relationship between statins treatment and good functional outcome was not significant. This finding has been previously reported in case of patients treated with tissue plasminogen activator, suggesting that statin use at stroke onset may be associated with increased rates of intracerebral hemorrhage after intravenous or intra-arterial thrombolysis (Citation30,Citation31). Our patients did not undergo thrombolysis, and the percentage of intracerebral hemorrhage after stroke was similar in the three groups. An explanation for our bad results regarding functional recovery could be a bigger neurologic deficit at admission than other studies published and an older age of the patients included, which would make it more difficult to achieve an effective functional outcome.
Nevertheless, the principal finding in this study is the pleiotropic effect that statins could develop in the improvement in both total and cardiovascular mortality beyond the lowering of cholesterol. As shown in regression analysis, both hsCRP and the gradient of hsCRP at 3 months after the event had implications for the prognosis independently of the cholesterol levels achieved under statins treatment. To our knowledge, there are no previous studies that report the levels of cholesterol and hsCRP after 90 days of treatment with statins in patients who have suffered an ischemic stroke. This finding adds evidence that higher levels of hsCRP are associated with high risk of death in cerebral infarction patients (Citation32,Citation33) and, which is more important, that by lowering hsCRP levels with statins we could reduce the mortality and readmission rates in these patients. It is known that inflammation plays an important role in the pathogenesis of brain damage (Citation34,Citation35), and anti-inflammatory treatment may add a better clinical outcome to acute ischemic stroke (Citation31). Statins have been reported to be anti-inflammatory (Citation36), while hsCRP has been widely accepted as an inflammatory biomarker for predicting prognostic outcome in cardiovascular and cerebrovascular diseases (Citation33,Citation37); therefore, as we show in this study, the role of statins after a stroke goes beyond a lowering of cholesterol levels but also has an anti-inflammatory effect that improves even more the survival of these patients.
Strengths of our study include its size, availability of cholesterol and hsCRP data after 3 months of follow-up, and availability of outcome data for 87.2% of patients. In addition, the patients included are in general older than in previous studies, which is interesting because of the absence of knowledge in this part of population. Furthermore, our statistical methods were rigorous and robust which minimized errors caused by outliers.
As other observational studies, ours has several limitations. Firstly, it has been controversial whether the dose of statins has an effect on the seriousness of stroke (38); however, we did not gather the category or dosage of statins, which made it impossible to assess the dose–effect relationship of the benefit of statins. Secondly, statins discontinuation was not included in this study, while statins withdrawal was associated with increased risk of death or dependency at 90 days (Citation26). Thirdly, although it is possible that unmeasured factors related to statin prescribing patterns may partially account for our findings, the association of statins therapy with improved outcome persisted after adjusting for other important determinants of prognosis, including age, stroke severity, functional status, and co-morbidities. Finally, as it was an observational study, there were differences between the statins use and non-statins use groups in risk factors, stroke severity, age, etc. These differences still led to bias although we adjusted for this in the multivariate models.
Conclusions
Our research shows that use of statins, either previous or early initiation, in patients after an ischemic stroke could improve their survival and readmission rates by lowering both cholesterol and inflammation, measured as hsCRP levels. These findings could propose that statin treatment should be started in patients with ischemic stroke, even with normal levels of cholesterol; however, further studies are needed to confirm our results.
Supplementary material available online
Supplementary Table I and II to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1010227.
iann_a_1010227_sm0156.pdf
Download PDF (34.8 KB)Declaration of interest: The authors report no conflicts of interest.
References
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58.
- Colhom HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96.
- Amarenco P, Labreuche J, Lavallée P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis. Systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–9.
- Biffi A, Devan WJ, Anderson CD, Cortellini L, Furie KL, Rosand J, et al. Statin treatment and functional outcome after ischemic stroke. Stroke. 2011;42:1314–19.
- Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset. Neurology. 2005;65:253–8.
- Martinez-Sanchez P, Rivera-Ordoñez C, Fuentes B, Ortega-Casarrubios MA, Idrovo L, Diez-Tejedor E. The beneficial effect of statins treatment by stroke subtype. Eur J Neurol. 2009;16:127–33.
- Ní Chróinin D, Callaly EL, Duggan J, Merwick A, Hannon N, Sheehan O, et al. Association between acute statin therapy, survival and improved functional outcome after ischemic stroke. Stroke. 2011;42:1021–9.
- Squizzato A, Romualdi E, Dentali F, Ageno W. Statins for acute ischemic stroke. Cochrane Database Syst Rev. 2011;(8):CD007551.
- Cappellari M, De Luca C, Tinazzi M, Tomelleri G, Carletti M, Fiaschi A, et al. Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J Neurol Sci. 2011;308:128–34.
- Ní Chroinin D, Asplund K, Asberg S, Callaly E, Cuadrado-Godia E, Díez-Tejedor E, et al. Statin therapy and outcome after ischemic stroke. Systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–56.
- Oliver MF. Cholesterol and strokes. Cholesterol lowering is indicated for strokes due to carotid atheroma. BMJ. 2000;320:459–60.
- Herbert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statin agents, risk of stroke, and total mortality: an overview of randomized trials. JAMA. 1997;278:313–21.
- Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–45.
- Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-zocor trial. Circulation. 2006;114:281–8.
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al.; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8.
- Arévalo-Lorido, Carretero-Gómez J, Calvo-Romero JM, Romero-Requena JM, Pérez-Alonso JL, Gutiérrez-Montaño C, et al.C-reactive protein in the acute phase of ischemic stroke. Med Clin (Barc). 2005;125: 766–9.
- Arévalo-Lorido, Carretero-Gómez J. C-reactive protein and carotid intima-media thickness in atherothrombotic ischemic stroke. Med Clin (Barc). 2009;133:496–500.
- Arévalo-Lorido, Carretero-Gómez J, Álvarez-Oliva A, Gutiérrez-Montaño C, Fernández-Recio JM, Najarro-Díez F. Mean platelet volume in acute phase of ischemic stroke, as predictor of mortality and functional outcome after 1 year. J Stroke Cerebrovasc Dis. 2013;22:297–303.
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
- Lyden PD, Lu M, Levine SR, Brott TG, Broderick J; NINDS rTPA Stroke Study Group.A modified National Institute of Health Stroke Scale for use in stroke clinical trials: preliminary reliability and validity. Stroke. 2001;32:1310–17.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
- Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-bind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51.
- Pocock SJ, McCormack V, Gueyffier F, Boutitie F, Fagard RH, Boissel JP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomized controlled trials. BMJ. 2001;323:75–81.
- Cantoni E, Ronchetti E. Robust inference for generalized linear models. J Am Stat Assoc. 2001;96:1022–30.
- Goldstein LB, Amarenco P, Zivin J, Messiq M, Altafullah I, Callaham A, et al. Stroke prevention by aggressive reduction in cholesterol levels investigators. Statin treatment and stroke outcome in the stroke prevention by aggressive reduction in cholesterol levels (SPARCLE) trial. Stroke. 2009;40:3526–31.
- Blanco M, Nombela F, Castellanos M, Rodríguez-Yañez M, García-Gil M, Leira R, et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69:904–10.
- Kennedy J, Hill MD, Rickburst KJ, Eliasziw M, Demchuk AM, Bucham AM; FASTER investigators. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomized controlled pilot trial. Lancet Neurol. 2007;6:961–9.
- Ovbviagele B, Saber JL, Starkman S, Kim D, Ali LK, Jahan R, et al. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–31.
- Chen P-S, Cheng C-L, Kao Yang Y-H, Yeh P-S, Li Y-H. Impact of early statin therapy in patients with ischemic stroke or transient ischemic attack. Acta Neurol Scand. 2014;129:41–8.
- Miedema I, Ugttenboogaart M, Koopman K, De Keyser J, Luijck GC. Statin use and functional outcome after tissue plasminogen activator treatment in acute ischaemic stroke. Cerebrovasc Dis. 2010;29: 263–7.
- Engelter ST, Soinne L, Ringleb P, Sarikaya H, Burdet R, Berrouschot J, et al. IV thrombolysis and statins. Neurology. 2011;77:888–95.
- Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Association of circulating inflammatory markers with recurrent vascular events after stroke: a prospective cohort study. Stroke. 2011;42:10–16.
- Idicula TT, Brogger J, Naess H, Waje-Andreassen U, Thomassen L. Admission C-reactive protein after acute ischemic stroke is associated with stroke severity and mortality: the “Bergen stroke Study”. BMC Neurol. 2009;9:18.
- Samson Y, Lapergue B, Hosseini H. Inflammation and ischaemic stroke: current status and future perspectives. Rev Neurol [Paris]. 2005;165: 1177–82.
- Lefer DJ. Statins as potent antiinflammatory drugs. Circulation. 2002; 106:2041–2.
- Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP pooling project members. Stroke. 2005;36:1316–29.
- Martínez-Sanchez P, Fuentes B, Martínez-Martínez M, Ruiz-Ares G, Fernández-Travieso J, Sanz-Cuesta BE, et al. Treatment with statins and ischemic stroke severity: does the dose matter? Neurology. 2013;80: 1800–5.