Abstract
Background. Abdominal aortic aneurysm (AAA) is characterized by inflammatory cell accumulation in AAA lesions that produce inflammatory cytokines and advance its pathogenesis. Peripheral cytokines may predict the degree or risk of AAA.
Methods and results. ELISA determined plasma interleukin-6 (IL6), IL10, IL17A, IFN-γ, and C-reactive protein (CRP) from 476 AAA patients and 200 controls. AAA patients had lower IL6, IFN-γ, IL10, IL17A, and higher CRP than controls. IL10 correlated positively with IFN-γ, IL17A, or IL6, but not CRP in control or AAA populations. IL10 associated negatively with systolic blood pressure, whereas CRP associated positively with diastolic blood pressure and body mass index. CRP was an independent AAA risk factor and correlated positively with aortic diameters before and after adjustments for other risk factors. IFN-γ, IL17A, and CRP correlated positively with cross-sectional AAA area after adjustment. IL10 correlated positively with AAA growth rate before and after adjustment. The risk of death doubled in AAA patients with CRP levels above the median.
Conclusions. Reduced IFN-γ, IL10, and IL17A in AAA patients, positive correlations of IFN-γ and IL17A with cross-sectional AAA area, IL10 with AAA growth rate, and IL10 with IFN-γ and IL17A suggest combined Th1, Th2, and Th17 immune responses in human AAAs.
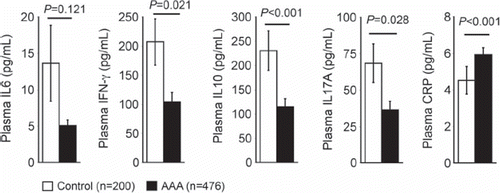
Plasma IL6, IL10, IFN-γ, and IL17A are reduced, but plasma CRP is increased in patients with AAA.
Among all tested biomarkers, plasma IFN-γ, IL17A, and CRP levels correlate positively with maximal cross-sectional AAA areas among AAA patients, IL10 correlates positively with maximal annual AAA growth rate, and CRP also correlates positively with maximal aortic diameter in the whole population after adjustment for common AAA risk factors.
Among all tested biomarkers, CRP is the only significant risk factor of human AAA after adjustment. AAA-associated risk of death is doubled among patients with plasma CRP levels above the median.
Introduction
Abdominal aortic aneurysm (AAA) is a chronic inflammatory disease involving extensive inflammatory cell infiltration to the arterial wall from the luminal intima to the peri-aortic adventitia (Citation1–3). Accumulation of these cells, including neutrophils, monocytes, macrophages, T cells, mast cells, and B cells, increases pro-inflammatory and anti-inflammatory cytokine levels (Citation4–6). These cytokines may promote further recruitment of inflammatory cells, activate inflammatory C-reactive protein (CRP) (Citation7–9), suppress collagen synthesis (Citation10), and regulate protease expression for aortic wall collagen and elastin degradation (Citation11,Citation12). Cytokines may also affect one another's production and activity (Citation13). Although many types of inflammatory cells produce the same cytokine, each source of an individual cytokine may affect AAAs differently. AAA patients exhibit elevated peripheral CD4+ and CD8+ T cells, which contribute to peripheral interferon-γ (IFN-γ) production (Citation14). The absence of CD4+ T cells engendered resistance to CaCl2-induced AAAs in mice. Intraperitoneal reconstitution of IFN-γ reversed AAAs in Cd4–/– mice (Citation15). CD8+ aortic elastase perfusion-induced AAAs in mice also requires T cell-derived IFN-γ (Citation16). Our prior study showed that mast cell-derived IFN-γ also contributed to the pathogenesis of elastase perfusion-induced AAAs (Citation17).
Most studies suggest an increase of pro-inflammatory cytokines, such as IL6, TNF-α, IL1β, IFN-γ, and IL17A, in either plasma or aortic tissue extracts from AAA patients (Citation18–20), particularly in those with ruptured AAAs (Citation21,Citation22). In contrast, AAA patient plasma or explant AAA lesion culture (Citation23,Citation24) often exhibits lower levels of anti-inflammatory cytokines, such as IL10, particularly in patients with ruptured AAAs (Citation22). Yet other studies showed that plasma IL6 levels remained stagnant between AAAs and controls, and did not correlate with AAA growth rate (Citation25,Citation26). Plasma anti-inflammatory IL10 levels correlated with CRP (Citation27), and AAA tissue extracts expressed higher IL10 levels than aortic occlusive disease (AOD) tissue extracts (Citation28). Patients with ruptured AAAs expressed higher plasma IL6 and IL10 levels than patients with non-ruptured AAAs (Citation29). Plasma TNF-α and IL8 levels were significantly elevated in patients with large or symptomatic AAAs (Citation30).
Most prior studies discussed above used small numbers of AAA patients. It is unknown if these conflicting conclusions arose from the size of the study population or variations among patients or studies. This study tested three pro-inflammatory cytokines (IL6, IL17A, and IFN-γ), one anti-inflammatory cytokine (IL10), and an inflammatory biomarker (CRP) in 476 AAA patients and 200 age-matched AAA-free controls who had undergone years of follow-up to test whether these cytokines associate with AAA risk, size, or expansion rate.
Materials and methods
Study population
In an ongoing randomized population-based screening trial for AAA, peripheral arterial disease (PAD), and hypertension in more than 50,000 men 65–74 years of age in the mid-region of Denmark (Citation31), baseline plasma samples were taken consecutively at diagnosis of 476 AAA patients and in 200 age-matched controls without AAA or PAD. AAA was defined as having a maximal aortic diameter greater than 30 mm, and PAD was defined as an ankle–brachial index (ABI) lower than 0.90 or > 1.4. AAA cases among first-degree relatives, smoking status, coexisting diabetes mellitus, hypertension, and use of β-blockers, angiotensin-converting enzyme (ACE) inhibitors, steroids, non-steroid anti-inflammatory drugs (NSAIDs), and statins were recorded. Body mass index (BMI) and systolic and diastolic blood pressure were also measured and recorded. Ankle systolic blood pressure was also measured, as previously validated and reported (Citation32), and maximal anterior–posterior diameter of the infrarenal aorta was measured in the peak of the systole from the inner edge to inner edge of aorta (Citation33). The lowest ABI was calculated as the lowest recorded ankle blood pressure divided by the brachial systolic blood pressure. To quantify the size of an AAA more accurately by taking the commonly observed ellipse-shaped configuration into consideration, patients with AAAs had a semi-automated measurement of the outer maximal area at the level of the largest Anteroposterior diameter by one observer at baseline. The intraobserver variation of this outer maximal AAA area is 0.99 in a test of 2 × 25 measurements (Citation34).
Patients with AAAs of less than 50 mm were offered annual control scans by the screening team; patients with AAAs measuring 50 mm or larger were referred for a computed tomography (CT) scan and vascular surgical evaluation. The interobserver variation of aortic diameter measurements was 1.52 mm (Citation33). Growth rates of small AAAs in patients kept under surveillance were calculated by individual linear regression analysis, using all observations. Vital surveillance of the aneurysmal cases was received from the nationwide patient administrative system. Blood samples were centrifuged at 3,000 g for 12 minutes, aliquoted, and stored at –80°C until analysis was performed. Informed consent was obtained from all subjects before participation, and the study was approved by the Local Ethics Committee of the Central Denmark Region, Denmark, and performed in accordance with the Helsinki Declaration. Use of non-coded human samples was also approved by the Partners Human Research Committee, Boston, Massachusetts, USA.
ELISA
Plasma IL6, IFN-γ, IL10, and IL17A levels were determined blindly using Human ELISA Ready-SET-Go!® kits (eBioscience Inc., San Diego, CA, USA) according to the manufacturer's instructions. Ultrasensitive plasma CRP was measured by the vario-method on an Architect c8000 analyzer according to the manufacturer's instructions (Abbott Laboratories, Abbott Park, IL, USA).
Statistics
Dichotomous variables were expressed as proportions and compared by the chi-square test, and reported as odds ratios (OR). Potential confounders were compared between cases and controls, as well as potential associations with the cytokines by Mann–Whitney U and Spearman's correlation analysis. Associations with a probability below 0.10 were considered to be potential confounders and used in multivariate analyses. The potential biomarkers were then tested with one-sample Kolmogorov–Smirnov test, and probability plots (not shown) were used to determine whether the cytokines, AAA size, and mean annual aneurysmal growth rate were normally distributed. Log transformation of the levels of the cytokines was necessary to secure normal distributions. The cytokines were then tested as independent predictors of AAA by univariate and multivariate logistic regression analysis, adjusting for the potential identified confounders. The associations between the potential serological biomarkers were then correlated to maximal anterior–posterior aortic diameter by Pearson's correlation analysis. Significant associations were tested by multivariate linear regression analysis adjusting for the aforementioned potential AAA confounders. Furthermore, AAA cases were classified as small (as defined by their maximal diameters between 30 and 49 mm) or large AAA (as defined by their maximal diameters at 50 mm and greater) and compared respectively to controls by a similar statistical approach. Subgroup analyses of all AAA cases concerning maximal outer area and growth rate were performed by Pearson's correlation analysis. Significant associations were tested by multivariate linear regression analysis, adjusting for the aforementioned potential AAA confounders. Growth rates (y) were calculated as the slope (α) of linear regression of all individually measured AAA diameters (x) over observation time in years (t), y = ax/t. The association of all measured variables above the median in cases with screening-detected AAAs and mid-term mortality was tested by Cox's proportional regression analysis univariately and multivariately adjusted for the above-mentioned potential confounders. Assumptions for proportional hazards were tested graphically by log-minus-log plots. SPSS 17.0 was used as statistical software.
Results
Case control study
Of more than 50,000 participants, 25,143 were randomized for population-based screening for PAD, AAA, and hypertension— among which 18,698 men (74%) attended (Citation31). Of the first 476 consecutively diagnosed cases of AAA (3.3%), 385 had small AAAs (aortic diameters smaller than 50 mm) and were offered surveillance ranging from 0.47 to 3.4 years, with an average of 1.9 ± 0.59 (mean ± SD) years. Patients with AAA measuring 50 mm or more were referred for a CT scan and the vascular surgical department to be evaluated for potential repair. Patients who did not receive a surgical referral were further followed by the screening team. Demographic factors and potential confounders, as well as aortic diameters, lowest ABI, growth rates, and serological findings, have been reported previously (Citation33). The mean ages were 69.9 ± 2.8 (mean ± SD) years and 69.6 ± 2.8 (mean ± SD) years among those without and with AAA, respectively.
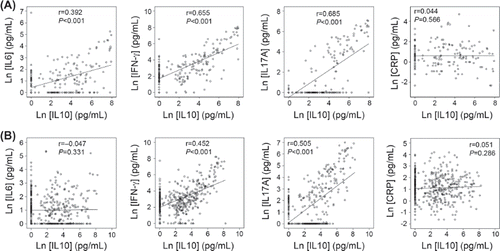
In this study, Student's t test demonstrated that 476 AAA patients had significantly lower plasma IFN-γ (103.78 ± 16.57 versus 206.94 ± 39.70 pg/mL, P = 0.021), IL10 (114.73 ± 16.83 versus 230.62 ± 40.75 pg/mL, P < 0.001), IL17A (36.48 ± 5.88 versus 68.38 ± 13.32 pg/mL, P = 0.028), and higher plasma CRP (5.92 ± 0.39 versus 4.52 ± 0.76 pg/mL, P < 0.001) levels than 200 age-matched controls. In contrast, plasma IL6 levels were also lower in AAA patients than in controls, but this did not reach statistical significance (5.07 ± 0.78 versus 13.63 ± 5.22 pg/mL, P = 0.121) (). Spearman's correlation test showed that anti-inflammatory IL10 associated positively and significantly with IL6, IFN-γ, and IL17A in the control group (), and positively and significantly with IFN-γ and IL17A in the AAA group (). In contrast, IL10 did not associate with CRP in either group (). In the control group, IL6 correlated positively and significantly with IFN-γ (Rho = 0.439, P < 0.001) and IL17A (Rho = 0.306, P < 0.001), but negatively with CRP (Rho = –0.169, P = 0.032). In AAA patients, IL6 correlated positively and significantly with IL17A (Rho = 0.207, P < 0.001), but not with IFN-γ (Rho = –0.009, P = 0.849) or CRP (Rho = 0.007, P = 0.889).
We examined the differences of familial disposition, current smoking habits, diabetes mellitus, hypertension, use of ACE inhibitors, use of β-blockers, use of low doses of aspirin or clopidogrel, use of statins, systolic and diastolic blood pressure (SBP and DBP), age, BMI, lowest ABI, and maximal aortic diameters between patients with and without (age-matched control) AAA. Chi-square test showed that smoking, hypertension, use of β-blockers, use of statins, and use of NSAIDs (non-steroidal anti-inflammatory drugs) were significant risks of human AAAs (), among which the use of NSAIDs was the strongest risk factor (OR 9.07, 95% CI 1.22–67.5, P < 0.001). Although we do not have a precise explanation for this, patients with arthrosis or rheumatic disease often receive NSAID treatment. In our cohort, we do not have patient information regarding arthrosis, but patients with painful walking without immediate relief at rest as in intermittent claudication had a significantly increased risk of AAA (OR 4.42, 95% CI 2.49–7.86, P < 0.001). Therefore, it is plausible that the strongest association between AAA and NSAIDs may be due to increased incidence of arthrosis symptoms among AAA patients, a hypothesis that merits further investigation. Among continuous variables, both SBP and DBP, age, and BMI were all significantly higher among AAA patients than in controls in a Mann–Whitney U test (). The same method found that none of the tested dichotomous variables significantly affected any of the tested cytokines (IL6, IFN-γ, IL10, and IL17A). Only smoking reduced plasma IL6 and increased plasma CRP, use of β-blockers increased plasma IL6, use of statins decreased plasma IFN-γ and CRP, and use of steroids increased plasma CRP to a possibility of below P < 0.10 (). These dichotomous variables were qualified as potential confounding factors of plasma cytokines. Among continuous variables, Spearman's correlation analysis demonstrated a significant but negative association of plasma IL10 and SBP. Such a negative correlation also applied to DBP and qualified DBP as a potential factor in affecting plasma cytokine levels. In contrast, plasma CRP showed a positive correlation with both DBP and BMI ().
Table I. Dichotomous and continuous variables between AAA patients and controls and their associations with plasma medians of IL6, IFN-γ, IL10, IL17A, and CRP levels.
Plasma cytokines as independent risk factors of human AAA
Univariate logistic regression analysis showed that both IFN-γ and IL10, after logarithmic conversion due to abnormal data distribution (one-sample Kolmogorov–Smirnov tests), were significant protecting factors of human AAA with OR at 0.91 (P = 0.044) and 0.91 (P = 0.011), respectively, but CRP was a significant risk factor with OR at 1.54 (P < 0.001) (). Multiple logistic regression analysis demonstrated that plasma CRP remained a significant risk factor of human AAAs after adjusting for smoking, diabetes mellitus, hypertension, use of β-blockers, statins, and NSAIDs, SBP, DBP, age, and BMI (OR 1.41, P < 0.001) (). Both IFN-γ (OR 0.98, P = 0.743) and IL10 (OR 0.96, P = 0.372) no longer associated with AAA risks after the adjustment (). Among all dichotomous variables, use of NSAIDs was the strongest risk factor for AAA (OR 9.07, P < 0.01). However, adjustment for NSAIDs alone weakly affected the association between AAA risks and IFN-γ (OR 0.91, 95% CI 0.83–1.01, P = 0.061) or IL10 (OR 0.92, 95% CI 0.85–0.98, P = 0.013).
Table II. Univariate and multivariate logistic regression analyses of logarithmization-transformed plasma medians of IL6, IFN-γ, IL10, IL17A, and CRP levels as independent risk factors of human aneurismal disease.
When AAA patients were subgrouped into small (maximal diameters between 30 and 49 mm) and large AAAs (maximal diameters at 50 mm and greater), we obtained similar conclusions. Univariate comparison by Student's t test of small and large AAAs with controls, respectively, showed significantly lower levels of IL10 and higher levels of CRP in both small and large AAAs than those in controls (). When large AAAs were compared with small AAAs, we did not detect significant differences among all tested molecules. In a multivariate logistic regression analysis after adjusting for common AAA risk factors, CRP was the only tested cytokine that remained a significant risk factor of small (OR 1.25, P = 0.046) and large (OR 1.47, P = 0.049) AAA. Such significances disappeared when large AAAs were compared with small AAAs ().
Table III. Comparison and risk assessment of five cytokines in controls versus cases of small and large AAA.
Plasma cytokines as independent risk factors of the natural history of human AAAs
For all 676 patients from this study, both univariate and multivariate Pearson's correlation analyses demonstrated that IL10 showed weak negative association with maximal aortic diameter (r = –0.075, P = 0.051) before adjustment for AAA risk factors smoking, diabetes mellitus, hypertension, use of β-blockers, statin, and NSAID, SBP, DBP, age, and BMI. Such weak significant and negative association between IL10 and maximal aortic diameter disappeared after the adjustment. Only CRP, but none of the other tested plasma cytokines (IL6, IL17A, and IFN-γ), associated positively with maximal aortic diameter in this population before (r = 0.202, P < 0.001) and after (r = 0.165, P < 0.001) adjusting for AAA confounding factors (). Yet the same analysis demonstrated that plasma IFN-γ levels from 476 AAA patients associated significantly and positively with maximal cross-sectional AAA area in a univariate analysis (r = 0.116, P = 0.044). Multivariate analysis after adjusting for aforesaid common AAA confounding factors and initial AAA size demonstrated increased significance of association of plasma IFN-γ (r = 0.133, P = 0.009), IL17A (r = 0.101, P = 0.044), and CRP (r = 0.097, P = 0.036) with maximal cross-sectional AAA area ().
Table IV. Univariate and multivariate Pearson's correlation analyses of the five cytokines’ potential associations with maximal aortic diameter in healthy and aneurysmal cases.
Table V. Univariate and multivariate Pearson's correlation analysis to determine the associations between the five cytokines and AAA size and AAA expansion rate.
Of 476 AAA patients, 385 had small AAAs and were monitored from 0.47 to 3.4 years (1.9 ± 0.59, mean ± SD, years). Pearson's correlation analysis also demonstrated that, in this AAA sub-population, plasma IL10 (r = 0.126, P = 0.028) and CRP (r = 0.121, P = 0.024) levels correlated significantly and positively with AAA maximal annual growth rate in a univariate analysis. In a multivariate analysis after adjusting for all listed potential AAA confounding factors and initial AAA sizes, however, only IL10 remained correlated with AAA maximal annual growth rate (r = 0.108, P = 0.036) ().
Although this study had only a very short period of follow-up (1.9 ± 0.59, mean ± SD, years), univariate Cox's proportional regression analysis demonstrated that plasma CRP levels above the median in cases with AAA significantly associated with mid-term mortality (hazard ratio (HR) 1.91, 95% CI 1.25–2.93, P = 0.003). Such significance remained after adjusting for the above-mentioned potential confounders (adjusted HR 2.20, 95% CI 1.38–3.51, P < 0.001) ().
Table VI. Univariate and multivariate Cox's proportional regression analysis to determine the associations of cytokines above the median in cases with screening-detected AAA and mid-term mortality.
Discussion
This study used 476 AAA patients screened from 50,000 men. This unprecedentedly large AAA population demonstrated reduced plasma levels of all four tested cytokines along with the elevated inflammatory biomarker CRP, regardless of pro-inflammatory (IL6, IL17A, and IFN-γ) or anti-inflammatory (IL10) properties, although the reduction of IL6 levels did not reach statistical significance (). These observations validated the findings that high plasma IFN-γ and IL10 levels were indeed protective markers from AAAs in humans before adjusting the confounding effects of other known AAA risk factors when both AAA and control patients were considered ().
It may be difficult to classify the cell types responsible for AAA lesion or peripheral IFN-γ, IL10, or IL6, although Th17 cells may express their unique IL17A (Citation35). Smooth muscle cells and endothelial cells also produce IFN-γ, IL10, or IL6 (Citation36,Citation37). T cells are one of the likely main sources of these cytokines. There has been historic dogma on whether T cell responses in AAA patients were Th1 preference or dominated Th2 type. Previous studies show that human AAA lesions bear both Th1 and Th2 type responses with increased Th1 cytokines IL2, IFN-γ, TNF-α, IL1α, and Th2 cytokines IL4, IL10, and IL13 (Citation38), but other reports suggest that Th1 cytokines are dominant in large AAAs. The expression of IFN-γ is increased in the peripheral and in AAA aortic tissues (Citation20,Citation39–41). Freshly isolated lymphocytes from human AAA aortic walls contain both CD4+ and CD8+ T cells. CD4+ T cells produce IFN-γ, but not IL4, reflecting a Th1 response (Citation39). Yet different studies suggest Th2-dominant immune responses in AAAs, resulting in expansive vascular remodeling and luminal ectasia. Human AAAs are characterized by the predominance of Th2 cytokine expression and a paucity of Th1 cytokines, especially IFN-γ (Citation42,Citation43). When wild-type mice and IFN-γ receptor-deficient mice received allograft aortic transplantation, wild-type mice developed intimal hyperplasia, whereas the IFN-γ receptor-deficient mice developed severe AAAs. When IFN-γ receptor-deficient recipient mice received treatment with an anti-IL4 monoclonal antibody or IFN-γ receptor, and IL4 double-deficient mice were used as recipient mice, both strategies nearly completely prevented AAA formation (Citation11). These observations supported a hypothesis that Th2 cytokines (e.g. IL4, IL5, IL10) and Th2 responses predominate in human AAA lesions, and Th1 responses predominate in stenotic atherosclerotic lesions (Citation44).
Most tested cytokines did not correlate with maximal aortic diameters, and only IL10 showed weak and negative correlation with maximal aortic diameters before adjusting for common AAA risk factors (). Instead, both plasma IFN-γ and IL17A levels correlated significantly and positively with a maximal cross-sectional area in a multivariate Pearson's correlation analysis when only 476 AAA patients were considered (). AAA formation includes both aortic wall expansion and inflammatory cell infiltration and proliferation, which are responsible for enlarged aortic diameters and lesion inflammation (Citation45,Citation46). Reduced plasma IFN-γ and IL17A in AAA patients (), insignificant correlation of these cytokines to maximal aortic diameters (), but positive and significant correlation with maximal cross-sectional AAA areas among 476 AAA patients () suggest that enriched inflammatory cells in AAA lesions and possibly in the peripheral from this AAA population are responsible, at least in part, for plasma IFN-γ and IL17A from AAA patients, although this hypothesis was not tested in this study.
Plasma IL10 levels were reduced in AAA patients, compared with those from AAA-free controls (), supporting an anti-inflammatory role of this cytokine against AAA formation (Citation47). Yet both univariate and multivariate Pearson's correlation analyses demonstrated a significant but positive correlation with AAA annual growth rate among 386 patients with small AAAs (), although this study had relatively short years of follow-up (0.47 to 3.4 years). Prior studies revealed a positive correlation of plasma IL10 with the well-known inflammation biomarker CRP from AAA patients (Citation27), and patients with ruptured AAAs had higher plasma IL6 and IL10 than those with non-ruptured AAAs (Citation29), suggesting a positive correlation of IL10 with inflammation. This hypothesis is consistent with our study that IL10 levels in both AAA patients () and AAA-free patients () correlated significantly and positively with plasma IFN-γ, IL17A, or IL6, although IL10 did not correlate with CRP in either group (). Significant correlations between IL10 and inflammatory cytokines IFN-γ, IL17A, or IL6, between maximal cross-sectional AAA area and IFN-γ and IL17A, and between maximal annual growth rate and IL10 together suggest that human AAAs have Th1, Th2, and even Th17 responses. However, positive correlations between IL10 and pro-inflammatory cytokines were reported to support a compensatory anti-inflammatory response of IL10 (Citation29). Similar observations have been reported in other biomarkers of human AAAs. Alpha-1 antitrypsin is a systemic elastase inhibitor, but it positively associated with AAA expansion rate along with matrix metalloproteinase-9 among a group of 121 men with small AAAs (Citation48). Therefore, the exact role of IL10 in human AAAs remains unclear from this study, although mice were prone to develop angiotensin II infusion-induced AAAs in the absence of IL10 (Citation49).
Among all tested variables, CRP remained the most powerful predictor of human AAA (), and associated with maximal aortic diameter () and maximal cross-sectional AAA area () after adjustment for common AAA confounding factors. It was the only significant risk factor of AAA-associated mortality among all tested variables (). Although plasma IL10 was reduced and CRP was increased in AAA patients, we did not detect any association between IL10 and CRP in either the AAA or non-AAA population (). This observation also supports a compensatory role of IL10 in human AAA, a hypothesis that merits further investigation in humans and experimental models.
Together, this study may favor all Th1, Th2, Th17 immune responses in human AAAs with reduced plasma Th1 cytokine IFN-γ, Th2 cytokine IL10, and Th17 cytokine IL17A, when patients with AAAs were compared with controls. Within the AAA populations, enhanced inflammatory cell infiltration (Citation1–6) in the aortic wall may enlarge AAA lesion cross-sectional area and expedite AAA growth. Observations from this relatively large randomized population-based study may potentially guide the next phase of study to the cell types that are more important to human AAA development than other human arterial diseases.
Acknowledgements
Mengyang Liao and Cong-Lin Liu contributed equally to this study. The authors thank Henriette Lindholt for technical assistance and Chelsea Swallom for editorial assistance.
Funding: This study is supported by the mid-region of Denmark and the European Commission Seventh Framework Programme, Health–2007–2.4.2–2 agreement number 200647 (J.S.L.); and by grants from the National Institutes of Health (HL60942, HL81090, HL88547) (G.P.S.).
Declaration of interest: The authors declare no conflicts of interest.
References
- Vanderlaan PA, Reardon CA. Thematic review series: the immune system and atherogenesis. The unusual suspects: an overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005; 46:829–38.
- Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, et al. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–405.
- Bobryshev YV, Lord RS. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis. 2001;154:15–21.
- Vainas T, Lubbers T, Stassen FR, Herngreen SB, van Dieijen-Visser MP, Bruggeman CA, et al. Serum C-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation. 2003;107:1103–5.
- Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:II224–7.
- Hamano K, Li TS, Takahashi M, Kobayashi T, Shirasawa B, Ito H, et al. Enhanced tumor necrosis factor-alpha expression in small sized abdominal aortic aneurysms. World J Surg. 2003;27:476–80.
- Tsipouras P, Schwartz RC, Liddell AC, Salkeld CS, Weil D, Ramirez F. Genetic distance of two fibrillar collagen loci, COL3A1 and COL5A2, located on the long arm of human chromosome 2. Genomics. 1988;3:275–7.
- Pickering JG, Ford CM, Chow LH. Evidence for rapid accumulation and persistently disordered architecture of fibrillar collagen in human coronary restenosis lesions. Am J Cardiol. 1996;78:633–7.
- Sukhova GK, Schönbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–9.
- Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995; 96:318–26.
- Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–8.
- Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–83.
- Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–72.
- Duftner C, Seiler R, Klein-Weigel P, Göbel H, Goldberger C, Ihling C, et al. High prevalence of circulating CD4 + CD28- T-cells in patients with small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2005;25:1347–52.
- Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4 + T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–12.
- Zhou HF, Yan H, Cannon JL, Springer LE, Green JM, Pham CT. CD43-mediated IFN-γ production by CD8 + T cells promotes abdominal aortic aneurysm in mice. J Immunol. 2013;190:5078–85.
- Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–68.
- Dawson J, Cockerill GW, Choke E, Belli AM, Loftus I, Thompson MM. Aortic aneurysms secrete interleukin-6 into the circulation. J Vasc Surg. 2007;45:350–6.
- Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjälä H, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997; 17:2843–7.
- Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126:S38–45.
- Treska V, Kocova J, Boudova L, Neprasova P, Topolcan O, Pecen L, et al. Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cytokines Cell Mol Ther. 2002;7:91–7.
- Cheuk BL, Cheng SW. Differential secretion of prostaglandin E(2), thromboxane A(2) and interleukin-6 in intact and ruptured abdominal aortic aneurysms. Int J Mol Med. 2007;20:391–5.
- Kadoglou NP, Papadakis I, Moulakakis KG, Ikonomidis I, Alepaki M, Moustardas P, et al. Arterial stiffness and novel biomarkers in patients with abdominal aortic aneurysms. Regul Pept. 2012;179:50–4.
- Vucevic D, Maravic-Stojkovic V, Vasilijic S, Borovic-Labudovic M, Majstorovic I, Radak D, et al. Inverse production of IL-6 and IL-10 by abdominal aortic aneurysm explant tissues in culture. Cardiovasc Pathol. 2012;21:482–9.
- Parry DJ, Al-Barjas HS, Chappell L, Rashid ST, Ariëns RA, Scott DJ. Markers of inflammation in men with small abdominal aortic aneurysm. J Vasc Surg. 2010;52:145–51.
- Jones KG, Brull DJ, Brown LC, Sian M, Greenhalgh RM, Humphries SE, et al. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation. 2001;103:2260–5.
- Muehling BM, Paintner A, Marx N, Barth TF, Babiak C, Orend KH. In vivo study on the expression pattern of resistin in patients with abdominal aortic aneurysm. Vasc Endovascular Surg. 2011;45:63–8.
- Davis VA, Persidskaia RN, Baca-Regen LM, Fiotti N, Halloran BG, Baxter BT. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–6.
- Wallinder J, Skagius E, Bergqvist D, Henriksson AE. Early inflammatory response in patients with ruptured abdominal aortic aneurysm. Vasc Endovascular Surg. 2010;44:32–5.
- Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med. 2000;38:1161–4.
- Grøndal N, Søgaard R, Henneberg EW, Lindholt JS. The Viborg Vascular (VIVA) screening trial of 65–74 year old men in the central region of Denmark: study protocol. Trials. 2010;11:67.
- Joensen JB, Juul S, Abrahamsen J, Henneberg EW, Lindholt JS. Doppler ultrasound compared with strain gauge for measurement of systolic ankle blood pressure. Angiology. 2008;59:296–300.
- Grøndal N, Bramsen MB, Thomsen MD, Rasmussen CB, Lindholt JS. The cardiac cycle is a major contributor to variability in size measurements of abdominal aortic aneurysms by ultrasound. Eur J Vasc Endovasc Surg. 2012;43:30–3.
- Behr-Rasmussen C, Grøndal N, Bramsen MB, Thomsen MD, Lindholt JS. Mural thrombus and the progression of abdominal aortic aneurysms: a large population-based prospective cohort study. Eur J Vasc Endovasc Surg. 2014;48:301–7.
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4 + effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
- Gerthoffer WT, Singer CA. Secretory functions of smooth muscle: cytokines and growth factors. Mol Interv. 2002;2:447–56.
- Vernier A, Diab M, Soell M, Haan-Archipoff G, Beretz A, Wachsmann D, et al. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect Immun. 1996;64: 3016–22.
- Lindeman JH, Abdul-Hussien H, Schaapherder AF, Van Bockel JH, Von der Thüsen JH, et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond). 2008;114: 687–97.
- Galle C, Schandené L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, et al. Predominance of type 1 CD4 + T cells in human abdominal aortic aneurysm. Clin Exp Immunol. 2005;142:519–27.
- Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45:574–80.
- Golledge AL, Walker P, Norman PE, Golledge J. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis Markers. 2009;26:181–8.
- Schönbeck U, Sukhova GK, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506.
- Chan WL, Pejnovic N, Liew TV, Hamilton H. Predominance of Th2 response in human abdominal aortic aneurysm: mistaken identity for IL-4-producing NK and NKT cells? Cell Immunol. 2005;233:109–14.
- Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, et al. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–213.
- Lukasiewicz A, Reszec J, Kowalewski R, Chyczewski L, Lebkowska U. Assessment of inflammatory infiltration and angiogenesis in the thrombus and the wall of abdominal aortic aneurysms on the basis of histological parameters and computed tomography angiography study. Folia Histochem Cytobiol. 2012;50:547–53.
- Hendy K, Gunnarson R, Golledge J. Growth rates of small abdominal aortic aneurysms assessed by computerised tomography—a systematic literature review. Atherosclerosis. 2014;235:182–8.
- Meng X, Yang J, Zhang K, An G, Kong J, Jiang F, et al. Regulatory T cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension. 2014;64:875–82.
- Lindholt JS, Vammen S, Fasting H, Henneberg EW, Heickendorff L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg. 2000;20:281–5.
- Ait-Oufella H, Wang Y, Herbin O, Bourcier S, Potteaux S, Joffre J, et al. Natural regulatory T cells limit angiotensin II-induced aneurysm formation and rupture in mice. Arterioscler Thromb Vasc Biol. 2013; 33:2374–9.

