Abstract
Introduction. We studied prevalence of hypovitaminosis D, its determinants, and whether achievement of recommended dietary vitamin D intake (10 μg/d) is associated with absence of hypovitaminosis D in adults.
Methods. The study is part of the Cardiovascular Risk in Young Finns Study. We collected serum samples of 25-hydroxyvitamin D as part of the 27-year follow-up (994 men and 1,210 women aged 30–45 years). Hypovitaminosis was defined as vitamin D concentration ≤ 50 nmol/L.
Results. Hypovitaminosis D was found in 38% of men and 34% of women. Dietary vitamin D intake (OR 0.90, 95% CI 0.86–0.93), use of vitamin–mineral supplements (0.66, 0.51–0.85), sunny holiday (0.55, 0.41–0.75), and oral contraceptive use in women (0.45, 0.27–0.75) were independently associated with reduced odds of hypovitaminosis. Increase in body mass index (1.06, 1.03–1.09), being a smoker (1.36, 0.97–1.92), investigation month (December versus other) (1.35, 1.12–1.61), and risk alleles in genotypes rs12785878 (1.31, 1.00–1.70) and rs2282679 (2.08, 1.66–2.60) increased odds of hypovitaminosis. Hypovitaminosis D was common also when recommended dietary intake was obtained (men 29%, women 24%).
Conclusion. Several factors were associated with hypovitaminosis D. The condition was common even when recommended vitamin D intake was reported. The results support the importance of vitamin D fortification and nutrient supplement use.
Hypovitaminosis D defined as serum 25(OH)D concentration < 50 nmol/L was common: 38% of men and 34% of women had hypovitaminosis in our nationally representative study sample.
The most important factors associated with serum 25(OH)D concentration were dietary vitamin D intake, the use of vitamin–mineral supplements, sunny holiday, and SNP rs2282679 coding for the vitamin D-binding protein; and in women the use of oral contraception.
When the recently recommended dietary intake of 10 μg/d was achieved, hypovitaminosis D was found in 29% of men and 24% of women.
Introduction
During the past decades there has been growing attention towards vitamin D and its functions. Its well-known importance for calcium metabolism and maintenance of bone health was observed nearly a century ago (Citation1), and even earlier the healing effect of ultraviolet light (UV light) (Citation2) and sunlight on rickets (Citation3) was observed. Along with the growing interest, new roles for vitamin D have been suggested. It has been reported that vitamin D may play an inhibiting role in the etiogenesis of diabetes (Citation4,Citation5), cardiovascular diseases (Citation6–9), and several cancers (Citation10). Currently, hypovitaminosis D is increasingly common worldwide, both in Western and developing countries (Citation11,Citation12).
Diet is an important source of vitamin D, especially for those individuals who are not exposed to sunlight (Citation13). Only a few foods, however, contain significant amounts of vitamin D naturally. The most important natural sources are fish, eggs, and offal, such as liver, and some mushrooms. With these natural sources alone it is difficult to maintain sufficient serum concentration which is estimated to be within the range of 50–75 nmol/L (Citation14). Therefore, foods are fortified with vitamin D. In Finland, liquid milk products (excluding organic products) and margarines are the main sources of vitamin D through fortification (Citation15).
Since UV light initiates cutaneous synthesis of vitamin D, individuals spending time in direct sunlight usually have adequate serum 25(OH)D concentrations (Citation16,Citation17). Increased body mass index (BMI) and smoking in turn associate with lower concentrations (Citation18,Citation19). Use of oral contraceptives in women is associated with higher concentrations of serum vitamin D (Citation20). Recent genome-wide association study has identified common genetic variants that influence serum 25(OH)D concentration (Citation21). These variants include single nucleotide polymorphism near genes DHCR7 (rs12785878), CYP2R1 (rs10741657), GC (rs2282679), and CYP24A1 (rs6013897) that are suggested to play a role in vitamin D synthesis (DHCR7 and CYP2R1) and metabolism (GC and CYP24A1).
The aim of this study was to examine comprehensively the determinants of serum 25(OH)D concentration in 2,204 participants of the Cardiovascular Risk in Young Finns Study. We specifically assessed prevalence and determinants of hypovitaminosis D, and examined whether the reported achievement of recommended dietary vitamin D intake was associated with a sufficient serum 25(OH)D concentration.
Subjects and methods
Setting and participants
The Cardiovascular Risk in Young Finns Study is an on-going multicenter follow-up study collecting data related to cardiovascular health. The project began in 1980 with a cross-sectional survey of 3,596 Caucasian participants 3–18 years of age (Citation22). The subjects were chosen randomly from the national register. The follow-ups were conducted in 1983, 1986, 2001, 2007, and 2011. In this study, the 27-year follow-up data collected in 2007 are used. The study comprised 2,204 subjects 30–45 years of age, of whom 994 were men and 1,210 women (). The subjects resided near the five follow-up centers across Finland. The subjects have signed an informed consent, and the study was approved by the local ethics committees.
Table I. Characteristics of the study population.
Serum 25(OH)D
The collection of serum samples was carried out from October to February. Serum 25-hydroxyvitamin D concentrations were determined using radioimmunoassay (DiaSorin, Stillwater, MN, USA). The inter-assay coefficient of variation was 8.0% (n = 137). The assay level was assessed by reference material SRM 968c Fat-Soluble Vitamins (National Institute of Standards and Technology, Gaithersburg, MD, USA) indicating an average bias of –2.5 nmol/L at nominal concentration 38 nmol/L. The laboratory and the method used to determine vitamin D were accredited according to standard ISO/IEC 17025:2005 by the Finnish Accreditation Service. We defined 25(OH)D deficiency as a concentration < 30 nmol/L and hypovitaminosis D as a concentration < 50 nmol/L. Concentrations ≥ 50 nmol/L were considered sufficient (Citation14,Citation23).
Determinants of serum 25(OH)D
Food consumption was assessed using a semi-quantitative, self-administered 131-item food frequency questionnaire (FFQ), developed and validated by the Institute of Health and Welfare (Citation24). In the FFQ, there were nine frequency categories ranging from never or seldom to at least six times a day. The subjects were asked to answer the survey based on their dietary habits during the previous 12 months. Reported frequencies and portions were transformed into grams/day. Mean daily consumption of foods and intakes of nutrients (including vitamin D) were calculated using the National Food Composition Database Fineli (Citation25). At the time of the data collection, liquid milk products (milk and sour milk, excluding organic milk) and margarines were fortified by 0.5 μg/100 mL and 10 μg/100 g, respectively. The recently recommended dietary intake of 10 μg/d was used as a cut-off value (Citation26). Additionally, the previously recommended intake of 7.5 μg/d (Citation27) was applied (in effect at the time of the data collection).
Use of vitamin–mineral supplements was inquired with a questionnaire. The survey did not separate vitamin D-containing supplements from other supplements. This approach has been used before (Citation11).
Daily alcohol consumption expressed as standard drinks per day (approximately 11 g of alcohol per drink) was calculated from the number of reported units of beer and other mild alcohol beverages, wines, and spirits during the previous week before the survey.
BMI was calculated from the measured height and weight (Citation29) as weight divided by the square of height (BMI = kg/m2). Waist circumference was measured midway between the lowest rib and the iliac crest as the average of two measurements with an accuracy of 0.1 cm.
Total testosterone concentrations were measured by Spectria Testosterone kit (Orion Diagnostica, Espoo, Finland) (Citation29).
Sun exposure was studied with a self-reported questionnaire by inquiring about the subject's habits on sun avoidance, holiday in a sunny location abroad, and a skiing or a downhill skiing winter holiday in the home country or abroad during the previous year.
The use of oral contraceptives, being pregnant or lactating, having given birth, smoking habits, and educational status were also assessed with a questionnaire. Oral contraceptive use, pregnancy, lactation, and given birth were inquired as yes/no. Subjects who smoked daily were considered as smokers. Educational status was based on subject's highest degree (grammar school, college or vocational school, and university degree).
Interest in health habits was inquired with a question ‘how much do you pay attention to your health habits’, on a scale from 1 to 5 (1 = hardly/not at all, 5 = very much).
Physical activity was assessed by standardized questionnaire. The metabolic equivalent index (MET index) was calculated as described previously (Citation30).
The investigation month variable was given values according to the decreasing amount of sunlight i.e. October and February had a value of 1, November and January had a value of 2, and December a value of 3 in the analyses.
Four vitamin D-related single nuclear polymorphisms (CYP2R1-rs10741657, DHCR7-rs12785878, GC-rs2282679, and CYP24A1-rs6013897) from genotyped data were chosen based on recent findings (Citation21). The genotyping process is described in detail elsewhere (Citation31).
Statistical analyses
Univariate linear regression analysis was used to determine the association of explanatory variables and serum 25(OH)D concentrations. Variables associated with serum vitamin D were entered into a multivariate linear regression analysis to study independent determinants of serum vitamin D. Two multivariable linear regression analysis models were constituted. In model 1, total intake of dietary vitamin D was used as a determinant. In model 2, the effect of the most important vitamin D food sources of the Finnish diet was studied separately (fortified milk, fish, and fortified margarines) (Citation32). R-square values were calculated for each variable to indicate their proportional influence on serum 25(OH)D concentration. Logistic regression model was used to study the determinants of hypovitaminosis D.
Cross-tabling (Fisher's exact test) was used to determine whether dietary vitamin D intake ≥ 10 μg/d (or ≥ 7.5 μg/d) was associated with serum 25(OH)D concentrations ≥ 50 nmol/L. All statistical analyses were conducted with SAS 9.2/9.3. P values < 0.05 were considered statistically significant.
Results
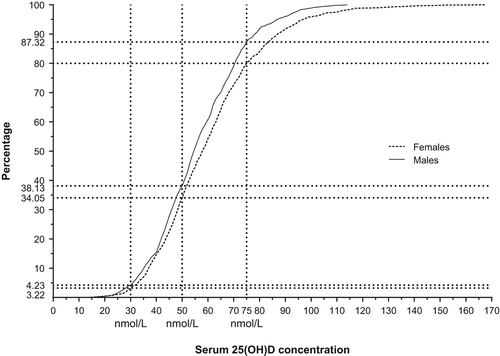
Mean ± SD serum 25(OH)D concentration was 57 ± 17 nmol/L in men and 61 ± 21 nmol/L in women (). In women using oral contraceptives, serum 25(OH)D concentration was 10 units higher than in non-users (69 ± 26 nmol/L versus 59 ± 19 nmol/L). Hypovitaminosis D was found in 38% of men and in 34% of women. For those not using any dietary supplements, the prevalence of hypovitaminosis was 43% in men and 38% in women. Vitamin D deficiency (serum 25(OH)D concentration < 30 nmol/L) was found in 4.2% of men and 3.2% of women.
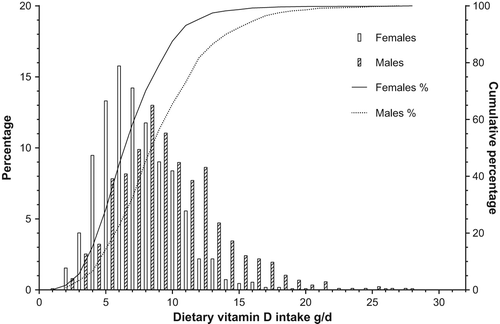
Figure 1. Cumulative distribution of serum 25(OH)D concentration and cut-off points for serum vitamin D deficiency (men: 4.2%, women: 3.2%), hypovitaminosis D (men: 38.1%, women: 34.1%), and sufficient concentration (men: 12.7%, women 20.0%). (Men n = 994; women n = 1,210).
Determinants of serum 25(OH)D concentration
Univariate associations
Univariate analyses studying the determinants of serum 25(OH)D concentration are shown in . Nearly all studied variables were associated with vitamin 25(OH)D concentration when men and women were studied combined (pooled data). The only exceptions were age, intake of sour milk products and eggs, and study center latitude. Age had a significant sex interaction (P = 0.0010); in the sex-stratified analyses it was directly associated with vitamin 25(OH)D concentration in men (β ± SE 0.30 ± 0.11, P = 0.0046) and inversely in women (β ± SE –0.23 ± 0.12, P = 0.049). In other analyses, no sex interactions were found. In men, serum testosterone concentration had a direct association with serum vitamin 25(OH)D (β ± SE 0.21 ± 0.10, P = 0.042). Use of oral contraceptives and lactation was associated with higher vitamin 25(OH)D concentration in women (β ± SE 9.11 ± 1.61, P < 0.0001 and β ± SE 6.56 ± 3.02, P = 0.030, respectively). Being pregnant or having given birth showed no association with serum vitamin 25(OH)D (β ± SE 3.49 ± 3.42, P = 0.31 and β ± SE –2.08 ± 1.38, P = 0.13, respectively).
Table II. Univariate linear regression analyses on determinants of serum 25(OH)D concentration.
Multivariate associations
In multivariate analyses (), sex was associated with serum 25(OH)D concentration. Men had 3.17 nmol/L lower concentration than women (P = 0.004). When women using oral contraceptives were excluded from the analysis, the sex difference disappeared [β(SE) –1.15 ± 1.07, P = 0.29]. One microgram increase in dietary vitamin D was associated with 0.87 nmol/L rise in the serum concentration (P < 0.0001). Use of any nutrient supplement was associated with 5.33 nmol/L higher serum 25(OH)D concentration. Increase in BMI was associated with lower vitamin D concentration. The results were similar when waist circumference was used instead of BMI (data not shown). Being interested in health habits had a positive association with serum vitamin D. A sunny holiday was associated with 6.06 nmol/L higher serum 25(OH)D concentrations (P < 0.0001), while sun avoidance was associated with 3.59 nmol/L lower serum vitamin D (P = 0.0003). Having had a skiing holiday was associated with 2.88 nmol/L higher concentrations. Investigation month in part determined serum vitamin D; the concentration was on average 2.80 nmol/L lower per month towards the winter solstice (P < 0.0001). Most of the selected genetic variables were associated with serum 25(OH)D concentrations.
Table III. Multivariable analysis on determinants of serum 25(OH)D concentration. Both total model and individual variable R2 values are shown.
In total the studied variables explained 18% of the serum 25(OH)D concentration (R2 = 0.1849). For single variables, the highest explanatory proportions were found for dietary vitamin D intake, nutrient supplement use, sunny holiday, and rs2282679. When the most important dietary vitamin D food sources were used instead of total dietary vitamin D intake, the results were essentially similar (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1020860). Fish and vitamin D-fortified milk and margarines were associated with higher serum 25(OH)D concentrations. Use of eggs and vitamin D-fortified sour milk products were not associated with serum 25(OH)D concentration.
Multivariate sex-stratified analyses
Similar to univariate analysis, age was associated with higher serum 25(OH)D concentration in men and lower concentration in women in sex-stratified multivariate analyses (). In men, alcohol consumption was associated with lower serum 25(OH)D concentrations, as was smoking in women. In women, interest in health habits was associated with higher serum 25(OH)D concentrations. The use of oral contraceptives was positively associated with serum 25(OH)D concentration also in the multivariate analysis.
Fortified dairy products and margarines as a source of dietary vitamin D
Mean ± SD dietary vitamin D intake was 9.0 ± 3.9 μg/d in men and 6.8 ± 2.9 μg/d in women. Dietary vitamin D intake from fortified dairy products and margarines was 4.4 ± 2.5 μg/d in men and 3.7 ± 2.0 μg/d in women. Hence, fortified dairy and margarines provided 49% and 54% of the food originating dietary vitamin D in men and women, respectively. Vitamin D intake from fortified milk alone was 2.4 ± 1.9 μg/d for men and 1.7 ± 1.4 μg/d for women (26% of food originating dietary vitamin D intake in men and 24% in women).
Determinants of hypovitaminosis D
Dietary vitamin D intake, use of nutrient supplements, and sunny holiday were independently associated with reduced odds of hypovitaminosis D (). Increase in BMI, use of alcohol, investigation month (December versus other), and risk alleles in genotypes rs12785878 and rs2282679 increased the odds of hypovitaminosis.
Table IV. Logistic regression analyses on determinants of hypovitaminosis D.
In sex-stratified analyses, the effects were generally in the same direction and of similar magnitude in men and women. In men, nutrient supplement use was associated with lower and rs12785878 and investigation month with higher odds of hypovitaminosis. In women, skiing holiday was associated with lower odds of hypovitaminosis D. In women, use of oral contraceptives was associated with lower odds of hypovitaminosis D (OR 0.45, CI 95% 0.27–0.75).
Recommended dietary vitamin D intake and hypovitaminosis D
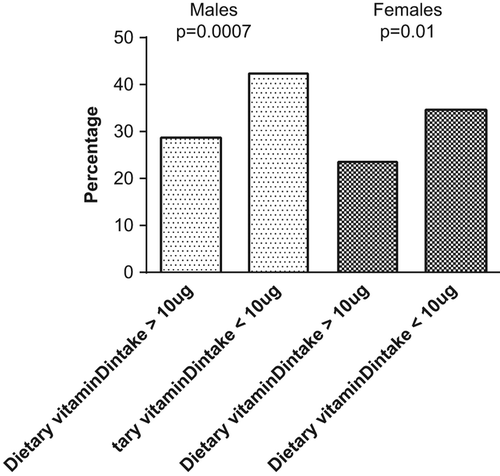
A total of 65.5% of men and 87.6% of women failed to achieve the recommended daily vitamin D intake of 10 μg () from food sources. Among those with dietary vitamin D intake ≥ 10 μg/d, 29% of men and 24% of women had hypovitaminosis (). Among those women with vitamin D intake ≥ 7.5 μg/d (prior dietary recommendation that was valid during the data sampling), use of oral contraceptives was associated with lower prevalence of hypovitaminosis D: 6.3% of the oral contraceptive users had hypovitaminosis compared to 29% of the non-users (P < 0.001). The analysis could not be done with the cut-off value ≥ 10 μg/d due to the low number of subjects.
Discussion
We observed that several lifestyle and genetic factors are associated with serum 25(OH)D concentrations among 30–45-year-old Finns. Although mean serum 25(OH)D concentration was sufficient both in men and women, hypovitaminosis D was common (38% in men and 34% in women). In individuals reporting recommended daily dietary vitamin D intake of 10 μg or more, the prevalence of hypovitaminosis D was slightly decreased (29% in men and 24% in women).
Dietary vitamin D intake was an important determinant of serum 25(OH)D concentration. On average, men reported an intake of 9.0 μg/d and women 6.8 μg/d, which is higher than found in the National FINDIET Survey conducted in 2007 (Citation33) or in North-America and Asia-Pacific countries (Citation34). The consumption of fish and vitamin D-fortified milk and margarine was associated with increased serum 25(OH)D concentrations, while no association was found for eggs and fortified sour milk. This lack of association may be due to the lesser use of eggs and sour milk in the diet resulting in lower amounts vitamin D derived from these sources. As food ingredients naturally containing significant amounts of vitamin D are scarce, it is difficult to obtain great amounts of vitamin D from the diet. In this study, 66% of men and 88% of women had vitamin D intake from food less than the recently recommended 10 μg/d. One way to enhance the intake of vitamin D at the population level is a wider fortification of foods (Citation35).
The higher intake of dietary vitamin D in men was related to higher use of fortified dairy products and also higher fish consumption. Despite higher dietary intake, men had lower serum 25(OH)D concentrations compared to women. However, when women using oral contraceptives were excluded from the analysis, there was no sex difference in serum vitamin D, suggesting that oral contraceptive use may in part explain the observed difference between men and women. The mechanisms that increase serum 25(OH)D concentrations in women using oral contraceptives containing estrogens are not completely understood. One explanation is that estrogen increases the concentration of circulating vitamin D-binding protein (Citation36). In our study, women using oral contraceptives had more seldom hypovitaminosis D (6.3% versus 33%), and their serum 25(OH)D concentrations were on average 10.2 nmol/L higher than in non-users. This finding is in accordance with previous studies (Citation21,Citation37,Citation38).
In women, those who had given birth had lower odds for hypovitaminosis D (). This may in part be due to frequent visits in maternity health clinics and child health clinics where mothers are informed of the importance of sufficient vitamin D intake.
BMI was associated with lower serum 25(OH)D concentration and higher odds of hypovitaminosis D. In line with this, prior data show that overweight or obese individuals more often have hypovitaminosis D (Citation39). This phenomenon is suggested to relate to volumetric dilution of vitamin D as it is a fat-soluble agent stored in the adipose tissue (Citation40). Hence, it is suggested that in overweight/obese individuals the larger storage capacity for vitamin D leads to lower circulating 25(OH)D.
Use of any vitamin–mineral supplement was associated with higher serum 25(OH)D concentration and lower odds of hypovitaminosis D. Although the supplements were not restricted to those containing vitamin D, the results suggest an important role for supplement use (Citation39,Citation41). The multivitamin or vitamin D supplements in Finland typically contain 10 or 20 μg vitamin D (oral communication, representative of University Pharmacy chain).
Having had a sunny holiday was an important determinant of serum 25(OH)D concentration. A week of sun-bathing can replenish the vitamin D reservoir notably (Citation13). In our study, a holiday in a sunny place was associated with 6.06 nmol/L elevated serum 25(OH)D concentration. Reporting sun avoidance was associated with lower serum concentrations in women. Our study population lives approximately between 60° N and 65° N, where the amount of UV-B light efficient enough to cause epidermal synthesis is available for approximately half the year (Citation42). Here we did not observe an association between study center location and serum 25(OH)D concentration. Several factors such as clothing, use of sunscreen, and cloudiness affect sun exposure and may also dilute the effect of latitude (Citation41).
Smoking was associated with lower serum 25(OH)D concentration. This is in accordance with previous studies (Citation20,Citation43), but there are also studies where no association was found (Citation44,Citation45). Alcohol consumption was associated with increased odds of hypovitaminosis D. The same association is described with alcohol use disorder patients (Citation46), but with moderate consumption the association between serum 25(OH)D concentration and alcohol intake has been direct in several studies (Citation46,Citation47).
The absorption mechanisms of dietary vitamin D in the intestine are probably passive diffusion (Citation48) and active transport linked to that of cholesterol (Citation49). Source of synthetic endogenous vitamin D is the epidermal photolytic non-enzymatic reaction (Citation16). Before that reaction, 7-dehydrocholesterol is reduced by 7-dehydrocholesterol reductase DHCR7 whose activity is affected by the point mutation rs12785878 (Citation21). In circulation, vitamin D is carried by vitamin D-binding protein DBP that is affected by rs2282679 (Citation21) and chylomicrons (Citation51). Vitamin D needs activation to gain its full physiological potential (Citation51). The penultimate step in activation takes place in the liver where an enzyme affected by the point mutation rs10741657 (Citation21)—25-hydroxylase CYP2R1—converts vitamin D into 25-OH-D3 (Citation52) which is converted into the active metabolite 1,25-(OH)-2-D3 by the renal hydroxylation enzyme CYP27B1. The active form of vitamin D is catabolized by CYP24A1 (Citation53), which is influenced by point mutation rs6013897 (Citation22). In our study, the effects of these point mutations on vitamin D metabolism were similar to those reported recently (Citation21).
In this study, approximately 20% of the variation in serum vitamin 25(OH)D concentration could be explained by the studied variables. In prior studies examining several determinants of serum 25(OH)D, explanatory ratios of 20%–30% have generally been reported (Citation54–56). Although rather low, our result is thus in accordance with previous data. In a recent study from the EPIC cohort, 32.8% of the variation in serum vitamin 25(OH)D concentration could be explained by the studied variables (Citation57). In that study, by far the most important determinant of 25(OH)D was season—assessed throughout the year—explaining nearly half of the found variation (14.6%). In our study, the blood samples to determine serum 25(OH)D concentration were taken from October to February, diminishing the chance to detect seasonal variation. In consequence, this may be one reason why we did not find a higher explanatory ratio. Also more precise data on sun exposure, e.g. clothing and sun protection habits, might have increased the explained variation in serum vitamin 25(OH)D concentration.
The prevalence of hypovitaminosis D is high in Western countries (Citation38). In NHANES 2000–2004 hypovitaminosis D was found in 18.5% of men and 21.9% of women in a cohort similar to ours (Citation11). In our study, the prevalence of hypovitaminosis D was even higher which may partly be due to the difference in data sampling period and the amount of sun exposure before measurements. The latter may be explained by the differences in latitudes as the data collection in NHANES took place 25–47°N (Citation11) and in our study 60–65°N. The fact that individuals reporting recommended daily dietary vitamin D intake also experienced hypovitaminosis D may in part relate to the dietary intake assessment method, which, compared to food records, overestimates the intake of vitamin D (Citation24). This may lead to overestimation of those fulfilling the recommended intake. Recently, it was suggested that the US and Canadian recommendation for vitamin D intake (15 μg/d at age 1–70 y) is based on a miscalculation and should be considerably higher (Citation58). The debate on these claims is on-going, and no conclusions have been made. Interestingly, it has been reported that despite vitamin D supplementation with 10 μg/d or more, 10% of participants had serum concentrations of less than 50 nmol/L (Citation59). It may be that, especially during winter months in some individuals, supplemental vitamin D is needed to avoid hypovitaminosis D.
Estimates for sufficient serum 25(OH)D concentration vary. Regarding bone health, values from a minimum of 25 to 100 nmol/L have been suggested (Citation60,Citation61). Hypovitaminosis D is often defined as serum 25(OH)D concentration < 50 nmol/L. Serum 25(OH)D concentration where parathyroid hormone begins to plateau is sometimes considered optimal. The estimates differ notably and range from 37.5 nmol/L to 122 nmol/L; 75 nmol/L has been considered the most precise estimate (Citation62–64). Even considering merely bone health, it is suspected that the widely spread hypovitaminosis D is a health concern of our time.
Since our study population is purely Caucasian, the results may not be generalizable to people of other ethnic origin. FFQ as a method to study dietary intake has known limitations, e.g. data on the absolute intakes should be interpreted cautiously, but it is considered well-suited and valid for large cohorts and group averages as in this study (Citation24). A limitation is that vitamin–mineral supplement use was not specific for vitamin D. However, a similar approach has been used previously (Citation11). Due to the cross-sectional study design the results do not infer causal relationships. As we aimed to investigate the role of several determinants of serum vitamin 25(OH)D, multiple analyses were performed, which increases the chance of false positive findings. This is regarded as a limitation of the study. The large number of studied determinants, assessed with well-established methods, and the vast nationally representative sample are the main strengths of this study.
Conclusions
Hypovitaminosis D, serum 25(OH)D concentration < 50 nmol/L, was common (38% in men and 34% in women) even when recommended 10 μg/d was obtained from the diet (29% in men and 24% in women). Dietary vitamin D intake, nutrient supplement use, and a sunny holiday were associated with lower odds for hypovitaminosis D, while BMI, alcohol consumption, investigation month, and risk alleles at rs12785878 and rs2282679 were associated with increased odds. Most of the dietary vitamin D was derived from fortified food sources. These results give evidence for the importance of vitamin D fortification and nutrient supplement use.
Supplementary material available online
Supplementary Table I & II, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1020860.
iann_a_1020860_sm9823.pdf
Download PDF (45.5 KB)Acknowledgements
Irina Lisinen and Ville Aalto are gratefully acknowledged for their assistance in statistical analyses.
Funding: The Young Finns Study has been financially supported by the Academy of Finland: grants 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi); the Social Insurance Institution of Finland; Kuopio, Tampere and Turku University Hospital Medical Funds (grant 9N035 for T.L.), Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation of Cardiovascular Research; Finnish Cultural Foundation; Tampere Tuberculosis Foundation and Emil Aaltonen Foundation (T.L), Yrjö Jahnsson Fondation (T.L.).
Declaration of interest: The authors report no conflicts of interest.
Notes on Contributors
1) designed research (project conception, development of overall research plan, and study oversight): MJ, KP, OR, JV, AV; 2) conducted research (hands-on conduct of the experiments and data collection): NHK, MJ, MK, TL, B-ML, JM, VM, OR; 3) provided essential reagents or provided essential materials (applies to authors who contributed by providing animals, constructs, databases, etc, necessary for the research): MK, JM, SM; 4) analyzed data or performed statistical analysis: MJ, OR, AV; 5) wrote paper (only authors who made a major contribution): MJ, KP, OR, AV; 6) had primary responsibility for final content: OR, AV; 7) other: GCM (contributed to critical revision of the manuscript). Presented critical comments on the manuscript: NHK, AJ, TL, KP, VM, SM, JV. Read and approved the final manuscript: KP, JM, SM, JV.
References
- McCollum EV, Simmonds N, Becker JE, Shipley PG. Studies on experimental rickets. Journal of Biological Chemistry. 1922;53:293–312.
- Huldschinsky K. Heilung von Rachitis durch Künstliche Höhensonne (German: Healing from rickets with the aid of artificial sunlight). Dtsch Med Wochenschr. 1919;45:90–1..
- Mozolowski W. Jedrezj Sniadecki (1768-1838) on the Cure of Rickets. Nature. 1939;143:121.
- Scragg R, Sowers M, Bell C; Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–18.
- Hyppönen E, Laara E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3.
- Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12.
- Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–60.
- Marniemi J, Alanen E, Impivaara O, Seppänen R, Hakala P, Rajala T, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15:188–97.
- Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliövaara M, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–9.
- Garland CF, Garland FC, Gorham ED. Calcium and vitamin D. Their potential roles in colon and breast cancer prevention. Ann N Y Acad Sci. 1999;889:107–19.
- Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr.2008;88:1519–27.
- Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103:620–5.
- Duplessis CA, Harris EB, Watenpaugh DE, Horn WG. Vitamin D supplementation in underway submariners. Aviat Space Environ Med. 2005;76:569–75.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB. IOM (Institute of Medicine). 2011. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium.2011.
- Helldán A, Raulio S, Kosola M, Tapanainen H, Ovaskainen M, Virtanen S. Finravinto 2012 -tutkimus/The National FINDIET 2012 Survey. 2012;16/2013 Juvenes Print – Suomen Yliopistopaino Oy, Tampere, Finland. Permanent link: http://urn.fi/URN:ISBN:978-952-245-951-0..
- Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr, Anderson RR, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5.
- de Gruijl FR. Sufficient vitamin D from casual sun exposure? Photochem Photobiol. 2011;87:598–601.
- Kamycheva E, Joakimsen RM, Jorde R. Intakes of calcium and vitamin d predict body mass index in the population of Northern Norway. J Nutr. 2003;133:102–6.
- Lamberg-Allardt CJ, Outila TA, Kärkkäinen MU, Rita HJ, Valsta LM. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res. 2001;16:2066–73.
- Sowers MR, Wallace RB, Hollis BW, Lemke JH. Parameters related to 25-OH-D levels in a population-based study of women. Am J Clin Nutr. 1986;43:621–8.
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8.
- Åkerblom HK, Viikari J, Uhari M, Räsänen L, Byckling T, Louhivuori K, et al. Atherosclerosis precursors in Finnish children and adolescents. I. General description of the cross-sectional study of 1980, and an account of the children’s and families’ state of health. Acta Paediatr Scand Suppl. 1985;318:49–63.
- Kauppinen-Mäkelin R, Tähtelä R, Löyttyniemi E, Kärkkäinen J, Välimäki MJ. A high prevalence of hypovitaminosis D in Finnish medical in- and outpatients. J Intern Med. 2001;249:559–63.
- Männistö S, Virtanen M, Mikkonen T, Pietinen P. Reproducibility and validity of a food frequency questionnaire in a case-control study on breast cancer. J Clin Epidemiol. 1996;49:401–9.
- National Public Health Institute of Finland. Fineli. Finnish Food Composition Database. Release 7. Helsinki, Finland, the National Public Health Institute, Nutrition Unit, 2007. Available at: http://www.fineli.fi/.
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012. Part 1. NNR 5 2013 Oct 03, 2013;Nord 2013:009.
- Nordic Council of Ministers. Nordic nutrition recommendations 2004. Integrating nutrition and physical activity. Århus, Denmark: Nordic Council of Ministers; 2004.
- Juonala M, Viikari JS, Hutri-Kähönen N, Pietikäinen M, Jokinen E, Taittonen L, et al. The 21-year follow-up of the Cardiovascular Risk in Young Finns Study: risk factor levels, secular trends and east-west difference. J Intern Med. 2004;255:457–68.
- Firtser S, Juonala M, Magnussen CG, Jula A, Loo BM, Marniemi J, et al. Relation of total and free testosterone and sex hormone-binding globulin with cardiovascular risk factors in men aged 24-45 years. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2012; 222:257–62.
- Mansikkaniemi K, Juonala M, Taimela S, Hirvensalo M, Telama R, Huupponen R, et al. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2012;44:733–44.
- Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383.
- Paturi M, Tapanainen H, Reinivuo H, Pietinen P. The National FINDIET 2007 Survey. 2008;23.
- Whiting SJ, Green TJ, Calvo MS. Vitamin D intakes in North America and Asia-Pacific countries are not sufficient to prevent vitamin D insufficiency. J Steroid Biochem Mol Biol. 2007;103:626–30.
- Hirvonen T, Sinkko H, Valsta L, Hannila ML, Pietinen P. Development of a model for optimal food fortification: vitamin D among adults in Finland. Eur J Nutr. 2007;46:264–70.
- Aarskog D, Aksnes L, Markestad T, Rodland O. Effect of estrogen on vitamin D metabolism in tall girls. J Clin Endocrinol Metab. 1983; 57:1155–8.
- Harris SS, Dawson-Hughes B. The association of oral contraceptive use with plasma 25-hydroxyvitamin D levels. J Am Coll Nutr. 1998; 17:282–4.
- Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–92.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
- Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20:1444–8.
- Välimäki VV, Löyttyniemi E, Välimäki MJ. Vitamin D fortification of milk products does not resolve hypovitaminosis D in young Finnish men. Eur J Clin Nutr. 2007;61:493–7.
- Brustad M, Alsaker E, Engelsen O, Aksnes L, Lund E. Vitamin D status of middle-aged women at 65-71 degrees N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004;7:327–35.
- Lorentzon M, Mellstrom D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503.
- Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, et al. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. 2007;261:558–65.
- McKinney K, Breitkopf CR, Berenson AB. Association of race, body fat and season with vitamin D status among young women: a cross- sectional study. Clin Endocrinol (Oxf). 2008;69:535–41.
- Naude CE, Carey PD, Laubscher R, Fein G, Senekal M. Vitamin D and calcium status in South African adolescents with alcohol use disorders. Nutrients. 2012;4:1076–94.
- Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002;21:536–44.
- Hirani V, Cumming RG, Blyth FM, Naganathan V, Le Couteur DG, Handelsman DJ, et al. Vitamin D status among older community dwelling men living in a sunny country and associations with lifestyle factors: the Concord Health and Ageing in Men Project, Sydney, Australia. J Nutr Health Aging. 2013;17:587–93.
- Hollander D, Muralidhara KS, Zimmerman A. Vitamin D-3 intestinal absorption in vivo: influence of fatty acids, bile salts, and perfusate pH on absorption. Gut. 1978;19:267–72.
- Reboul E, Goncalves A, Comera C, Bott R, Nowicki M, Landrier JF, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol Nutr Food Res. 2011;55:691–702.
- Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–5.
- Lund J, DeLuca HF. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966;7:739–44.
- Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004;324:451–7.
- Masuda S, Byford V, Arabian A, Sakai Y, Demay MB, St-Arnaud R, et al. Altered pharmacokinetics of 1a,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D- 24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146:825–34.
- Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, Liu S, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–35.
- Bertrand KA, Giovannucci E, Liu Y, Malspeis S, Eliassen AH, Wu K, et al. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br J Nutr. 2012;108:1889–96.
- Freedman DM, Cahoon EK, Rajaraman P, Major JM, Doody MM, Alexander BH, et al. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide U.S. study. Am J Epidemiol. 2013;177:180–92.
- Kuhn T, Kaaks R, Teucher B, Hirche F, Dierkes J, Weikert C, et al. Dietary, lifestyle, and genetic determinants of vitamin D status: a cross-sectional analysis from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Eur J Nutr. 2014;53:731–41.
- Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014; 6:4472–5.
- Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22:1389–99.
- Basha B, Rao DS, Han ZH, Parfitt AM. Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am J Med. 2000;108:296–300.
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293: 2257–64.
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83.
- Kinyamu HK, Gallagher JC, Rafferty KA, Balhorn KE. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites. Am J Clin Nutr. 1998;67: 342–8.
- Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–8.