Abstract
Sepsis is a systemic uncontrolled inflammatory response in the presence of an infection. It remains a major cause of morbidity and mortality in hospitalized patients. According to its severity, sepsis can progress to three different states: severe sepsis, septic shock, and multiple organ dysfunction syndrome, related to organ dysfunction and/or tissue hypoperfusion. Different processes underlie its pathophysiology; among them are oxidative stress, endothelial and mitochondrial dysfunction, and angiogenesis-related factors. However, no studies have integrated these elements in sepsis. The main difficulty in sepsis is its diagnosis. Currently, the potential of inflammatory biomarkers in septic patients remains weak. In this context, the research into new biomarkers is essential to aid with sepsis diagnosis and prognostication. Furthermore, even though the current management of severe forms of sepsis has been effective, morbimortality remains elevated. Therefore, it is essential to explore alternative approaches to therapy development. The aim of this review is to present an update of evidence supporting the role of oxidative stress and angiogenesis-related factors in the pathophysiology of the different forms of sepsis. It proposes a novel convergence between both elements in their role in the disease, and it will cover their utility as new diagnostic tools, predictors of outcome, and as novel therapeutic targets.
Oxidative stress and angiogenesis-related factors play an important role in both mitochondrial and endothelial dysfunction in sepsis, but they have not been previously related to each other; hence, a novel integration of both elements is proposed.
Currently, standard diagnosis of sepsis is mainly based on inflammatory biomarkers, but their potential remains weak, which makes the research into new biomarkers essential, such as those of oxidative stress or angiogenesis.
The use of complementary therapies, such as mitochondrial antioxidants or anti-angiogenic molecules, might improve treatment efficacy of severe forms of sepsis.
Introduction
Sepsis is a systemic illness caused by microbial invasion of normally sterile parts of the body (Citation1). It is a clinical syndrome that complicates severe infection and is characterized by vasodilation, leukocyte accumulation, and increased vascular permeability in those tissues that are remote from the infection, and its diagnosis depends on several clinical criteria (Citation2).
Sepsis implicates the presence of both infection and a systemic inflammatory response, and there are three definitions related to it: severe sepsis, where sepsis is complicated by organ dysfunction or tissue hypoperfusion; septic shock, which is defined as sepsis-induced persistent arterial hypotension unexplained by other causes and despite adequate volume resuscitation (Citation3); and multiple organ dysfunction syndrome (MODS), defined as the progressive, potentially reversible dysfunction of two or more organ systems after acute, life-threatening disruption of systemic homeostasis (Citation4).
These definitions correlate with an increasing severity which, in turn, correlates with increasing mortality: the number rises from 25%–30% for severe sepsis up to 40%–70% for septic shock (Citation1).
Sepsis remains a major cause of morbidity and mortality in hospitalized patients in several industrialized nations, being the second leading cause of death among patients in non-coronary intensive care units (Citation5), with an increasing mortality and with as many deaths annually as those from acute myocardial infarction (Citation6).
The Surviving Sepsis Campaign has as objective to build awareness of the challenges associated with sepsis, where the fundamental challenge is the difficulty in its diagnosis (Citation2,Citation7). Once sepsis diagnosis is suspected, prompt confirmation is required for instauration of adequate therapeutic actions (Citation8).
Biomarker research is essential in sepsis to potentially diagnose, monitor, stratify and predict outcome (Citation9). Since the potential of inflammatory biomarkers for diagnosis of infection in patients with severe sepsis and septic shock remains undefined (Citation2), it is necessary to explore alternative sepsis-related biomarkers.
The aim of this review is to present an update of the evidence supporting the role of oxidative stress and angiogenesis-related factors (ARF) in the pathophysiological processes of sepsis, severe sepsis, septic shock, and MODS. It proposes a novel convergence between both elements in their role in the disease. Moreover, this review will cover their utility as new diagnostic tools, predictors of outcome, and as novel therapeutic targets in septic patients.
Pathophysiology of sepsis
A consensus conference defined sepsis as the systemic inflammatory response syndrome (SIRS) that occurs during infection (Citation10). This syndrome may be initialized by intense local inflammation to inactivate and clear the invading microbes (Citation11).
The pathogen-associated molecular patterns (PAMPs) of bacteria (mainly lipopolysaccharides (LPS) and exotoxins (Citation6)) produce infection, and the host response against these micro-organisms involves the activation of macrophages, dendritic cells (Citation10), and polymorphonuclear leukocytes (PMNs) through the triggering of cytosolic nuclear factor-κB (NF-κB)—activated by toll-like receptors (TLRs), that recognize PAMPs—by inducing the production of inflammatory cytokines, chemokines, adhesion molecules, and nitric oxide (NO) (Citation11).
The balance of pro-inflammatory (TNFα, IL-1, IL-2, IL-6, IL-8, IFNγ, PAF) and anti-inflammatory cytokines (IL-6, IL-10) regulates the activation of NF-κB and, therefore, the inflammatory processes (Citation12). When the balance is broken, and the release of pro-inflammatory mediators is excessive and prolonged, a hyper-inflammatory response occurs, and when this response exceeds the boundaries of the local environment, systemic inflammatory response syndrome (SIRS) occurs, leading to sepsis (Citation13). Pro-inflammatory cytokines may also delay apoptosis in activated macrophages and neutrophils, thereby prolonging or augmenting the inflammatory response, thus contributing to the development of multiple organ failure (Citation14). Genetic factors, such as polymorphisms of genes that encode cytokines, may influence whether persons have marked pro-inflammatory or anti-inflammatory responses to infection () (Citation10).
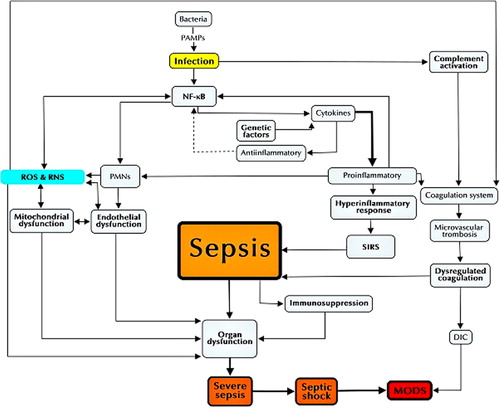
The infection may also trigger systemic activation of the complement system. Complement products, such as C5a, along with cytokines and oxidants activate the coagulation system through induction of tissue factor from endothelial cells and monocytes, and cause microvascular thrombosis by fibrin deposition, resulting in disseminated intravascular coagulation (DIC), frequently present in septic patients (Citation11).
During sepsis, the endothelium is one of the first targets of the inflammatory response, being activated by pro-inflammatory cytokines by up-regulating adhesion molecules (ICAM-1, VCAM-1). When pathogens invade a tissue, endothelial cells are induced locally to release inflammatory mediators, to recruit leukocytes, and to promote clotting as a means of walling off the infection. During this process, endothelial cells may experience apoptosis or necrosis as tissue is reabsorbed and repaired (Citation15).
Leukocytes are recruited by chemokines and adhesion molecules, and extravasate into surrounding tissue, producing proteases, cytotoxic enzymes, and reactive oxygen and nitrogen species (ROS and RNS, respectively), such as NO (Citation15,Citation16). This increased activation of leukocytes leads to a state known as oxidative stress, which contributes to endothelial and vascular smooth cell injuries () (Citation16).
It is important to emphasize that these pathophysiological mechanisms are involved in sepsis but might differ depending on the pathogenic micro-organism and the patient. For example, sepsis involving superantigen-producing bacteria is characterized by an undifferentiated T lymphocyte activation, leading to faster onset of septic shock and MODS (Citation17). Furthermore, the genetic profile seems to be relevant, determining susceptibility for severe sepsis and even treatment response (Citation18).
Oxidative stress in sepsis
Oxidative stress is a harmful state that causes biological damage. It is produced when pro-oxidant molecules exceed the protective antioxidant mechanism of the cell (Citation19). There are two variants of these pro-oxidant molecules: ROS and RNS. The first group includes the superoxide anion radical (O2•–), hydrogen peroxide (H2O2), and the hydroxyl radical (OH•) (Citation16,Citation20), while the second group includes mainly the nitric oxide (NO•) and the peroxynitrite anion (ONOO–) (Citation21). On the other hand, antioxidant mechanisms are present in cells to remove ROS/RNS or even prevent their generation. Antioxidant defenses of the cell include enzymatic components—such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GSH-Pxs), heme oxygenase (HO), among others—and non-enzymatic molecules (e.g. reduced glutathione (GSH), vitamin C, and vitamin E) (Citation22).
ROS and RNS easily react with components of the cell, generating DNA fragmentation, nitration or nitrosylation of proteins, and lipid peroxidation, thus altering the normal functioning of the cell (Citation23). Pro-oxidant agents are involved in several physiological processes (Citation24) and in many pathophysiological conditions, such as ischemia-reperfusion (Citation25) and inflammation (Citation26).
In sepsis conditions, an over-production of RNS and ROS takes place, both in the circulation and in the affected organs (Citation27). This is probably caused by the high levels of cytokines (IL-1B, IL-6, TNFα) and PAMPs that stimulate the activation of endothelial and immune cells, increasing their formation of ROS/RNS (Citation28). In addition, hypoxia produced in more severe forms of sepsis has been correlated with an increase of ROS production in cells (Citation29). Several mechanisms might generate this phenomenon: 1) mitochondrial inhibition of the electron transport chain (ETC) increasing its generation of ROS (Citation30); 2) increased formation of xanthine oxidase (XO) (Citation19); 3) increased activity of NADPH-oxidase (Citation31); and 4) an augmented expression of the inducible nitric oxide synthase (iNOS) (Citation32).
In this context, ROS and RNS have been associated with an amplification of the pro-inflammatory state of sepsis in the surrounding tissues. This might explain the capacity of some reactive species (especially H2O2, NO•, and hypochlorous acid (HOCl)) to diffuse across cellular membranes, promoting a secondary reaction that increases the production of RNS/ROS in these cells (Citation21). One of these reactions is the increase of activity of some transcription factors such as NF-κB and AP1 (Citation33). Also, it has been proved that H2O2 is capable of increasing endothelium permeability and expression of adhesive molecules (Citation34), thus augmenting the migration and activation of immune cells.
Therefore, production of ROS/RNS is increased in sepsis and contributes to the pro-inflammatory state, thus generating a positive feedback system. In addition, ROS and RNS have a central role in two pathophysiologic processes involved in the genesis of severe sepsis and MODS: mitochondrial and endothelial dysfunction.
Oxidative stress-related injury
Mitochondrial dysfunction
Mitochondria are crucial organelles involved in many cellular activities, oxidative phosphorylation/energy production from oxygen in the form of ATP being one of the most remarkable (Citation35). In this process, ROS are naturally generated as by-products of the incomplete four-electron reduction of molecular oxygen to water (Citation27). As knowledge of sepsis has increased, more evidence has been gathered about the central role that mitochondrial dysfunction plays in the pathophysiology of severe sepsis (Citation27). In several studies a decrease in the functioning of mitochondria (Citation36) was noticed.
The state of sepsis might induce several mechanisms of mitochondrial impairment. As described before, hypoxemia increases generation of ROS, thus augmenting oxidative damage in the mitochondria. In addition, the presence of cytokines increases the concentration of NO• and ONOO– radicals in the mitochondria, due to an augmented activity of mitochondrial NOS (mtNOS, a subform of NOS, outside the mitochondria) and the induction of iNOS (Citation37). NO• has the ability to inhibit the complex IV of the ETC (Citation38), and ONOO– is able to damage the structure of complex I through nitrosylation, both of them altering the functioning of ETC and thus increasing its ROS formation (Citation39). Finally, the increase of ROS/RNS generates DNA damage, especially in the mitochondrial DNA due to its proximity to the source of this species, reducing the capacity of the mitochondria to re-establish the normal functioning of the ETC and the antioxidant system, and thus giving rise to further ROS production, which generates a perpetuating cycle (Citation40).
Oxidative stress, nitric oxide, and a fall in ATP concentration can initiate the mitochondrial permeability transition, resulting in activation of the caspase cascade (Citation21,Citation27), culminating in apoptosis or programmed cell death (Citation27).
Mitochondrial dysfunction produced by these mechanisms alters the use of oxygen by cells. It has been proposed that this incapacity to produce ATP, rather than a decrease of the oxygen availability, is a central factor in the pathophysiology of MODS (Citation10). This phenomenon is known as cytopathic hypoxia (Citation41). This hypothesis is supported by the unsatisfactory results in clinical studies using the increase of oxygen delivery to tissues as a therapeutic measure (Citation42,Citation43) and by a recent autopsy study conducted by Hotchkiss et al., which showed discordance between histological findings and the degree of organ dysfunction seen in patients who died of sepsis. In this last investigation, cell death in the heart, kidney, liver, and lung was relatively minor and did not reflect the clinical evidence of more profound organ dysfunction, which led them to speculate that much of organ failure in patients with sepsis can be explained by ‘cell hibernation’ (Citation10).
Endothelial dysfunction
The endothelium modulates vascular homeostasis through the maintenance of vasomotor tone, blood fluidity, cellular and nutrient trafficking, local balance in pro-inflammatory and anti-inflammatory mediators, angiogenesis, and programmed cell death, among other processes (Citation15).
Endothelial dysfunction was initially identified as an impaired vasodilatation to specific stimuli such as acetylcholine or bradykinin. Nonetheless, a broader understanding of this term includes not only a reduced vasodilation, but impairment of the global function of the endothelium, generating a pro-inflammatory and prothrombic state in the vascular system (Citation44). Thereby, malfunctioning of the microvascular endothelium produces alterations of the microcirculatory system (Citation45), leading to a heterogeneous capillary perfusion and reduced capacity for tissue oxygen extraction (Citation46).
The pathophysiology of endothelial dysfunction is complex and involves multiple factors including oxidative stress, imbalance of angiogenic growth factors and vascular tone-regulating molecules (such as angiotensin II), and insulin resistance. However, the decline of the NO levels—which acts as a vasodilator, inhibits growth and inflammation, and has anti-aggregant effects on platelets—appears to be common among these factors (Citation44). This phenomenon results from an impaired generation of NO by endogenous nitric oxide synthase (eNOS) and/or decreased NO bioavailability (Citation47), both produced mainly through ROS ability to uncouple eNOS and oxidize NO (Citation48).
In a sepsis state, as a consequence of the increase of cytokines and reactive species, the normal functioning of the endothelial cells is impaired, triggering apoptosis and endothelial dysfunction (Citation47), thus amplifying the pro-coagulant and pro-inflammatory states of sepsis, increasing endothelial permeability, and altering the vasomotor response (Citation6,Citation16,Citation49). These alterations seem to be ubiquitous in sepsis animal models (Citation50,Citation51), and it has been observed in human tissues as well (Citation45), unchaining a global microcirculatory failure (Citation46). Microcirculatory dysfunction is known to be a critical element in the pathogenesis of severe sepsis and septic shock (Citation52). Furthermore, an association has been proven between the severity of microcirculatory dysfunction with development of MODS (Citation52,Citation53) and poor outcome of septic patients (Citation45,Citation52).
Angiogenesis-related factors
Pro- and anti-angiogenic factors regulate the complex process known as angiogenesis, corresponding to the development of new blood vessels out of existing ones in physiological, compensatory, or pathologic conditions (Citation54).
As previously mentioned, endothelial dysfunction plays a key role in the pathogenesis of sepsis (Citation16) and subsequent associated mortality. However, endothelial progenitor cells from the bone marrow ameliorate the dysfunction through angiogenesis in ischemic areas and in damaged small vessels (Citation10).
Some angiogenic growth factors, such as VEGF, and angiopoietins, may have a pivotal role in the endothelial dysregulation seen in sepsis, or they may simply reflect an attempt by the host to repair endothelial damage (Citation10).
VEGF is a diffusible endothelial cell-specific mitogen and pro-angiogenic factor that increases vascular permeability (Citation54), and mediates endothelial proliferation, migration, and survival (Citation55,Citation56). During sepsis, VEGF contributes to vascular leak, propagation of host response (Citation57), and may be associated with hypotension (Citation58), while its soluble receptor, sFlt-1, is an anti-inflammatory peptide that inhibits VEGF activity (Citation57).
In response to several agents often associated with sepsis, such as Gram-negative bacterial LPS, Gram-positive bacterial components, and TNFα, VEGF production or secretion of the intracellular pool is increased. In addition, hypoxia increases VEGF production via hypoxia-inducible factor-1α (HIF-1α) transcription factor (Citation58).
The endothelial-specific angiopoietin (Ang)/Tie-2 ligand- receptor system is another regulator of endothelial responsiveness, angiogenesis, and vascular integrity (Citation59,Citation60). Ang-1 is a Tie-2 agonist and is important for blood vessel stability, inhibiting vascular leakage, and suppressing inflammatory gene expression. Ang-2 is a Tie-2 antagonist and promotes endothelial activation, destabilization, inflammation, and, in the presence of VEGF, cell survival (Citation55,Citation56,Citation59).
In sepsis, Ang-1 and Ang-2 plasma levels are altered in the presence of LPS. Recently, investigators have found that LPS causes decreases in Ang-1 and Tie-2 expression, while increasing the levels of Ang-2 in murine models of sepsis (Citation61).
The imbalance of ARF and their receptors, including the Ang/Tie-2 and VEGF/VEGF receptor pathways, has been shown to predict poor prognosis in sepsis and has been implicated as a contributing factor in multiple organ dysfunction (Citation62). Therefore, oxidative stress and ARF, taken together, seem to play a fundamental role in severity of sepsis.
Sepsis progression to MODS
In addition to the above-mentioned, sepsis induces extensive lymphocyte and dendritic cell apoptosis and an anti-inflammatory immunosuppressive state that may cause leukocyte dysfunction, believed to be another reason for injury to organs, contributing to the development of organ failure (Citation10,Citation63). When sepsis is complicated by organ dysfunction, it is known as severe sepsis ().
Organ dysfunction in severe sepsis seems to be multifactorial, where mitochondrial and endothelial dysfunctions are essential in its pathophysiology. Also, they seem to influence each other in a synergic relation (). A decreased mitochondrial dysfunction correlates with increased NO/NOO production, acidosis, and a depletion of ATP, all stimuli that potentially activate ATP-dependent potassium channels in the vascular smooth muscle. This phenomenon hyperpolarizes these cells, which inhibits the voltage-dependent calcium channels, leading to hypotension and vascular hypo-reactivity (Citation64). In turn, microcirculatory dysfunction leads to hypoxic, pro-coagulant, and pro-inflammatory conditions, as explained before, promoting or increasing the mitochondrial damage.
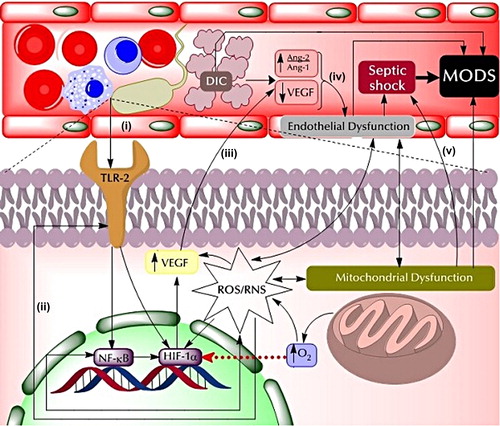
In some cases, as severe sepsis progresses, the pro-coagulant state produces a serious clinical complication known as disseminated intravascular coagulation (DIC). DIC is characterized by the widespread fibrin deposition in microvessels as a result of coagulation activation, inhibition of anticoagulation and fibrinolysis, and consumption of clotting factors and platelets (Citation65). DIC is closely related to organ failure, resulting from extensive formation of fibrin clots, microvascular occlusion, and reduced oxygen delivery to cells and tissues (Citation60). On the other hand, in sepsis conditions, pro-angiogenic factor VEGF plasma levels are elevated, as they are at the beginning of severe sepsis (Citation66–68).
During the onset of sepsis, the complex relationships between microregional inflammation, microvascular heterogeneity, hypoxia-inducible gene expression, and organ function are not well understood in general (Citation50). Therefore, it is possible to hypothesize that this rise in VEGF levels can be explained by increased levels of HIF-1α and elevated ROS/RNS production. HIF-1α is a transcriptional factor, inducible for hypoxia, which promotes the up-regulation of several genes, such as VEGF (Citation69).
As described before, microcirculatory dysfunction generates hypoxic conditions in severe sepsis, which may explain, in part, the increased HIF-1α activity. Moreover, oxidative stress may also contribute to this phenomenon, not only by inducing endothelial dysfunction and NF-κB transcription, but also through stimulation of HIF-1α transcription (Citation70) and TLR activation. By-products of oxidative damage of proteins generates ω-carboxyethylpyrrole (CEP) adducts, which are responsible for the activation of TLR-2. TLR-2 leads to activation of NF-κB and modulates HIF-1α expression, thus contributing to high VEGF levels (Citation70). NF-κB can also modulate HIF-1α expression () (Citation69).
Nonetheless, several studies have shown that low VEGF levels are associated with more severe forms of sepsis, organ dysfunction, and mortality (Citation56,Citation66), which seems to be contradictory to previous studies which suggested that VEGF levels correlated with the severity of organ dysfunction (Citation58,Citation71). One explanation is that DIC-induced platelet consumption and elevation of sFlt-1 receptor are the main causes of low VEGF levels in patients with DIC associated with severe sepsis (Citation60). Nevertheless, this mechanism might not be able to justify the low levels of VEGF in all critical septic patients. A different time pattern may explain differences between studies (Citation66). As previously described, there is reduced oxygen utilization by cells in severe sepsis conditions secondary to mitochondrial dysfunction. Thus, as severity of sepsis progresses in time, this may imply a rise in cellular O2 concentration, which may decrease HIF-1α survival, thus reducing VEGF production.
With a diminished production of VEGF, the endothelial cell apoptosis rate will increase, reinforcing endothelial dysfunction (Citation72).
Also, DIC is one of the main conditions that give rise to an imbalance in Ang-1 and Ang-2 in severe sepsis (Citation60). The latter mediates microvascular and hemodynamic alterations in this clinical condition (Citation73).
Therefore, decreased VEGF and Ang-1 and increased Ang-2 levels contribute to endothelial dysfunction that in association with mitochondrial dysfunction leads to septic shock, mainly by decreasing myocardial activity (Citation74) and increasing permeability and vasoplegia (Citation16).
The progression of severe sepsis, septic shock, endothelial dysfunction, and mitochondrial dysfunction, in addition to DIC, alters at a global level the functioning of cells and tissues, ultimately resulting in MODS () (Citation75), where circulation (Citation76), lung (Citation77,Citation78), kidney (Citation79), liver (Citation80), gastrointestinal tract (Citation81), and/or nervous system (Citation82) can be affected.
Once sepsis is suspected, prompt confirmation is required for instauration of adequate therapeutic actions (Citation8). As the understanding of pathophysiology of sepsis increases, more diagnostic tools have been developed, such as biomarkers.
Biomarkers in sepsis
A biomarker is a molecule that can be measured in a biological sample in an objective, systematic, and precise way; its levels are indicators of a process (be it normal or abnormal), and they can be used to monitor response to treatment (Citation83). The most important factors that characterize the ideal biomarker are the sensitivity, specificity, and predictive value (Citation84). It has to be capable of establishing an early diagnosis, quantifying the severity and stratifying risk, and monitoring evolution of bacterial infection and its response to treatment (Citation85).
There have been numerous biomarkers investigated to aid with diagnosis and prognostication in sepsis, with the majority suffering from lack of sensitivity or specificity. Also, there is no biomarker clearly identified as being able to differentiate sepsis from SIRS (Citation86). Inflammatory biomarkers such as CRP, IL-6, IL-8, IL-18, NK cells and procalcitonin (PCT) have been shown to be elevated in sepsis, but are inadequate to discriminate independently as diagnostic tools. Despite PCT having been heralded as the most promising biomarker for bloodstream infections (Citation87,Citation88), an elevation of PCT is not as specific for infection as was believed (Citation89), because, for example, it cannot accurately distinguish sepsis from SIRS (Citation9). Also, it is not useful as a marker in the diagnosis and assessment of viral infection (Citation90) and does not reliably predict the outcome in patients with sepsis (Citation91).
C-reactive protein (CRP), an acute-phase reactant produced by hepatocytes and released in response to inflammation or tissue injury, is an established biomarker of infection and inflammation (Citation87,Citation89). However, in some studies, CRP has shown low discriminatory capacity to differentiate sepsis from SIRS, and has also been shown to be a poor predictor of mortality compared to other markers, but it has presented a high diagnostic accuracy when combined with another infection marker (Citation87,Citation92).
Another widely used biomarker is lactate, a marker of organ dysfunction. Serum levels of lactate can reflect tissue hypoperfusion and anaerobic metabolism in severe forms of sepsis, and it has been seen as a correlation between serum lactate and outcome, with the duration and degree of hyperlactatemia as important predictors of morbidity and mortality (Citation88,Citation89,Citation92,Citation93). However, a recent review suggested that plasma or serum lactate measurement could not provide specific prognostic information for individual patients, because of the extensive overlap of levels among patients with different outcomes (Citation94).
Currently, the inflammatory biomarker with the best demonstrated prognostic performance in terms of mortality is the mid-regional proadrenomedullin (MR-ProADM), which predicted the development of septic shock (Citation83,Citation88,Citation95).
There are biomarkers that focus on the coagulation system, such as D-dimer, which is the most commonly used fibrin-related marker, and is utilized to monitor hemostatic abnormalities associated with sepsis, such as DIC, predicting sepsis severity and survival (Citation96).
Other kinds of biomarkers are related to activated neutrophils and monocytes, complement proteins, immunosuppression, organ dysfunction, and endothelial dysfunction (). Nevertheless, none of them has proven to be really effective on its own.
Table I. Biomarkers of sepsis. Different types of biomarkers have been proposed. Despite no single biomarker of sepsis being ideal, many of them are helpful identifying critically ill patients.
In this context, other biomarkers, such as of oxidative damage or of endothelial damage (like ARF) may provide additional information in sepsis pathophysiology (Citation8).
Oxidative stress biomarkers
The most commonly used biomarkers of oxidative damage are lipid peroxidation end-products such as malondialdehyde (MDA) and F2-isoprostanes (Citation97). A multicenter study performed by Lorente et al. found that patients with severe sepsis showed higher serum MDA levels than healthy controls, that non-surviving septic patients showed higher serum MDA levels than survivors, and that serum MDA levels could be used to predict outcomes in septic patients. Elevated serum MDA levels probably represent an unbalanced oxidant state (Citation98).
Because of their stability and high specificity, F2-isoprostanes are currently considered to be the most reliable biomarkers of in vivo oxidative stress and lipid peroxidation. It has been determined that elevated levels of plasma F2-isoprostane were associated with renal, hepatic, and coagulation failure (Citation99).
Other indicators of oxidative stress have been measured, such as the thiobarbituric acid reactant substances (TBARS), XO, and myeloperoxidase (MPO) activity, an enzyme released by activated neutrophils. TBARS have been found to be elevated in patients with SIRS and organ dysfunction, while XO activity has been found to be higher in non-survivor patients. MPO activity has been found to be significantly higher in sepsis compared to healthy controls (Citation100).
In addition, it has been reported that there is an increased plasma concentration of nitrite plus, nitrate, and NO reaction products in patients with septic shock (Citation20).
On the other hand, plasma antioxidant potential in septic shock patients has been shown to be lower than that in healthy subjects at the beginning of the disease. This is noticed in lower levels of antioxidant vitamin concentrations such as retinol, tocopherol, β-carotene, lycopene, and ascorbic acid, as well as GSH and selenium (Citation8,Citation20). Also, it has been found that plasma carbonyls and SOD activity are significantly increased in non-survivors (Citation101).
Therefore, increased oxidative stress is associated with poor outcome in critically ill patients and may be a prognostic indicator. It has been seen that oxidative damage markers are more useful than antioxidant protection markers in predicting outcome (Citation102).
Angiogenesis-related biomarkers
It is known that an increase in the number of failing organs during the first 48 hours following admission to an intensive care unit (ICU) is a good indicator of mortality in septic patients (Citation103). Consequently, the early diagnosis of sepsis and prevention of organ dysfunction progression to MODS is the primary goal in the treatment of septic patients (Citation7).
In sepsis, endothelial activation and dysfunction are critical determinants of the host response and represent an explanation for the complex sepsis pathophysiology (Citation72). Therefore, biomarkers reflecting the state of endothelial cells might be useful in the tracking of sepsis (Citation104).
Clinical data from adult studies support the association of elevated plasma angiogenic growth factor concentrations with sepsis (Citation56).
Different studies have shown that plasma VEGF levels from patients with severe sepsis are increased and associated with disease severity (Citation58,Citation66), and that low VEGF levels are associated with organ dysfunction and unfavorable outcome (Citation60,Citation66). Moreover, elevated levels of VEGF at onset in severe sepsis but lower levels along the course of disease have been reported (Citation67).
In the lung, the organ primarily injured in sepsis (Citation105), VEGF is abundantly expressed, and its actions affect lung development and structural maintenance of the adult lung (Citation78). One of the most serious complications of sepsis is acute lung injury (ALI) that leads to acute respiratory distress syndrome (ARDS) (Citation61). Different studies have shown a decreased pulmonary expression of VEGF, an increased expression of plasma VEGF, and higher levels of sFlt-1 in critically ill septic patients with ALI and/or ARDS (Citation61,Citation77,Citation78,Citation105). It has also been reported that VEGF plays a role in patients with sepsis and with kidney damage (Citation79). However, VEGF does not seem to be a useful tool for the prediction of unfavorable outcome in the critical care setting (Citation66,Citation106).
In contrast, sFlt-1 receptor, a critical regulator of circulating VEGF bioavailability (Citation108), has been shown to be strongly prognostic in sepsis (Citation57). It has been described that sFlt-1 levels are higher in septic shock patients, being an independent predictive factor for mortality and the presence of concomitant multiorgan dysfunction (Citation109). This could be explained by several hypotheses: 1) sFlt-1 levels reflect the vigor of the anti-inflammatory response in sepsis, 2) elevated sFlt-1 levels may cause immune depression by interfering with sFlt-1-mediated signaling in monocytes, and 3) sFlt-1 may interfere with endothelial repair by inhibiting VEGF signaling in endothelial cells (Citation53).
Placental growth factor (PlGF), a member of the VEGF family, was reported to potentiate the angiogenic response to VEGF in pathological angiogenesis (Citation109). Studies have shown that VEGF levels were increased in patients with severe sepsis, but decreased in patients with organ failure without infection. In contrast, PlGF levels were increased in both groups of patients. These results suggest that the up-regulation of PlGF represents an adaptive host response to infection (Citation68,Citation109).
Ang-1 and Ang-2 are two of the most widely studied biomarkers of endothelial activation/dysfunction in infectious diseases (Citation110). As previously described, angiopoietins are endothelial-derived vascular growth factors that play opposing roles during sepsis (Citation93). Several studies have documented that, in different kinds of sepsis, Ang-2 levels are higher, and Ang-1 levels are lower in septic patients (Citation56,Citation60,Citation111,Citation112). It has been reported that Ang-1 is independently associated with mortality (Citation56,Citation72), remaining as a significant predictor of outcome (Citation59), whereas Ang-2 plays a pivotal role in the development of organ dysfunction, thus leading to a poor outcome (Citation111,Citation112). Ang-2 or Ang-2/Ang-1 ratio could promote inflammation and increase the vascular permeability during DIC associated with severe sepsis, resulting in organ damage and a poor outcome (Citation62,Citation112).
Angiopoietins seem to be of interest as prognostic severe sepsis biomarkers of disease severity and patient outcome (Citation113). Ang-2 might present a reliable marker reflecting the direct status of the endothelium that correlates with sepsis severity and outcome, whereas Ang-1 levels might have a high predictive outcome value, when determined at admission to the ICU (Citation72).
Other ARF have been documented, such as fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and plasminogen activator inhibitor (PAI-1). Lower plasma FGF and PDGF concentrations were associated with an unfavorable outcome in children with severe bacterial infection (Citation55,Citation56).
Anti-angiogenic factor PAI-1 levels have been shown to be increased in post-traumatic sepsis, and they correlated with MODS in septic DIC patients (Citation72,Citation114).
Currently, there is much enthusiasm and interest in the clinical utility of sepsis biomarkers (Citation88). However, this utility is limited by the lack of standardization in analytical assays and of data regarding receiver operating characteristics and of validation (Citation115).
In this context, several clinical trials have been performed using panels of biomarkers, with different combinations (Citation96). A recent study by Wong et al. tested and validated a multibiomarker-based risk model that estimates mortality probability in adults with septic shock (Citation116). Thus, the combination of several biomarkers might increase sensitivity and specificity (Citation96).
New therapeutic strategies in severe forms of sepsis
Current management of severe sepsis focuses on adequate fluid resuscitation, appropriate and early antibiotic therapy, and close monitoring of hemodynamic conditions, using vasopressors agents if necessary (Citation2). However, although this treatment has been effective, morbidity and mortality of sepsis still remain elevated (Citation42,Citation43). This is probably because an adequate blood pressure and normal oxygen saturation do not necessarily correlate with an adequate oxygen distribution at the microvasculature level or correct oxygen utilization by cells, which are the main issues in the pathophysiology of severe sepsis, as seen before. In this way, therapeutic agents for both microcirculatory and mitochondrial dysfunction might ameliorate these phenomena, improving treatment efficacy.
The use of antioxidant molecules has been proposed as a promising tool to reduce the mitochondrial damage seen in the septic state, mainly using vitamins A, E, and C, selenium, and iron-chelating agents (Citation20,Citation117). Promising results have been achieved in animal models (Citation58); nevertheless, the evidence of the beneficial effect of antioxidant supplementation in critically ill patients is still controversial (Citation118). For example, a recent well designed controlled multicenter study showed that antioxidants (selenium, zinc, and vitamins E and C) had no effect on 28-day mortality or any other secondary end-point included (Citation119). However, a previous meta-analysis by Manzanares et al. which evaluated relevant clinical outcomes with antioxidant micronutrients (vitamins and trace elements) supplementation versus placebo, including 21 clinical trials performed in critically ill adult patients between 1980 and 2011, found that combined antioxidants were associated with a significant reduction in mortality (Citation120). This contradictory finding might be explained by several reasons, including: ignorance of the optimal composition, dose, timing, and duration of therapy (Citation121), limited effects on the intracellular levels of antioxidants (Citation122), and ineffectiveness in altering the intracellular and mitochondrial redox status (Citation21,Citation27).
Therefore, antioxidants that specifically target mitochondria have been suggested as a novel therapy. Many different strategies have been used to accomplish this effect, including: an antioxidant that naturally acts/accumulates in the mitochondria or binding to a carrier molecule (cationic compounds such as rhodamine 123 and triphenylphosphonium) that directs them to this organelle, employing molecules that stimulate the production of antioxidant defenses in the mitochondria, and decreasing ROS generation with co-factor or substrates of the ETC () (Citation27). Numerous studies on animal models have been performed with promising results: decreased oxidative stress and cellular injury markers (Citation123,Citation64), reduced apoptosis (Citation123), ameliorated organ dysfunction (Citation124), and prolonged or improved survival (Citation125).
Table II. Novel therapeutic options for sepsis.
Regarding the microcirculatory dysfunction correction, it is important to mention that the formal treatments for sepsis shock (vasopressor agents and fluids) have demonstrated some efficacy in septic patients (Citation126). In addition, several novel strategies have proved to be helpful in animal models, among which the utilization of tetrahydrobiopterin (BH4), vitamin C, and pro/anti-angiogenic agents stands out (). BH4 is an important co-factor of eNOS, which is degraded by peroxynitrite, present in oxidative stress condition. This leads to an uncoupled state of eNOS which instead of producing NO produces superoxide anions. Excellent results have been achieved in animal models using this molecule, improving endothelial dysfunction (Citation127), microcirculatory perfusion (Citation128), organ function, and increased survival rate (Citation129). Vitamin C also improved microcirculation in animal models of sepsis (Citation130), partly by increasing BH4 bioavailability (Citation127). Finally, pro/anti-angiogenic agents appear to be a novel therapeutic option by reducing the endothelial dysfunction due to the imbalance of ARF. The anti-VEGF antibody, bevacizumab (Bev), was recently used in murine models of sepsis, resulting in a decreased expression of pro-inflammatory cytokines and decreased permeability of lungs, spleen, and kidneys. Mortality showed interesting results: at a low concentration of Bev the survival rate was significantly higher, but at higher levels no difference was achieved (Citation131). Other molecules used are synthetic Tie-2 agonist (vasculotide) and a recombinant Ang-1, resulting in an improved microvascular endothelial barrier and reduced mortality in clinically relevant murine sepsis models () (Citation59,Citation132).
No clinical studies have been published yet using these novel therapies on septic patients, therefore whether these treatments are or are not useful in human sepsis remains an unanswered question.
Conclusions and future perspectives
Sepsis is a complex pathophysiological phenomenon involving many factors, including oxidative stress and ARF. Although it is known that oxidative stress has a great influence in sepsis in both mitochondrial and endothelial dysfunction, and that ARF play a more important role in endothelial dysfunction, to date no reviews have integrated both phenomena in sepsis.
In the context of severe sepsis progression to MODS, this review proposes that HIF-1α is more sensitive to the increase of free O2 following mitochondrial dysfunction, than to the action of NF-κB and TLR, which brings consequently a decrease in VEGF levels, leading to an alteration on the endothelial integrity. Minimal or basal levels of VEGF would be necessary to maintain the correct endothelial function.
As both oxidative stress and angiogenesis are common mechanisms of sepsis, independently of the origin and severity of this condition, biomarkers associated with these two processes would be expected to be useful in an optimal sepsis diagnosis and prognosis. For example, as both processes are easily detectable at subclinical sepsis stages, it would be reasonable to hypothesize that biomarkers related with oxidative stress and angiogenesis would have the capacity to establish an early diagnosis.
Moreover, considering that oxidative stress represents a predominance of pro-oxidant species relative to antioxidant molecules, sepsis severity progression would be expected to correlate positively with levels of pro-oxidant species and negatively with the levels of antioxidant species, which could be useful to quantify the severity of the condition. In the case of angiogenesis-related factors, these could also quantify the severity and stratify risk, e.g. VEGF. Considering the proposed integration model, where VEGF increases at the onset of severe sepsis and decreases as severity of sepsis progresses, a cut-off point could be deduced from further clinical trials with VEGF, which allows separating the onset of severe sepsis from septic shock and/or MODS.
On the other hand, as both oxidative stress and angiogenesis are present throughout the progression of sepsis, changes of biomarkers regarding these components could be used to monitor evolution of the disease and its response to treatment.
However, as currently there are only a few studies performed in sepsis patients with biomarkers of oxidative stress and factors associated with angiogenesis, most of them focused on epidemiological outcomes, every proposal of biomarker aimed at evaluate clinical evolution must be tested in new controlled trials. Also, clinical trials should evaluate if any of these biomarkers are capable of differentiating infection from non-infectious states, and if they are capable of differentiating between pathogens (e.g. viral versus bacterial).
In any case, considering that it would be near impossible to find a single biomarker that is ideal in every way, the development of a panel of biomarkers related to oxidative stress and angiogenesis becomes essential. The work by Wong et al. could be used as a model for new combinations, using a basis of admission lactate concentrations, age, and chronic disease status, plus markers that have been shown to have a high prognostic value, such as MR-ProADM (inflammatory), sFlt-1, Ang-1 and 2 (ARF), MDA (oxidative stress), as well as some inflammatory biomarkers from the study by Wong et al. that have been tested, calibrated, and validated, such as CCL3, HSPA1B, and IL-8 (Citation116).
Finally, given that elevated morbidity and mortality associated to sepsis persist despite current therapies, a complementary antioxidant and angiogenesis-related therapy is proposed. As there is a close relationship between endothelial and mitochondrial dysfunction, which influence each other, focusing on a single phenomenon may be insufficient: a dual therapy is a reasonable option in the attempt to reduce the progression of severe sepsis to MODS. However, there are still some questions that remain and should be the focus of future research, in parallel to continuing testing of antioxidants as co-therapy to sepsis and to developing a biomarker panel based in antioxidant and angiogenesis-related biomarkers ().
Table III. Remaining questions and focus of future research.
Funding: No funding declared.
Declaration of interest: The authors report no conflicts of interest.
References
- Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–83.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637.
- Vincent JL. Clinical sepsis and septic shock—definition, diagnosis and management principles. Langenbecks Arch Surg. 2008;393:817–24.
- Barie PS, Hydo LJ, Pieracci FM, Shou J, Eachempati SR. Multiple organ dysfunction syndrome in critical surgical illness. Surg Infect (Larchmt). 2009;10:369–77.
- Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis. 2005;41:S490–7.
- Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170: 1435–44.
- Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: raising awareness to reduce mortality. Crit Care. 2003;7:1–2.
- Rodrigo R, Saa D, Galleguillos F, Céspedes C. Septic shock: clinical diagnosis and risk factors. In: Johnston MC, Knight JE, editors. Septic shock: symptoms, management and risk factors. 1st ed. pp. 51–74. Hauppage, NY: Nova Science Publishers; 2012.
- Kaplan JM, Wong HR. Biomarker discovery and development in pediatric critical care medicine. Pediatr Crit Care Med. 2011;12: 165–73.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
- Okazaki Y, Matsukawa A. Pathophysiology of sepsis and recent patents on the diagnosis, treatment and prophylaxis for sepsis. Recent Pat Inflamm Allergy Drug Discov. 2009;3:26–32.
- Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–32.
- Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17:175–81.
- Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM 2nd, Buchman TG, et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–21.
- Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77.
- Boisramé-Helms J, Kremer H, Schini-Kerth V, Meziani F. Endothelial dysfunction in sepsis. Curr Vasc Pharmacol. 2013;11:150–60.
- Alouf JE, Müller-Alouf H. Staphylococcal and streptococcal superantigens: molecular, biological and clinical aspects. Int J Med Microbiol. 2003;292:429–40.
- Christaki E, Giamarellos-Bourboulis EJ. The beginning of personalized medicine in sepsis: small steps to a bright future. Clin Genet. 2014; 86:56–61.
- Karapetsa M, Pitsika M, Goutzourelas N, Stagos D, Tousia Becker A, Zakynthinos E. Oxidative status in ICU patients with septic shock. Food Chem Toxicol. 2013;61:106–11.
- von Dessauer B, Bongain J, Molina V, Quilodrán J, Castillo R, Rodrigo R. Oxidative stress as a novel target in pediatric sepsis management. J Crit Care. 2011;26:103.e1–7.
- Andrades MÉ, Morina A, Spasić S, Spasojević I. Bench-to-bedside review: sepsis - from the redox point of view. Crit Care. 2011;15:230.
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264.
- Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800.
- Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271(4 Pt 2):H1626–34.
- Granger DN, Stokes KY, Shigematsu T, Cerwinka WH, Tailor A, Krieglstein CF. Splanchnic ischaemia-reperfusion injury: mechanistic insights provided by mutant mice. Acta Physiol Scand. 2001;173: 83–91.
- Yang Y, Bazhin AV, Werner J, Karakhanova S. Reactive oxygen species in the immune system. Int Rev Immunol. 2013;32:249–70.
- Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64.
- Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–80.
- Araneda OF, Tuesta M. Lung oxidative damage by hypoxia. Oxid Med Cell Longev. 2012;2012:856918.
- Schumacker PT. Lung cell hypoxia: role of mitochondrial reactive oxygen species signaling in triggering responses. Proc Am Thorac Soc. 2011;8:477–84.
- Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–41.
- Hecker M, Cattaruzza M, Wagner AH. Regulation of inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Gen Pharmacol. 1999;32:9–16.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
- Millar TM, Phan V, Tibbles LA. ROS generation in endothelial hypoxia and reoxygenation stimulates MAP kinase signaling and kinase- dependent neutrophil recruitment. Free Radic Biol Med. 2007;42: 1165–77.
- Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst). 2006;5:145–52.
- Fredriksson K, Hammarqvist F, Strigård K, Hultenby K, Ljungqvist O, Wernerman J, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291: E1044–50.
- Finocchietto PV, Franco MC, Holod S, Gonzalez AS, Converso DP, Antico Arciuch VG, et al. Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death. Exp Biol Med (Maywood). 2009;234:1020–8.
- Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369: 136–9.
- Franco MC, Antico Arciuch VG, Peralta JG, Galli S, Levisman D, López LM, et al. Hypothyroid phenotype is contributed by mitochondrial complex I inactivation due to translocated neuronal nitric-oxide synthase. J Biol Chem. 2006;281:4779–86.
- Fariss MW, Chan CB, Patel M, Van Houten B, Orrenius S. Role of mitochondria in toxic oxidative stress. Mol Interv. 2005;5:94–111.
- Garrabou G, Morén C, López S, Tobías E, Cardellach F, Miró O, et al. The effects of sepsis on mitochondria. J Infect Dis. 2012;205: 392–400.
- Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–55.
- Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12:919–24.
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92.
- De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5:73–9.
- Nencioni A, Trzeciak S, Shapiro NI. The microcirculation as a diagnostic and therapeutic target in sepsis. Intern Emerg Med. 2009; 4:413–18.
- Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346:1999–2001.
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–4.
- Kaymak C, Basar H, Sardas S. Reactive oxygen species (Ros) generation in sepsis. FABAD J Pharm Sci. 2011;36:41–7.
- Bateman RM, Tokunaga C, Kareco T, Dorscheid DR, Walley KR. Myocardial hypoxia-inducible HIF-1alpha, VEGF, and GLUT1 gene expression is associated with microvascular and ICAM-1 heterogeneity during endotoxemia. Am J Physiol Heart Circ Physiol. 2007; 293:H448–56.
- Wester T, Häggblad E, Awan ZA, Barratt-Due A, Kvernebo M, Halvorsen PS, et al. Assessments of skin and tongue microcirculation reveals major changes in porcine sepsis. Clin Physiol Funct Imaging. 2011;31:151–8.
- Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care. 2013;28:538.e9–14.
- Shapiro NI, Arnold R, Sherwin R, O’Connor J, Najarro G, Singh S, et al. The association of near-infrared spectroscopy-derived tissue oxygenation measurements with sepsis syndromes, organ dysfunction and mortality in emergency department patients with sepsis. Crit Care. 2011;15:R223.
- Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002;9(2 Suppl):36–44.
- Bouïs D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res. 2006;53:89–103.
- Mankhambo LA, Banda DL; IPD Study Group, Jeffers G, White SA, Balmer P, Nkhoma S, et al. The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care. 2010;14:R91.
- Schuetz P, Jones AE, Aird WC, Shapiro NI. Endothelial cell activation in emergency department patients with sepsis and non-sepsis related hypotension. Shock. 2011;36:104–8.
- van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–8.
- David S, van Meurs M, Kümpers P. Does low angiopoietin-1 predict adverse outcome in sepsis? Crit Care. 2010;14:180.
- Jesmin S, Wada T, Gando S, Sultana SS, Zaedi S. The dynamics of angiogenic factors and their soluble receptors in relation to organ dysfunction in disseminated intravascular coagulation associated with sepsis. Inflammation. 2013;36:186–96.
- Novotny NM, Lahm T, Markel TA, Crisostomo PR, Wang M, Wang Y, et al. Angiopoietin-1 in the treatment of ischemia and sepsis. Shock. 2009;31:335–41.
- Zhang RY, Zhang H, Huang J, Qu HP, Tang YQ. Angiogenic factors in sepsis: are we ready for the new therapeutic era? Crit Care. 2014;18:403.
- Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47.
- O’Brien A, Stidwill RP, Clapp LH, Singer M. Variable effects of inhibiting iNOS and closing the vascular ATP-sensitive potassium channel (via its pore-forming and sulfonylurea receptor subunits) in endotoxic shock. Shock. 2009;31:535–41.
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–92.
- Karlsson S, Pettila V, Tenhunen J, Lund V, Hovilehto S, Ruokonen E, et al. Vascular endothelial growth factor in severe sepsis and septic shock. Anesth Analg. 2008;106:1820–6.
- Liu Y, Song S-D, Wang H-X. [A clinical study of the serum vascular endothelial growth factor in patients with severe sepsis]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21:172–4.
- Yano K, Okada Y, Beldi G, Shih SC, Bodyak N, Okada H, et al. Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med. 2008;205:2623–31.
- Zepeda AB, Pessoa A Jr, Castillo RL, Figueroa CA, Pulgar VM, Farías JG. Cellular and molecular mechanisms in the hypoxic tissue: role of HIF-1 and ROS. Cell Biochem Funct. 2013;31:451–9.
- Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–31.
- Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. 2005;24:508–12.
- Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16:11–21.
- Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013;123:3436–45.
- Gibot S, Lévy B, Nevière R, Cariou A, Lesur O. Myocardial dysfunction and septic shock. Med Sci (Paris). 2004;20:1115–18.
- Métais C, Wiel E, Vallet B. Role of endothelial dysfunction in sepsis mortality. Therapie. 2004;59:31–40.
- Reid VL, Webster NR. Role of microparticles in sepsis. Br J Anaesth. 2012;109:503–13.
- Manoilescu I, Teleman S, Cojocaru E, Mihăilă D, Plămădeală P. Vascular endothelial growth factor (VEGF) expression in the lung in toxic septic shock. Rom J Morphol Embryol. 2011;52:309–13.
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290: L209–21.
- Cardinal-Fernández P, Ferruelo A, El-Assar M, Santiago C, Gómez-Gallego F, Martín-Pellicer A, et al. Genetic predisposition to acute kidney injury induced by severe sepsis. J Crit Care. 2013;28: 365–70.
- Moreau R, Arroyo V. Acute on chronic liver failure: a new clinical entity. Clin Gastroenterol Hepatol. 2014 Feb 28. [Epub ahead of print]
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1.
- Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37:S331.
- León C, Loza A. Sepsis biomarkers. Simplifying the complex? Enferm Infecc Microbiol Clin. 2014;32:137–9.
- Atkinson AJ Jr, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69: 89–95.
- Julián-Jiménez A, Candel-González FJ, González del Castillo J. [Usefulness of inflammation and infection biomarkers in the Emergency Department]. Enferm Infecc Microbiol Clin. 2014;32: 177–90. [In Spanish]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15.
- Nelson GE, Mave V, Gupta A. Biomarkers for sepsis: a review with special attention to India. Biomed Res Int. 2014;2014:264351.
- Walley KR. Biomarkers in sepsis. Curr Infect Dis Rep. 2013;15: 413–20.
- Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50:23–36.
- Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17–29.
- Charles PE, Gibot S. Predicting outcome in patients with sepsis: new biomarkers for old expectations. Crit Care. 2014;18:108.
- Samraj RS, Zingarelli B, Wong HR. Role of biomarkers in sepsis care. Shock. 2013;40:358–65.
- Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother. 2014;46:1–12.
- Borthwick HA, Brunt LK, Mitchem KL, Chaloner C. Does lactate measurement performed on admission predict clinical outcome on the intensive care unit? A concise systematic review. Ann Clin Biochem. 2012;49:391–4.
- Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnosis and biomarkers. Clin Microbiol Rev. 2012;25:609–34.
- Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis. 2006;17:445–51.
- Bernal ME, Varon J, Acosta P, Montagnier L. Oxidative stress in critical care medicine. Int J Clin Pract. 2010;64:1480–8.
- Lorente L, Martín MM, Abreu-González P, Domínguez-Rodriguez A, Labarta L, Díaz C, et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit Care. 2013;17:R290.
- Ware LB, Fessel JP, May AK, Roberts LJ 2nd. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36:12–17.
- Kothari N, Keshari RS, Bogra J, Kohli M, Abbas H, Malik A, et al. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care. 2011;26: 435.e1–7.
- Forceville X, Mostert V, Pierantoni A, Vitoux D, Le Toumelin P, Plouvier E, et al. Selenoprotein P, rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur Surg Res. 2009;43:338–47.
- Mishra V, Baines M, Wenstone R, Shenkin A. Markers of oxidative damage, antioxidant status and clinical outcome in critically ill patients. Ann Clin Biochem. 2005;42(Pt 4):269–76.
- Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
- Zhang RY, Liu YY, Qu HP, Tang YQ. The angiogenic factors and their soluble receptors in sepsis: friend, foe, or both? Crit Care. 2013; 17:446.
- Tsokos M, Pufe T, Paulsen F, Anders S, Mentlein R. Pulmonary expression of vascular endothelial growth factor in sepsis. Arch Pathol Lab Med. 2003;127:331–5.
- Yang KY, Liu KT, Chen YC, Chen CS, Lee YC, Perng RP, et al. Plasma soluble vascular endothelial growth factor receptor-1 levels predict outcomes of pneumonia-related septic shock patients: a prospective observational study. Crit Care. 2011;15:R11.
- Ostrowski S, Sørensen AM, Windeløv NA, Perner A, Welling K-L, Wanscher M, et al. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scand J Trauma Resusc Emerg Med. 2012; 20:27.
- Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, Trzeciak S, et al. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39:427–32.
- Smadja DM, Borgel D, Diehl JL, Gaussem P. Vascular endothelial growth factor, as compared with placental growth factor, is increased in severe sepsis but not in organ failure. J Thromb Haemost. 2012; 10:974–6.
- Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507–16.
- Davis JS, Weo TW, Piera KA, Woodberry T, Celermajer DS, Stephens DP, et al. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care. 2010;14:R89.
- Wada T, Jesmin S, Gando S, Yanagida Y, Mizugaki A, Sultana SN, et al. Angiogenic factors and their soluble receptors predict organ dysfunction and mortality in post-cardiac arrest syndrome. Crit Care. 2012;16: R171.
- Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39:702–10.
- Raeven P, Salibasic A, Drechsler S, Weixelbaumer KM, Jafarmadar M, van Griensven M, et al. A non-lethal traumatic/hemorrhagic insult strongly modulates the compartment-specific PAI-1 response in the subsequent polymicrobial sepsis. PLoS One. 2013;8:e55467.
- Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis- a systematic review. Crit Care. 2012;16:R7.
- Wong HR, Lindsell CJ, Pettilä V, Meyer NJ, Thair SA, Karlsson S, et al. A multibiomarker-based outcome risk stratification model for adult septic shock. Crit Care Med. 2014;42:781–9.
- Lehmann C, Sharawi N, Al-Banna N, Corbett N, Kuethe JW, Caldwell CC. Novel approaches to the development of anti-sepsis drugs. Expert Opin Drug Discov. 2014;9:523–31.
- Mishra V. Oxidative stress and role of antioxidant supplementation in critical illness. Clin Lab. 2007;53:199–209.
- Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al.; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–97.
- Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK. Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit Care. 2012;16:R66.
- Manzanares W, Langlois PL, Hardy G. Update on antioxidant micronutrients in the critically ill. Curr Opin Clin Nutr Metab Care. 2013;16:719–25.
- Lane N. A unifying view of ageing and disease: the double-agent theory. J Theor Biol. 2003;225:531–40.
- Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, et al. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem. 2004; 279:37575–87.
- Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–65.
- Macias CA, Chiao JW, Xiao J, Arora DS, Tyurina YY, Delude RL, et al. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg. 2007;245:305–14.
- Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36:949–55.
- Mittermayer F, Pleiner J, Schaller G, Zorn S, Namiranian K, Kapiotis S, et al. Tetrahydrobiopterin corrects Escherichia coli endotoxin-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289: H1752–7.
- Tyml K, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit Care Med. 2008;36:2355–62.
- He X, Su F, Velissaris D, Salgado DR, de Souza Barros D, Lorent S, et al. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit Care Med. 2012;40:2833–40.
- Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med. 2005;33:1823–8.
- Jeong SJ, Han SH, Kim CO, Choi JY, Kim JM. Anti-vascular endothelial growth factor antibody attenuates inflammation and decreases mortality in an experimental model of severe sepsis. Crit Care. 2013; 17:R97.
- Kumpers P, Gueler F, David S, Slyke PV, Dumont DJ, Park JK, et al. The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit Care. 2011;15:R261.
- Austenaa LM, Carlsen H, Hollung K, Blomhoff HK, Blomhoff R. Retinoic acid dampens LPS-induced NF-kappaB activity: results from human monoblasts and in vivo imaging of NF-kappaB reporter mice. J Nutr Biochem. 2009;20:726–34.
- Durant R, Klouche K, Delbosc S, Morena M, Amigues L, Beraud JJ, et al. Superoxide anion overproduction in sepsis: effects of vitamin e and simvastatin. Shock. 2004;22:34–9.
- Wilson JX, Wu F. Vitamin C in sepsis. Subcell Biochem. 2012;56: 67–83.
- Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–26.
- Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201: 96–103.
- Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263: 709–16.
- Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Beitz DC. Effect of a mitochondria-targeted vitamin E derivative on mitochondrial alteration and systemic oxidative stress in mice. Br J Nutr. 2011;106: 87–95.
- Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, et al. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc. 2005;127:12460–1.

