Abstract
Objectives. Little is known about the association of reduced pulmonary function and the risk of sudden cardiac death (SCD). Our aim was to examine the relation of forced expiratory volume (FEV1), forced vital capacity (FVC), and the ratio of FEV1 to FVC with SCD in a population-based sample of men.
Methods. This study was based on 1250 men 42–60 years of age without chronic obstructive pulmonary disease, asthma, and lung cancer. During the 20-year follow-up, 95 SCDs occurred. FEV1, FVC, and ratio of FEV1 to FVC were used as lung function tests.
Results. As a continuous variable, each 10% increase in the percentage predicted FEV1 was associated with 18% (adjusted risk 0.82, 95% CI 0.73–0.93, P < 0.002) reduced risk for SCD. Subjects with most reduced (lowest quintile) FEV1 had a 3.5-fold increased risk for SCD (95% CI 1.42–8.41, P = 0.006), after adjustment for conventional risk factors. Similar results were observed with FVC. The results remained statistically significant among non-smokers and smokers respectively.
Conclusion. Our study shows that reduced lung function is a robust predictor of SCD in middle-aged men. Lung function test may be useful in risk stratification for SCD in general population.
This prospective study shows that poor lung function is associated with an increased risk of sudden cardiac death.
The current study demonstrates that lung function test provided prognostic value beyond that predicted by common cardiovascular risk factors among men with no history of chronic obstructive pulmonary disease, asthma, and lung cancer.
Pulmonary function test with spirometry, a common clinical tool, can be useful in risk stratification for sudden cardiac death.
Introduction
Sudden cardiac arrest accounts for one-half of all deaths related to coronary heart disease (CHD) and presents as the first manifestation of CHD in about 20%–30% of the deaths (Citation1). A large majority of sudden cardiac deaths (SCD) occur among more general segments of the population hence requiring screening methods applicable to the general population (Citation2,Citation3). Little is known about the relationship between impaired pulmonary function tests and the risk of SCD among the general population, although some studies suggest that reduced lung function is a risk predictor for cardiovascular mortality (Citation4–7). Furthermore we also wanted to study the impact of lung disease both among smokers and non-smokers and SCD.
This prospective population-based study was designed to determine if impaired pulmonary function (FEV1), forced vital capacity (FVC) and the ratio of FEV1 to FVC are risk predictors for SCD in the general male population.
Methods
Study population
The focus of this prospective study is on non-invasive risk markers for SCD in the general population. The study population is a representative sample of men living in the city of Kuopio and its surrounding rural communities, who were 42–61 years of age at baseline examinations performed between March 1984 and December 1989 (Citation8). Of 3235 potentially eligible men, 2682 (83%) volunteered to participated in this study. The Kuopio Ischemic Heart Disease study was approved by the Research Ethics Committee of the University of Kuopio, and each participant gave written informed consent. All men who visited the clinics between 1984 and 1989 participated in the measurements. The study reported here is based on data obtained from 1441 participants who had data on lung function test measurements at baseline. Men with chronic obstructive pulmonary disease (COPD) or asthma (n = 129), pulmonary tuberculosis (n = 45), and lung cancer (n = 18) were excluded from the present study. Main analyses were performed among men without previous pulmonary diseases; during supplementary analyses men with pulmonary diseases were included to study the prognostic value of pulmonary function in the presence of these diseases. The study reported here is based on data obtained from 1250 participants who had available data on lung function test at baseline.
Assessment of lung function
Lung function was measured via the forced vital capacity manoeuvre, in which the maximal volume of air is exhaled during a forced expiration starting from a position of full inspiration and ending at complete expiration. Spirometry was performed with standardized equipment (Medica, Kuopio, Finland). The measurements used in this study are forced expiratory volume (FEV1) and forced vital capacity (FVC); the total volume of the gas exhaled was also examined as well as the ratio of FEV1 to FVC. Lung function was measured by a trained certified nurse.
Assessment of risk factors
The lifelong exposure to smoking (cigarette pack-years) was estimated as the product of the number of years smoked and the number of tobacco products smoked daily at the time of examination (Citation9,Citation10). Resting blood pressure was measured between 8.00 and 10.00 a.m. with a random-zero sphygmomanometer (Citation11). The use of medications and the diagnosis of diseases were collected at baseline examination by an internist (Citation9,Citation10). Echocardiographic studies were performed with an ATL Ultra-mark IV system with the use of 2D-guided M-mode measurements with a 3.0- or 3.5-MHz transducer. Echocardiographic images with the assessment of left ventricular function were obtained from the parasternal window and a perpendicular projection across the heart, with participants lying in a modified left lateral decubitus position.
The cholesterol contents of serum lipoprotein fractions and triglycerides were measured enzymatically (Boehringer Mannheim, Mannheim, Germany). Serum high-density lipoprotein (HDL)-cholesterol and its sub-fractions were separated from fresh serum samples using ultracentrifugation and precipitation. Serum C-reactive protein was measured with an immune-metric assay (Immulite High Sensitivity C-reactive protein Assay, DPC, Los Angeles, CA, USA). The use of medications and baseline diseases were assessed by self-administered questionnaires. Alcohol consumption was assessed using the Nordic Alcohol Consumption Inventory (Citation10). Body mass index (BMI) was computed as the ratio of weight in kilograms to the square of height in meters. The standardized cycle exercise testing protocol comprised direct analyses of respiratory gases (Medical Graphics, St. Paul, MN, USA). Standard resting 12-lead electrocardiography (ECG) was recorded with the definition of left ventricular hypertrophy (LVH) according to voltage criteria (Sokolow–Lyon).
Classification of sudden cardiac death
All deaths that occurred by the end of 2011 were checked from the hospital documents, wards of health centres, and death certificates. Deaths were coded using the Ninth International Classification of Diseases codes or the Tenth International Classification of Diseases codes. The sources of information were interviews, hospital documents, death certificates, autopsy reports, and medico-legal reports (Citation10,Citation11). There were no losses to follow-up. A death was determined as SCD when it occurred either within 1 hour of the onset of an abrupt change in symptoms or within 24 hours of onset of symptoms when clinical findings did not reveal a non-cardiac cause of sudden death. The deaths due to aortic aneurysm rupture, cardiac rupture or tamponade, and pulmonary embolism, cancer or other non-cardiac co-morbidities were not included as SCD. The diagnostic classification of events was based on symptoms, electrocardiographic findings, cardiac enzyme elevations, autopsy findings (80%), and history of CHD together with the clinical and ECG findings of the paramedic staff. We have available all hospital documents including medical records, laboratory and ECG findings from hospital and paramedical staff, and the use of medications and defibrillator. The documents related to the deaths were cross-checked in detail by two physicians. An independent events committee blinded to clinical data performed classification of deaths.
Statistical analysis
Descriptive data are presented as means and percentages. Risk factors for main outcomes were analysed using multivariate Cox model. FEV1, FVC, and the ratio of FEV1 to FVC were entered into forced SPSS Cox proportional hazards’ models. Cox models were adjusted for age and other demographic and clinical factors previously reported to be predictive of SCD and the clinical relevance. The multivariate model was further adjusted for alcohol consumption, cigarette smoking, serum low- and high-density lipoprotein cholesterol, systolic blood pressure, type 2 diabetes, body mass index, C-reactive protein, previous myocardial infarction, and cardiorespiratory fitness. The analyses were repeated among smokers (n = 401) and non-smokers (n = 849) separately. Relative risks (RR) with 95% confidence intervals (CI), adjusted for clinical risk factors, were estimated as antilogarithms of the coefficients from multivariable models. Analyses were also performed among men with lung diseases. The linearity assumption was satisfied for all continuous variables, and it was assessed with Martingale residuals for each continuous variable against survival time. A P value of less than 0.05 was considered statistically significant. These statistical analyses were performed using SPSS 20.0 for Windows.
Incremental value of FEV1 added to other risk predictors was evaluated using the C-index. The C-index was calculated to assess the model discrimination, the ability of the model correctly to identify subjects with respect to SCD (Citation12). The Harrell C-index was used as the primary measure of discrimination. Additionally we calculated the integrated discrimination improvement for the model with and without FEV1 (Citation13,Citation14). Previously mentioned predicted probabilities of SCD were calculated on the basis of a 20-year follow-up period.
Results
Baseline characteristics
Baseline characteristics in the population are shown in . The mean value for FEV1 was 96.6% (range 15.4%–226.7%). The important differences between the two groups were that among men with spirometry were younger in age (52.2 years versus 54.3 years, P < 0.001), with slightly lower systolic blood pressure (132.5 versus 136.3 mmHg), total cholesterol 5.78 versus 6.1 mmol/L), and low-density lipoprotein (LDL-) cholesterol (3.88 versus 4.21 mmol/L), with fewer years smoked (8.0 versus 9.35 years), and fewer cases of type 2 diabetes (5% versus 6%) compared to those with no spirometry performed. Men who died due to SCD were older, had higher systolic and diastolic blood pressure, more serum HDL-cholesterol and higher triglycerides levels, were more likely to have prevalent CHD, previous MI, hypertension, and type 2 diabetes, and smoked more as compared to those who did not die due to SCD.
Table I. Baseline demographic characteristics among men with and without sudden cardiac death.
Outcome events and risk factors for sudden cardiac death
The average follow-up time to death or the end of follow-up was 20 years (range 0.3–25.7 years). Among men with no history of COPD, asthma, and lung cancer a total of 95 SCDs occurred. There were 52 SCD cases among 849 non-smokers, whereas 43 cases occurred among 401 smokers.
The C-index for the total model discrimination was 0.795 (95% CI 0.755–0.847). After FEV1 was added into the multivariate model, the C-index increased from 0.787 (95% CI 0.753–0.844) to 0.795, showing a slight incremental value of FEV1 in predicting SCD. The integrated discrimination improvement was 0.014 (95% CI 0.004–0.024), and the relative integrated discrimination improvement was 0.11, showing a significant level of discrimination. Consistently, binary R2 increased from 0.156 to 0.167 after FEV1 was added into the multivariable model.
In the multivariate model, the strongest risk factors for SCD were age (RR 1.04, 95% CI 1.00–1.08, P = 0.020), smoking (RR 1.25, 95% CI 1.12–1.39, P < 0.001), previous MI (RR 5.60, 95% CI 3.47–9.03, P < 0.001), and SBP (RR 1.22, 95% CI 1.08–1.37, P = 0.001) in addition to FEV1. Similarly among smokers the strongest risk factors for SCD were previous MI (RR 5.84, 95% CI 2.78–12.27, P < 0.001) and SBP (RR 1.25, 95% CI 1.03–1.53, P = 0.022) in addition to FEV1. Among non-smokers the strongest risk factors for SCD were age (RR 1.06, 95% CI 1.00–1.12, P = 0.025), previous MI (RR 6.34, 95% CI 3.30–12.19, P < 0.001), and CRP (RR 1.08, 95% CI 1.00–1.16, P = 0.031) including FEV1.
Lung function test and the risk of sudden cardiac death
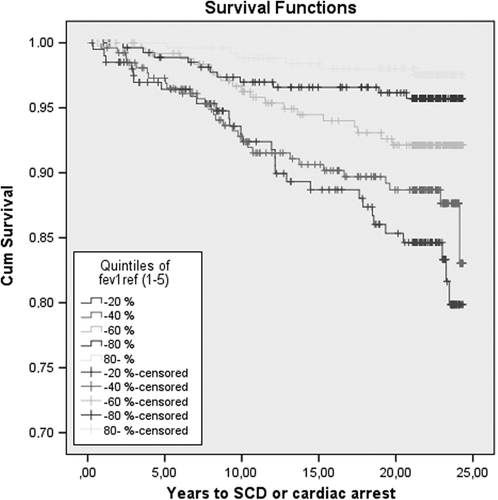
The risk of SCD among quintiles of FEV1 is shown in . As a continuous variable, each 10% increase in percentage predicted FEV1 was associated with 26% (age-adjusted risk 0.74, 95% CI 0.66–0.83, P < 0.001) reduced risk for SCD. After further adjustment for established risk factors (age, smoking, alcohol, SBP, BMI, serum LDL and HDL-cholesterol, previous MI, diabetes, and CRP) each 10% increase in predicted FEV1 was associated with 20% (adjusted risk 0.80, 95% CI 0.70–0.91, P < 0.001) reduced risk. LVH was not related to the risk of SCD. The risk of SCD among those in the highest FEV1 was increased 2.71-fold (95% CI 1.00–7.47, P = 0.050) after additional adjustment for LVH. Furthermore, among men with the assessment of echocardiography (n = 802), FEV1 was associated with a 2.20-fold (95% CI 1.05–4.62, P = 0.031) increased risk of SCD when left ventricular function and other risk factors were adjusted.
Table II. Forced expiratory volume (percentage) and the risk of sudden cardiac death among 1250 men.
Comparisons with FVC and the ratio of FEV1 to FVC
In a multivariate adjusted model FVC was associated with 3.10-fold (95% CI 1.34–7.25, P = 0.008) increased risk for SCD among men in the lowest quintile (13.62%–80.34%) as compared to the highest quintile (> 105.23%). The Kaplan–Meier survival curves with in the quintiles of FEV1 and SCD are shown in . Since FEV1 and FVC behaved so similarly, no association was observed with the ratio of FEV1 and FVC (HR 1.45, 95% CI 0.72–2.95, P = 0.302) in the lowest quintile.
Lung function test and the risk of sudden cardiac death among men with lung disease
FEV1 was associated with 2.65-fold (95% CI 1.25–5.56, P = 0.01) increased risk for SCD among men with asthma, COPD, and lung cancer in lowest quintile lowest quintile (< 71.8%) as compared to highest quintile (≥ 118.9%) in a multivariate model. In analyses using FVC instead of FEV1 the lowest quintile (13.62%–80.34%) yielded almost identical results with fully adjusted HR of 2.20 (95% CI 1.11–4.36, P = 0.02) as compared to the highest quintile. When further adjusting for smoking status (current, former, never) in addition to pack-years the results remained statistically significant. No association was observed between the ratio of FEV1 and FVC and SCD.
Lung function test and the risk of sudden cardiac death among non-smokers
As a continuous variable, each 10% increase in FEV1 was associated with 25% (age-adjusted risk 0.75, 95% CI 0.64–0.87, P < 0.001) reduced risk for SCD (non-smokers: n = 849; 52 cases). In a multivariate-adjusted model, every 10% increase in the predicted FEV1 was associated with 16% (0.84, 95% CI 0.70–0.99, P = 0.05) reduced risk for SCD. In a multivariate-adjusted model the risk was increased 3.62-fold (95% CI 1.04–12.69, P = 0.042) for SCD in the lowest quintile.
Lung function test and the risk of sudden cardiac death among smokers
Every 10% increase in FEV1 was related to 21% (age-adjusted risk 0.79, 95% CI 0.65–0.95, P = 0.020) reduced risk for SCD. In a multivariate adjusted model, every 10% increase in FEV1 was associated with 20% (adjusted risk 0.80, 95% CI 0.65–0.98, P = 0.04) reduced risk for SCD. After adjusting for age, low FEV1 was associated with a 5.71-fold (95% CI 1.25–26.13, P = 0.025) increased risk for SCD as compared to the highest quintile. In a multivariate-adjusted model the respective risk was increased 5.01-fold (95% CI 1.09–23.01, P = 0.041) for SCD in the lowest quintile.
Discussion
This prospective study shows that poor lung function is associated with an increased risk of SCD in the general population. The current study demonstrates that a lung function test provided prognostic value beyond that predicted by common cardiovascular risk factors among men with no history of COPD, asthma, and lung cancer. Our findings indicate that airflow obstruction is an important risk factor for SCD both in non-smokers and smokers. In our study the risk of SCD was also increased among men with obstructive lung disease.
Some underlying risk factors for SCD may be in part similar to common cardiovascular risk factors for CHD (Citation15,Citation16), but the assessment of airflow obstruction can provide additional prognostic information for SCD in conjunction with traditional risk factors in the general population. Previous research suggests that the systemic inflammation or visceral adiposity commonly present in COPD leads to the increased risk, and that treatment aimed at decreasing inflammation in those with COPD may decrease the development of cardiac diseases (Citation17). Vascular inflammation may also contribute to impaired airway vascular smooth muscle relaxation in COPD, and thus the elimination of external toxic agents may also play a role in the prevention of cardiac diseases (Citation18–20). However, in this study firstly, the association between impaired pulmonary function and SCD remained significant, although the level of C-reactive protein was taken into account. Secondly, both FEV1 and FVC were significantly and inversely related to the risk of SCD after adjusting for other possible confounders, suggesting the protective effect of lung function on SCD risk. Findings from our study show that cigarette smoking has an adverse effect on SCD. The risk for SCD was increased considerably among smokers as compared to non-smokers. Pulmonary function appears to be associated not only with cardiovascular risk factors but also clinical cardiovascular disease as our study showed that coronary heart disease was one of the important risk predictors.
The pathophysiological mechanisms by which lower FEV1 and FVC may be associated with an increased risk of SCD is not very clear. The strong association between lung function and SCD observed in smokers suggests that a low FEV1 may be a marker of ischemic myocardial disease, resulting in greater susceptibility to SCD, particularly in men with underlying CHD. This concept of FEV1 as a measure of physiological reserve capacity to withstand pathological insults to the cardiovascular circulation seems tenable, particularly in view of the stronger inverse association between FEV1 and SCD. Reduced lung volume may also reflect impaired cardiac function due to an occult coronary disease (Citation4), and it has been shown that left ventricular dysfunction is associated with an increased risk of SCD (Citation4,Citation21). Depressed LV ejection fraction is a well-known predictor of mortality; however, we found that FEV1 was an independent risk predictor for SCD when left ventricular function was additionally adjusted for. Poor lung function with bronchial wall oedema can lead to airway obstruction inducing myocardial ischemia (Citation22,Citation23). It has also been suggested that the increased risk may be associated with alcohol intake, which has been shown to influence lung function, which may in turn cause SCD (Citation24, Citation25); however, our findings were independent of alcohol intake.
On the basis of this long-term follow-up study it is possible to conclude that impaired pulmonary function is a predictor for SCD. A strength of our study is that the study is based on a representative population-based sample of middle-aged men, with a high participation rate. A limitation of the present study is that we have included only middle-aged men, and no women and elderly in the cohort. Furthermore, the study design does not allow generalization to other races. We do not know whether the reduction of pro-inflammatory markers would reduce the risk of SCD. The single assessment of lung function may lead to an underestimation, rather than an overestimation of the prognostic significance of lung function. However, an unanswered question is whether the new findings can be used to implement a recommendation for the routine use of spirometry for SCD risk assessment in the general population.
These findings emphasize the importance of identifying high-risk persons and the prognostic utility of pulmonary function test in the prediction of SCD in the general population. Our study suggests that lung function test with spirometry, which is a common clinical tool, can be used as a predictive measure for SCD.
Funding: This study was supported by the Finnish Medical Foundation, Helsinki, Finland.
Declaration of interest: No potential conflicts of interest relevant to this article are reported.
References
- Zipes DP, Wellen HJ. Sudden cardiac death. Circulation. 1998;98:2334–51.
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–82.
- Muller D, Agrawal R, Arntz HR. How sudden is cardiac death? Circulation. 2006;114:1146–50.
- Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294: 1071–5.
- Engström G, Lind P, Hedblad B, Wollmer P, Stavenow L, Janzon L, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation. 2002;106:2555–60.
- Schünemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118: 656–64.
- Bang KM, Gergen PJ, Kramer R, Cohen B. The effect of pulmonary impairment on all-cause mortality in a national cohort. Chest. 1993;103: 536–40.
- Salonen JT. Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Clin Res. 1988;20:46–50.
- Lakka TA, Venäläinen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction in men. N Engl J Med. 1994;330:1549–54.
- Laukkanen JA, Mäkikallio TH, Rauramaa R, Kiviniemi V, Ronkainen K, Kurl S. Cardiorespiratory fitness is related to the risk of sudden cardiac death. A population-based follow-up study. J Am Coll Cardiol. 2010;56: 1476–83.
- Kurl S, Mäkikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–94.
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87.
- Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al.; American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16.
- Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72.
- Albert CM, Ruskin JN. Risk stratifiers for sudden cardiac death (SCD) in the community: primary prevention of SCD. Cardiovasc Res. 2001;50:186–96.
- Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, et al.; American Heart Association; American College of Cardiology Foundation; Heart Rhythm Society. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol. 2008;52:1179–99.
- Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155:842–8.
- Sparrow D, Weiss ST, Vokonas PS, Cupples LA, Ekerdt DJ, Colton T. Forced vital capacity and the risk of hypertension: the Normative Aging Study. Am J Epidemiol. 1988;127:734–41.
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102: 2165–68.
- Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7.
- Kannel WB, Wolf PA, Verter J. Manifestations of coronary disease predisposing to stroke. JAMA. 1983;250:2942–46.
- Cabanes LR, Weber SN, Matran R, Regnard J, Richard MO, Degeorges ME, et al. Bronchial hyperresponsiveness to methacholine in patients with impaired left ventricular function. N Engl J Med. 1989;320:1317–22.
- Cabanes LR, Costes F, Weber S, Regnard J, Benvenuti C, Castaigne A, et al. Improvement in exercise performance by inhalation of methoxamine in patients with impaired left ventricular function. N Engl J Med. 1992;326:1661–5.
- Lange P, Groth S, Mortensen J, Applegard M, Nyboe I, Jensen G, et al. Pulmonary function is influenced by heavy alcohol consumption. Am Rev Respir Dis. 1987;137:1119–23.
- Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100:944–50.