Abstract
Aim. To evaluate the relation of proprotein convertase subtilisin-kexin type 9 (PCSK9) levels to coronary artery disease (CAD).
Methods. A total of 1031 consecutive individuals (552 CAD and 479 controls) were prospectively enrolled. The associations of plasma PCSK9 levels with the incidence and severity of CAD were investigated. Further, mediator analysis was performed to detect the potential mechanisms of the associations.
Results. No difference in PCSK9 levels between CAD patients and controls was detected (median 224.75 versus 224.64 ng/mL, P > 0.05). However, the CAD group had higher PCSK9 levels than the control group when adjusting for the confounding factors (228.03 ± 1.01 versus 219.28 ± 1.02 ng/mL, P = 0.019). PCSK9 levels were also associated with the severity of CAD assessed by the Gensini score (GS) system (P for trend < 0.05). Logistic regression analysis showed that PCSK9 levels were associated with an increased CAD risk (OR 3.296 and 5.130 for the incidence and severity, respectively). Importantly, mediator analysis indicated that the effects of PCSK9 levels on CAD were mediated by lipid (around 20%) and inflammation (around 15%).
Conclusions. PCSK9 levels were positively associated with the severity of CAD; the relatively important mechanisms including lipid and inflammation pathways were partly involved in this association.
Genetic variations in the PCSK9 gene are associated with dyslipidemia and atherosclerotic cardiovascular risk; hence, the determination of PCSK9 levels in patients with coronary artery disease (CAD) is of clinical interest.
Our data indicated that PCSK9 levels were positively associated with the severity of CAD, suggesting that PCSK9 is a potentially useful biomarker for predicting the severity of CAD.
The study further confirmed by mediator analysis that the effects of PCSK9 levels on CAD were mediated by increased lipid and inflammation.
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) was firstly characterized as a novel proteinase K-like subtilase by Seidah and colleagues (Citation1). Shortly thereafter, it was reported as a third genetically validated target, after low-density lipoprotein (LDL) receptor (LDLR) and apolipoprotein B (apoB), associated with autosomal dominant hypercholesterolemia (ADH), a form of familial hypercholesterolemia (Citation2). In the following years, experimental studies established the central role of PCSK9 in regulation of cholesterol homeostasis by degradation of hepatic LDLR, resulting in increased LDL cholesterol (LDL-C) concentrations (Citation3). The functional role of PCSK9 in atherogenic dyslipidemia and elevated coronary artery disease (CAD) risk had also been well-documented by genome-wide association and Mendelian randomization studies, which had confirmed that the gain- of-function mutations of PCSK9 were associated with hypercholesterolemia and sharply increased risk of CAD, whereas the loss-of-function mutations were accompanied by remarkable reductions in CAD risk (Citation4–6). Several investigations using the PCSK9-knockout mouse model furthered the understanding of the association between PCSK9 and CAD (Citation7,Citation8). These findings generated an intense attention on the potential proatherosclerotic properties of PCSK9.
Furthermore, the determination of plasma PCSK9 levels in different populations is of considerable interest for clinical practice, given the pathophysiological role of PCSK9 as a secreted factor and the recent finding that plasma PCSK9 levels predicted recurrent clinical events in patients with stable cardiovascular disease treated with low-dose atorvastatin (Citation9). The PCSK9 level as a potential useful biomarker for CAD might be more interesting and worthy of investigation.
However, there are few studies regarding the PCSK9 level as biomarker for CAD to date. It also remains unknown how much of the effect of PCSK9 levels on CAD is attributable to the mediation of atherogenic dyslipidemia or other pathways. Therefore, in the current study, we sought to evaluate the associations of PCSK9 levels with the incidence and severity of CAD, then to investigate the underlying mechanisms of the associations.
Materials and methods
Study design and population
The present study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (Fu Wai Hospital & National Center for Cardiovascular Diseases, Beijing, China). Informed written consent was obtained from all patients enrolled in this study.
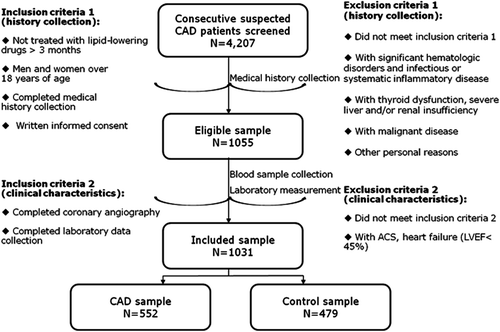
In a group of patients scheduled for coronary angiography because of their angina-like chest pain and/or positive treadmill exercise test and/or clinically suspected CAD in our division, we consecutively recruited 1031 individuals who had no lipid-lowering therapy (552 CAD and 479 controls). Essentially, the present study was a prospective study with angiography and lipid-lowering therapy being parts of the screening process (the flow chart shown in ). Because lipid-lowering medication could increase the PCSK9 levels (Citation10), confounding the study results, the individuals taking any lipid-lowering agent within 3 months prior to entering the study were excluded from this study. Additionally, patients with acute coronary syndrome (ACS), heart failure (left ventricular ejection fraction, LVEF < 45%), history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), significant hematologic disorders, infectious or systemic inflammatory disease, thyroid dysfunction, severe liver and/or renal insufficiency, and malignant disease were excluded from the study.
Figure 1. Flow chart of the selection of the present study population including inclusion and exclusion criteria.
Hypertension was defined as repeated systolic and/or diastolic blood pressure ≥ 140 and/or ≥ 90 mmHg on two different occasions or if patients were currently taking anti-hypertensive drugs. Diabetes mellitus (DM) was diagnosed as fasting serum glucose levels ≥ 7.0 mmol/L in multiple determinations or patients were being treated with insulin or oral hypoglycemic agents. Hyperlipidemia was considered to be present in patients if they had fasting total cholesterol (TC) ≥ 200 mg/dL or triglyceride (TG) ≥ 150 mg/dL.
Laboratory examinations
Blood samples were obtained for all patients from the cubital vein after a 12-hour overnight fast, and collected into EDTA-containing tubes. Then the samples were stored at –80°C until analysis.
The concentrations of plasma TC, TG, high-density lipoprotein cholesterol (HDL-C), LDL-C, and apoB were measured using an automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan). Of these, TC, TG, HDL-C, and LDL-C were measured using an enzymatic assay, and apoB was measured using a turbidimetric immunoassay. The white blood cell (WBC) count was determined using an automated blood cell counter (Beckman Coulter Ireland Inc., Mervue, Galway, Ireland). The levels of plasma fibrinogen were measured by the Clauss method. The concentrations of high-sensitivity C-reactive protein (hs-CRP) were determined using immunoturbidimetry (Beckmann Assay 360, Bera, CA, USA). The levels of plasma PCSK9 were measured using a standard method named the high-sensitivity, quantitative sandwich enzyme immunoassay (Quantikine ELISA, R & D Systems Europe Ltd, Minneapolis, USA) as in previous studies (Citation11–13). A monoclonal antibody specific for PCSK9 was used in the ELISA assay. The lower limit of detection was 0.096 ng/mL.
Assessment of the severity of CAD
The study patients were referred for coronary angiography, which was performed using the standard Judkin technique with filming of multiple views of each vessel. The severity of CAD was assessed according to the Gensini score (GS) system (Citation14). The GS was computed by assigning a severity score to each coronary stenosis according to the degree of luminal narrowing and its geographic importance. Firstly, reductions in the lumen diameter or roentgenographic appearances of the coronary artery lesion were evaluated as 1 for 1%–25% stenosis, 2 for 26%–50% stenosis, 4 for 51%–75% stenosis, 8 for 76%–90% stenosis, 16 for 91%–99% stenosis, and 32 for total occlusion. Then, the scores were multiplied by a factor that represented the importance of the lesion's position in the coronary arterial system. They were 5 for the left main coronary artery, 2.5 for the proximal left anterior descending or proximal left circumflex artery, 1.5 for the mid-region, 1 for the distal left anterior descending or mid-distal region of the left circumflex artery, and 0.5 for small vascular branches. Patients with angiography-proven CAD were also subdivided into three subgroups according to GS tertiles. In patients who were going to undergo PCI or CABG, their angiographic GSs were measured before the revascularization procedures.
Statistical analysis
The values were expressed as mean ± SD or median (Q1–Q3 quartiles) for the continuous variables and number (percentage) for the categorical variables. Pearson correlation analysis was used to determine the correlations between two normal distribution variables. Both the lipid status and the inflammation status were assessed by a number of correlated markers. We performed principal component analysis to extract from the individual markers of atherogenic lipid profile (TG, TC, LDL-C, non-HDL-C, apoB) and inflammation (WBC, hs-CRP, fibrinogen) to the relevant weighted multi-marker indexes. A general linear model was used to study PCSK9 levels over the quartiles of the multi-marker indexes of lipid and inflammation with adjustments for confounding factors, and to calculate the adjusted mean values, respectively. The same model was used to study the levels of PCSK9, atherogenic lipids, and inflammation markers over the groups regarding the status and severity of CAD. Log-normalization was used for variables with positively skewed distribution (PCSK9, TG, hs-CRP). Multiple logistic regression analysis was performed to investigate the strength of association between exposure factors and CAD. Additionally, we examined the multi-marker indexes as potential mediators of the relationship between PCSK9 levels and CAD using mediation analysis. The lipid and inflammation pathways were of special interest because they were associated with both PCSK9 levels and CAD. Briefly, the mediation analysis was based on three models: one for explaining the outcome (CAD status) of an exposure (different levels of PCSK9), one for explaining mediator (atherogenic lipids and inflammation, respectively) of the PCSK9 levels, and the third for estimating the association of mediator and PCSK9 levels with CAD. The analysis was performed in the whole sample with adjustment for age and sex. A P value < 0.05 was considered statistically significant. The statistical analysis was performed with SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
A total of 1031 individuals were included in the present study. Among them, 552 (53.5%) had significant angiographically documented CAD, with > 50% diameter stenosis in ≥ 1 major epicardial coronary artery. Therefore, in the current study population (n = 1031), there were 552 patients with varying degrees of CAD and 479 controls (). The main demographic and clinical characteristics of the study subjects are listed in .
Table I. Baseline characteristics of the study population. Bold values in the Table indicate statistical significance.
Associations of PCSK9 levels with the incidence and severity of CAD
As shown in , no difference in PCSK9 levels between CAD patients and the controls was detected, but significant differences were found in patients’ characteristics between the groups including age, gender distribution, body mass index (BMI), hypertension, diabetes, smoking, and family history of CAD, which might confound the study result. For instance, the proportion of men in the CAD group was significantly larger than in the control group. Of note, it has been well-documented by previous studies that men have a much lower PCSK9 level than women (Citation15–17). Therefore, we further investigated the associations when adjusting for the confounding factors ().
Table II. Adjusted associations of PCSK9, atherogenic lipids, and inflammation with CAD. Bold values in the Table indicate statistical significance.
Firstly, we used three models to perform gradual adjustments. Significantly, the PCSK9 levels were higher in the CAD group than in the control group in all the three models (all P < 0.05). The atherogenic lipids such as non-HDL-C, LDL-C, and apoB and the inflammation markers including hs-CRP and fibrinogen also exhibited differences between the groups.
Secondly, we explored whether PCSK9 levels were increased with GS tertiles. We found that PCSK9 levels were also associated with the severity of CAD assessed by GS (P for trend < 0.05). Even after adjustment for age, gender, BMI, hypertension, and diabetes, in addition to smoking and family history of CAD, the levels of PCSK9 in the high, intermediate, and low GS groups remained significantly different (229.99 ± 1.01 versus 233.35 ± 1.01 versus 213.30 ± 1.01 ng/mL, respectively).
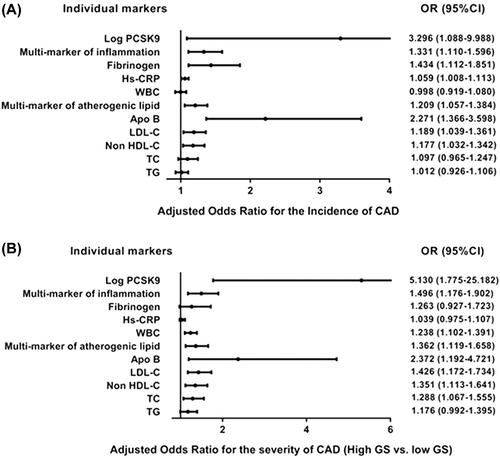
Furthermore, logistic regression analysis correcting for age and gender was performed, suggesting that the atherogenic lipid index (OR for the incidence of CAD, 1.209 [1.057–1.384]; for the severity of CAD, 1.362 [1.119–1.658]), the inflammation markers index (OR for the incidence of CAD, 1.331 [1.110–1.596]; for the severity of CAD, 1.496 [1.176–1.902]), and the PCSK9 levels (OR for the incidence of CAD, 3.296 [1.088–9.988]; for the severity of CAD, 5.130 [1.775–25.182]; log-transformed data) were significantly associated with increased risk of CAD ().
Figure 2. Adjusted odd ratios for incident CAD (A) and severity of CAD (high GS versus low GS) (B) according to the individual markers of lipid and inflammation, PCSK9 levels (log-transformed data), and the multi-marker indexes. Logistic regression analysis was performed, and models were adjusted for age and gender.
Associations of PCSK9 levels with atherogenic lipids and inflammation
Overall, the individual markers of lipid and inflammation including levels of TG, TC, non-HDL-C, LDL-C, apoB, WBC, hs-CRP, and fibrinogen were all significantly and positively correlated with the PCSK9 levels (). Both the atherogenic lipids and inflammation status can be assessed by a number of correlated markers. We extracted from the individual markers of atherogenic lipid profile (TG, TC, LDL-C, non-HDL-C, apoB) and inflammation (WBC, hs-CRP, fibrinogen) to the weighted multi-marker indexes respectively, as shown in . Significantly, the PCSK9 levels were increased with the elevated quartiles of atherogenic lipids index (207.97 ± 1.02, 215.28 ± 1.02, 220.80 ± 1.02, 240.99 ± 1.02 ng/mL) and inflammation index (215.28 ± 1.02, 220.29 ± 1.02, 220.80 ± 1.02, 239.88 ± 1.02 ng/mL) even after adjustment for confounding factors including age, sex, BMI, hypertension, diabetes, smoking, and family history of CAD (). Thus, there was a significant difference among the quartiles in the levels of PCSK9 (P < 0.001).
Figure 3. Correlations of lipid and inflammation markers with plasma PCSK9 levels in overall study population (A–H). Regression lines were calculated by Pearson correlation analysis. Coefficients and P value are presented.
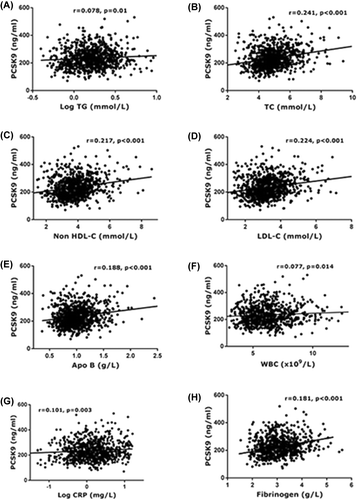
Table III. Relationships of PCSK9 with multi-marker indexes of atherogenic lipids and inflammation. Bold values in the Table indicate statistical significance.
Lipid and inflammation as mediators of the relationship between PCSK9 levels and CAD
To estimate the effect of PCSK9 levels on CAD via lipid/inflammation pathways, we performed mediation analysis (which assesses an intermediate variable as a mediator in the pathway between a risk factor and an outcome, estimating the extent to which the effect of the risk factor occurs through the mediator). The mediation analysis was performed in a model with the incidence or severity of CAD as the dependent variable, PCSK9 level as the independent variable, and lipid/inflammation index as the mediator variable, with age and sex as covariates (). In the model with lipid index as mediator, data showed that the effects of PCSK9 on the incidence and severity of CAD were mediated partly by lipid and the effect size on both was 17%. In the model with inflammation index as mediator, the effects of PCSK9 on CAD were also found to be mediated partly by inflammation, and the effect size was 10% for the incidence and 18% for the severity of CAD, respectively.
Table IV. Mediator analysis corroborating the association of PCSK9 with CAD. Bold values in the Table indicate statistical significance.
Discussion
The present study, for the first time, has suggested the associations of plasma PCSK9 levels with lipid/inflammation and CAD in humans. The main findings were: 1) plasma PCSK9 levels were higher in patients with CAD when adjusting for the confounding factors between the groups, although no difference in PCSK9 levels between CAD patients and controls was detected; 2) the PCSK9 levels were increased with the severity of CAD as assessed by GS in both crude and adjusted models; 3) plasma PCSK9 levels were significantly associated with circulating lipid-related and inflammatory markers; 4) evidence from mediation analysis indicated that lipid and inflammation pathways were partly involved in the effect of PCSK9 levels on CAD, and the effect sizes for the incidence and the severity of CAD by lipid were both 17%, while those by inflammation were 10% for the incidence and 18% for the severity of CAD, respectively. These findings provide a useful insight for understanding the mechanisms underlying the relationship of plasma PCSK9 and CAD. Importantly, the present study, from a clinical insight, investigated the association of PCSK9 levels with the severity of CAD in patients who had no lipid-lowering therapy, suggesting that PCSK9 might become a useful biomarker for predicting the severity of CAD.
The discovery of PCSK9 has changed our understanding of cholesterol metabolism from a system exclusively controlled by intracellular processes, to one eminently regulated by a circulatory protein (Citation3). Moreover, the demonstration that PCSK9 mutations influence atherosclerosis and CAD risk is another significant breakthrough in the clinical interest in PCSK9 (Citation2,Citation18,Citation19). The mutations are associated with either a specific hypo- or hyper-cholesterolemic phenotype with decreased or increased CAD risk. For instance, the Atherosclerosis Risk in Communities (ARIC) study has demonstrated that carriers of PCSK9 loss-of-function variants R46L and Y142X or C679X exhibit a significantly reduced risk of CAD. The Y142X or C679X nonsense variants occurred in 2.6% of Afro-American black subjects and were associated with a 28% reduction in mean LDL-C and an 88% reduction in CAD risk during a 15-year-long follow-up. In the same study, the R46L variant occurred in 3.2% of white subjects and was associated with a 15% reduction in mean LDL-C and a 47% reduction in the risk of CAD (Citation4). Therefore, it is of considerable interest to measure circulating levels of PCSK9 from a clinical perspective in humans.
In the present study, from a clinical insight, we focused on the role of PCSK9 levels in CAD and suggested PCSK9 as a novel biomarker. Firstly, consistent with other common biomarkers such as CRP, circulating PCSK9 could be quantitatively detected in patients’ plasma using a clinically feasible method and demonstrated differences in patients with different status of severity of CAD. Secondly, our data suggested a role of circulating PCSK9 in lipid metabolism and inflammation. A positive correlation between PCSK9 and LDL-C levels was found almost systematically since the well-demonstrated function of PCSK9 as a secret factor was directed toward LDLR degradation (Citation18). Importantly, PCSK9 levels were found to be positively associated with inflammatory makers including WBC, fibrinogen, and CRP (Citation11,Citation12). In fact, there have been several experimental studies regarding a connection between PCSK9 and inflammation. Previous studies have recognized that inflammation stimulated the expression of PCSK9 in human liver cells (Citation20), although there was no report that inflammatory cells could express PCSK9 so far. And also, PCSK9 blockade could lower levels of inflammatory cytokine and improve inflammatory response in both cultured cells (Citation21) and animal models (Citation22). More interestingly, the PCSK9-blocking antibody had been found to reduce monocyte recruitment and improve atherosclerotic lesion composition by increasing the smooth muscle cell and collagen content and decreasing the macrophage and necrotic core content (Citation23). Taken together, studies have shown that the inflammatory pathways might also be implicated in mediating the effects of PCSK9 on vascular biology. Additionally, the recent finding that plasma PCSK9 levels predicted recurrent clinical events in stable CAD patients with well-controlled LDL-C has made the determination of circulating PCSK9 more interesting in clinical practice as a potential useful biomarker (Citation9). It is plausible to postulate that the harmful effect of elevated PCSK9 levels on CAD risk is worthy of a careful look, and measuring PCSK9 levels might be a useful means to predict CAD risk.
Several limitations need to be mentioned in the present study. Firstly, the current study examined the relationship between PCSK9 levels and CAD only in Chinese people, and whether this differs across populations, regions, and time periods remains to be investigated. A recent study performed by Almontashiri et al. reported that plasma PCSK9 levels were elevated in acute myocardial infarction but not correlated with the severity of CAD in two similar cross-sectional cohorts of angiographic patients and controls, from the geographically distinct populations of the Ottawa Heart Study and the Emory Cardiology Biobank Study (Citation24). Although their studies were to some extent similar to the present study in the methodology of PCSK9 measurement and the enrolled patients without statin use, they assessed the severity of CAD using the number of coronary arteries with stenosis, and patients with DM were excluded from their study (Citation24). Secondly, in the present study, patients with CAD had significantly different characteristics from the controls. Compared to the controls, patients with CAD were older, more often of male sex, had higher BMI, more DM, and so on. The differences in groups might have some confounding effect on the study results. Therefore, we investigated the associations of PCSK9 with CAD in various adjusted models to minimize the confounding effects. Thirdly, only plasma PCSK9 levels were evaluated, and no genotypic detection for the loss-of-function or gain-of-function of PCSK9 was performed. Plasma PCSK9 levels do not fully represent function/biological effect, since some mutations that occur naturally have allowed a better understanding of PCSK9 biology (Citation25,Citation26), such as mutations that affect PCSK9 circulating half-life (Citation27), binding affinity to the LDLR (Citation28), and so on. Moreover, it has recently been demonstrated in a large cohort that five key factors drive CAD, namely diet, alcohol consumption, smoking, physical activity, and abdominal adiposity (Citation29). Not all of them were investigated in this study. Finally, despite the PCSK9 levels being different between CAD and non-CAD groups, or in patients with low, intermediate, and high GS, after the adjustment for confounding factors, the statistical p-value seemed marginal, and no significant statistical difference in levels of PCSK9 between the groups of high and intermediate GS was found. The relatively small sample size of the present study might be a potential explanation. However, there remained a significant and independent tendency of increase in PCSK9 levels with GS tertiles.
Conclusions
In summary, the present study suggests that plasma PCSK9 level is a useful biomarker for the severity of CAD, and the lipid and inflammation pathways are partly involved in the effect of PCSK9 levels on CAD.
Acknowledgements
Sha Li and Yan Zhang contributed equally to this study.
Funding: This work was partially supported by the National Natural Science Foundation of China (81070171, 81241121), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), the Capital Special Foundation of Clinical Application Research (Z121107001012015), the Capital Health Development Fund (2011400302), and the Beijing Natural Science Foundation (7131014) awarded to Dr Jian-Jun Li, MD, PhD.
Declaration of interest: The authors have no conflicts of interest to disclose.
References
- Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–33.
- Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
- Lambert G, Charlton F, Rye KA, Piper DE. Molecular basis of PCSK9 function. Atherosclerosis. 2009;203:1–7.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
- Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–42.
- Ason B, van der Hoorn JW, Chan J, Lee E, Pieterman EJ, Nguyen KK, et al. PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE. J Lipid Res. 2014;55: 2370–9.
- Denis M, Marcinkiewicz J, Zaid A, Gauthier D, Poirier S, Lazure C, et al. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012;125:894–901.
- Werner C, Hoffmann MM, Winkler K, Bohm M, Laufs U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. 2014;62:94–102.
- Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–9.
- Li S, Guo YL, Xu RX, Zhang Y, Zhu CG, Sun J, et al. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis. 2014;234:441–5.
- Zhang Y, Zhu CG, Xu RX, Li S, Guo YL, Sun J, et al. Relation of circulating PCSK9 concentration to fibrinogen in patients with stable coronary artery disease. J Clin Lipidol. 2014;8:494–500.
- Guo YL, Liu J, Xu RX, Zhu CG, Wu NQ, Jiang LX, et al. Short-term impact of low-dose atorvastatin on serum proprotein convertase subtilisin/kexin type 9. Clin Drug Investig. 2013;33:877–83.
- Sinning C, Lillpopp L, Appelbaum S, Ojeda F, Zeller T, Schnabel R, et al. Angiographic score assessment improves cardiovascular risk prediction: the clinical value of SYNTAX and Gensini application. Clin Res Cardiol. 2013;102:495–503.
- Baass A, Dubuc G, Tremblay M, Delvin EE, O’Loughlin J, Levy E, et al. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. 2009;55:1637–45.
- Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Q, et al. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 2010;213:632–6.
- Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–43.
- Cariou B, Le May C, Costet P. Clinical aspects of PCSK9. Atherosclerosis. 2011;216:258–65.
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–5.
- Feingold KR, Moser AH, Shigenaga JK, Patzek SM, Grunfeld C. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun. 2008;374:341–4.
- Tang Z, Jiang L, Peng J, Ren Z, Wei D, Wu C, et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kappaB activation in THP-1-derived macrophages. Int J Mol Med. 2012;30:931–8.
- Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6:258ra143.
- Kuhnast S, van der Hoorn JW, Pieterman EJ, van den Hoek AM, Sasiela WJ, Gusarova V, et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res. 2014;55: 2103–12.
- Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, Dandona S, Roberts R, Quyyumi AA, et al. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One. 2014;9:e106294.
- Lopez D. PCSK9: an enigmatic protease. Biochim Biophys Acta. 2008;1781:184–91.
- Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–36.
- Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat. 2005;26:497.
- Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol. 2007;14:413–19.
- Akesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64:1299–306.

