Abstract
Background. There is little quantitative information about the development of chronic obstructive pulmonary disease (COPD) among adult smokers and of what happens to patients who have already developed COPD.
Objectives. To examine the development and performance of COPD status over time, and the clinical characteristics of new COPD cases according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2007 and 2011 classifications.
Methods. Healthy asymptomatic smokers were recruited through newspaper announcements. They filled in questionnaires and had an individualized assessment of their health history during all three visits (visit 1, visit 2 after three years, visit 3 after six years).
Results. Of the eligible 621 heavy smokers, 572 attended visit 2. A total of 513 subjects completed the 6-year follow-up examination. According to GOLD 2007, COPD was present in 22.8% (n = 117) of these smokers. The severity of COPD changed during the years of follow-up. Furthermore, health status and prevalence of chronic respiratory symptoms both in the smokers with normal lung function and in the COPD groups varied over the time period.
Conclusions. GOLD 2011 recognized the complex patient subgroups better than GOLD 2007. Variability in chronic symptoms or in health status correlated poorly with the severity of airway limitation.
The clinical features of COPD vary over time regardless of the degree of severity in airway limitation.
Introduction
Chronic obstructive pulmonary disease (COPD) is known to be an under-diagnosed and life-threatening complex disease accompanied by several serious co-morbidities. COPD is emerging as a major disease burden all around the world (Citation1). The revised strategy recommendations of the Global Initiative for Chronic Obstructive Lung Disease in 2011 (GOLD 2011) not only include a classification of COPD severity confirmed by flow-volume spirometry with a bronchodilatation test (BD spirometry), but they also evaluate the risk of future exacerbations and the level of symptoms (dyspnoea, sputum production, and chronic cough) (Citation2). COPD is defined by a not fully reversible airway limitation in spirometry. However, COPD is a complex disease consisting of variable airway and parenchymal (emphysema) manifestations with different inflammatory cellular and molecular components (Citation3,Citation4).
Both the GOLD 2007 (Citation5) and the GOLD 2011 (Citation2) recommendations have been shown to be valuable in predicting both mortality and the risk of hospitalizations (Citation6). Some recent studies have evaluated the value of these GOLD recommendations in predicting the natural course of COPD (Citation7–9). Recently, one focus of COPD research has been on revealing the disease heterogeneity or phenotypes of COPD, i.e. not only severities according to spirometry but also identifying clinically important subgroups: emphysema (Citation4), the asthma–COPD overlap syndrome (ACOS) (Citation10), or manifestations with other chronic diseases (Citation11–13). However, the exacerbation rates in COPD patients seem to vary with time even in individuals displaying the same spirometry results (Citation14). This is also the case with the prevalence of prolonged respiratory symptoms. Some of those COPD signs may be more significant than others in clinical terms, i.e. they are associated with a worse outcome in diseased individuals (Citation15,Citation16).
In this study, we used the data of a 6-year follow-up study of adult heavy smokers in Northern Finland. The goals of this study were: 1) to monitor the course of observed new COPD cases, 2) to describe severities and clinical features of COPD using both GOLD 2007 and GOLD 2011 classifications and the change during a 3-year follow-up using GOLD 2007 criteria, and 3) to identify subgroups of COPD with clinically meaningful characteristics during the follow-up period.
Material and methods
Study design and participants
This study is a non-randomized longitudinal study with three examinations sessions (baseline, 3 years, 6 years) taking place over a 6-year follow-up period. The study subjects were volunteer adult daily smokers, who felt themselves healthy without any chronic diseases and were symptom-free at the baseline visit despite having smoked over 20 pack-years. The participants were 621 smokers who were recruited through newspaper announcements. The sample size was governed by the resources of this study. The first 3 years of the study were focused on exploring the success in smoking cessation of these smokers without knowledge of possible airway limitations, whereas variations in the disease severity of COPD were the main focus during the last 3 years of the follow-up. The baseline examinations and questionnaires were conducted from November 2003 to October 2006 by the same study nurse (Citation17). The exclusion criteria consisted of chronic pulmonary or other diseases requiring regular medication, allergies, risk factors for other pulmonary diseases, e.g. known exposures, bronchiectasis, malignancies, and also previous lung tuberculosis. The presence of any lung infections during the last 2 months before entering the study was also an exclusion criterion. All visits consisted of a personal interview, and the participants filled in questionnaires which enquired about their detailed updated smoking history and their assessment of prolonged respiratory symptoms (chronic cough and sputum production). All the assessments were conducted by the same experienced nurse during the three visits, and there were no missing data except in measuring the transfer factor from COPD cases.
This study was approved by the ethics committee of Lapland Hospital District in Rovaniemi, Finland. All participants gave their written informed consents.
Assessment of COPD status
The main outcome variable of this study was COPD status. Individuals with COPD were identified according to international GOLD 2007 recommendations based on forced expiratory volume in 1 second divided by forced vital capacity (FEV1/FVC) < 0.70. The flow-volume spirometry was conducted both before and after bronchodilatation with 0.4 mg salbutamol (BD spirometry) on both the second (3 years) and the third (6 years) visits. The participants fulfilling the COPD criteria were subdivided into severities of COPD stages I –IV according to the GOLD 2007 criteria, using the BD-FEV1 level expressed as a percentage of the predicted value (Citation5). Individuals without any airway limitation were further categorized into two groups based on their reported chronic cough and sputum production symptoms. Chronic cough was assessed via the question: ‘Do you usually have a cough?’ (no/yes), and sputum production by the question: ‘Do you usually bring up phlegm from your chest ?’ (no/yes). Subjects with FEV1/FVC ≥ 0.70 and no symptoms were labelled as being in the normal lung function group (NLF). The second group, the previous GOLD stage 0, consisted of subjects with FEV1/FVC ≥ 0.70 and both prolonged cough and sputum production as evidence of chronic bronchitis.
In some cases, the value of the post-bronchodilatator FEV1/FVC is maintained, i.e. > 0.70, but FEV1 is reduced, i.e. < 0.80, and thus these subjects are excluded from GOLD COPD recommendations and are designated as the so-called unclassified COPD (GOLD-U) (Citation18). The asthma–COPD overlap syndrome (ACOS) was classified according to both GOLD 2007 criteria based on FEV1/FVC < 0.70 in BD spirometry and an increase of ≥ 12% and ≥ 200 mL in FEV1. Furthermore, asthma was excluded by clinical data and by conducting 2 weeks’ peak flow measurement with sympathomimetic therapy (salbutamol) in every case.
The modified British Medical Research Council (mMRC) grade 0–4 was also used to assess dyspnoea (Citation19), and the COPD Assessment Test (CAT score) (Citation20) was applied to measure the impact of COPD on the quality of life and well-being (Citation21). The total CAT scale (range 0–40) was calculated by adding the scores from eight sub-items. The exacerbation history was collected as self-reported outpatient episodes along with details of physician-prescribed antibiotics or peroral steroids. Hospitalizations for COPD exacerbations were based on physician-charted records of hospital admissions. Finally, the study nurse phoned each COPD subject at the end of the year 2013 in order to double-check the frequency of the outpatient exacerbation rate during the last follow-up year.
According to the GOLD 2011 recommendations (Citation2), participants were classified according to their mMRC grade and CAT score into a fewer-symptoms category (mMRC < 2 and CAT score < 10) or to a more-symptoms category (mMRC ≥ 2 or CAT score ≥ 10). Then by applying the GOLD 2007 airway limitation stages the participants were stratified into a low-risk group (GOLD stages I–II and ≤ 1 exacerbation per year indicating low risk) or into a high-risk group (GOLD stages III–IV or ≥ 2 exacerbations per year indicating high risk). Finally, we combined the symptom and risk groups into four grades A, B, C, and D of COPD categories based on the GOLD 2011 recommendations.
The COPD subjects underwent a measurement of diffusing capacity (transfer factor) and diffusion capacity per alveolar volume (transfer coefficient) at the end of the study by the helium single-breath technique (VMAX Assembly Encore 22, SensorMedics Co.,Viasys Healthcare System, 2002). All values of the transfer factor < 0.80 of predicted were considered as abnormal. We used the national criteria in the classification of abnormal values in the transfer factor (Citation22).
Other variables
The basic subject characteristics included gender, smoking status, age, body mass index (BMI, kg/m2), and pack-years. In the statistical analysis, age, BMI, and pack-years from the third visit (6 years) were used. Causes of death were ascertained from hospital discharge records. The study nurse phoned all of the subjects lost from follow-up to find out their reasons for non-participation.
Statistical analysis
First, the incidence of COPD during the follow-up period was evaluated graphically. The basic characteristics of the study subjects, lung functions, and symptoms were compared between GOLD 2007 COPD staging severities. In the cross-tabulation, statistical significances of the associations between GOLD 2007 stages and categorical variables (gender, cough, and sputum production) were evaluated with the chi-square test or chi-square test for linear trend. Normal plots and Shapiro–Wilk test were used to evaluate the normality of quantitative variables. All basic continuous clinical variables (age, BMI, pack-years, lung function, and CAT score) were normally distributed, and the differences between COPD groups were evaluated by comparing mean values with one-way ANOVA, linear trend test, and Scheffe's pairwise comparisons. We also conducted a dichotomous logistic regression analysis to evaluate the independent effect of these clinical variables on the probability of COPD. For the subjects in GOLD 2007 stages I–IV, the percentage of dyspnoea (mMRC ≥ 2), decreased quality of life (CAT ≥ 10), and exacerbations were calculated. The distribution of the GOLD 2011 COPD grades according to the GOLD 2007 stages was determined by using cross-tabulation. Charts were used to compare the distributions of CAT scores and mMRC grades between COPD status groups. The statistical significance of differences between mean values of transfer coefficient by GOLD 2007 stages was evaluated with one-way ANOVA. A total of 20 COPD subjects had missing transfer coefficient value due to a variety of reasons, and they were not included in the emphysema component analysis. A scatterplot diagram was used to visualize graphically the GOLD-U participants with airway limitations. IBM SPSS Statistics 22.0 was used for the statistical analysis.
Results
Distribution of COPD stages
In , the history and frequency of the observed COPD cases in all of the heavy smokers (n = 621) during the follow-up period are presented graphically. Of the eligible 621 smokers, 572 attended the second visit organized 3 years after the baseline examination. The present study is based on data from these 572 (92.1%) subjects for whom there was complete information from the second visit and who were invited to attend the 6-year follow-up examination (visit 3).
Figure 1. Changes in chronic obstructive pulmonary disease (COPD) severities during the 6-year follow-up. COPD was classified according to the recommendations of the 2007 Global Initiative for Chronic Lung Disease (GOLD). The second visit (Visit 2) was organized 3 years after the baseline examination, and the last visit (Visit 3) after 6 years of follow-up.
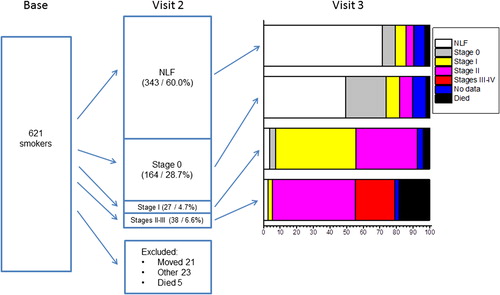
The post-bronchodilator spirometric findings of these participants revealed that 343 (60.0%) subjects had normal lung function, 164 (28.7%) could be classified as having the earlier GOLD stage 0, and 65 (11.4%) had COPD according to the GOLD 2007 criteria (stages I–IV). During the interval before the second visit (3 years), 49 participants were lost from the follow- up: 23 subjects moved away from the region, 5 died of lung cancer, and 21 subjects dropped out for miscellaneous reasons.
The follow-up status at the third visit (6 years) for all those 572 subjects who had attended the second visit (3 years) identified 39 (12.5%) new COPD cases (22 with stage I, 16 with stage II, and 1 with stage III) (). In addition, the disease had proceeded to COPD in 26 (17.6%) cases (13 with stage I, and 13 with stage II) in those subjects who had been classified in the GOLD stage 0 at the second visit (3 years previously). Furthermore, 10 of the 27 stage I COPD cases diagnosed at the second visit had proceeded to disease stage II, but none to the more severe stages III or IV. In addition, 59 participants dropped out from the study after the second visit: 18 subjects had moved away from the region, 19 dropped out for unknown reasons, and 22 had died, 8 of whom were patients with COPD.
A total of 513 subjects completed the 6-year examination and were subjected to post-bronchodilator spirometry. Of these 513 participants, 10 (1.9%) had severe COPD (GOLD stage III– IV), 58 (11.4%) had moderate COPD (GOLD stage II), and 49 (9.6%) mild COPD (GOLD stage I). describes the rate of COPD GOLD stages subdivided by gender and other basic characteristics of the study subjects. Males were more likely to have COPD (84 in GOLD stages I–IV, 27.8%) than females (33, 15.7% respectively) (P value of chi-square test = 0.002). The subjects with the more severe COPD disease were older than the smokers with normal lung function (P values of Scheffe's test < 0.001). The multivariable logistic regression analysis revealed that age (odds ratio [OR] for an increase of 10 years 1.93, 95% confidence interval [CI] 1.47–2.55, P value of maximum likelihood test < 0.001), gender (OR for male 1.7, 95% CI 1.03–2.77, P = 0.034), and pack-years (OR for an increase of 10 pack-years 1.16, 95% CI 1.01–1.33, P = 0.040) all exerted independent effects on the probability of suffering COPD (stages I–IV); BMI also had an independent effect, but it was not statistically significant (OR for an increase of 5 kg/m2 0.78, 95% CI 0.60–1.01, P = 0.066).
Table I. Basic clinical characteristics of 513 study subjects from Northern Finland subdivided by GOLD 2007 COPD stages at the end of the 6-year follow-up study.
All of the participants had smoked for a long time: the mean value of pack-years was 34.8 (SD 16.2). In addition, the intensity of smoking habits increased with the severity of COPD disease when expressed as a mean value of pack-years (P value of ANOVA test < 0.001). The number of COPD cases was higher among those subjects who continued smoking (25.2%) compared to those who stopped smoking (20.1%) or to those who had tried to quit but relapsed (9.6%) during the follow-up (P < 0.001).
Incidence of COPD according to the GOLD 2011 recommendations
A total of 117 (22.8% of the 513) participants fulfilled COPD criteria according to post-bronchodilator spirometry findings at the end of the 6-year follow-up (). There were several changes noted between the GOLD 2007 disease stages and the GOLD 2011 A–D grades in these individuals. Furthermore, if these newer recommendations were applied, then more COPD cases (n = 68) were diagnosed as grade B, in comparison with only 58 subjects who had been classified as having the moderate COPD stage II with the earlier GOLD 2007. A total of 30 (61.2%) subjects in GOLD 2007 stage I COPD had mMRC ≥ 2 or CAT ≥ 10, and thus were placed into grade B by the GOLD 2011 criteria (). On the other hand, 12 (20.7%) subjects who had been diagnosed as stage II with GOLD 2007 displayed fewer symptoms and thus were classified as grade A. The CAT score contributed more information to the spirometry results than the mMRC grade, thus conferring clinical value into the COPD classification. In total, 22.2% (n = 26) COPD subjects had experienced one to three exacerbations, and 8 of these patients had required hospitalization due to a COPD exacerbation during the last year before the end of the study.
Table II. Distribution of GOLD 2011 grades, symptoms, exacerbations, and hospitalizations subdivided according to GOLD 2007 stages of the 117 participants with chronic obstructive pulmonary disease after 6-year follow-up.
Distribution of COPD by symptoms
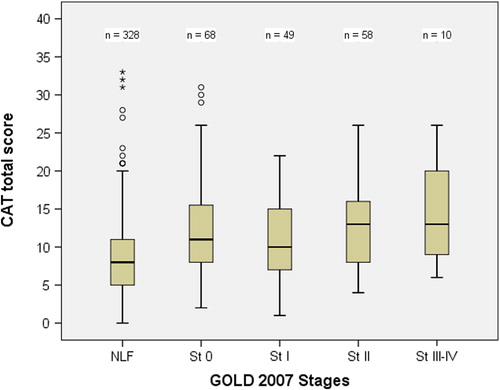
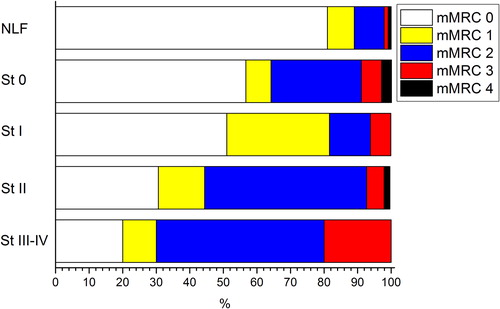
illustrates the total CAT score distribution as a box plot according to GOLD 2007 COPD stages. The subjects with more severe COPD had higher CAT scores (P value of ANOVA linear trend test < 0.001). The percentage distribution of mMRC grades subdivided into the various GOLD 2007 COPD stages in shows that the proportion of subjects with more severe dyspnoea was significantly higher in the moderate (stage II) and severe (stages III and IV) COPD stages (P value of chi-square test for trend < 0.001).
Emphysema with COPD
The emphysema component was assessed by measuring the transfer factor in 97 of the total 117 GOLD 2007 COPD subjects. The other 20 COPD subjects had missing values due to a variety of reasons. In stage III–IV COPD subjects (n = 8), the mean value (standard deviation) of the transfer coefficient was 51.4 (12.0), which was statistically significantly lower than in stage I subjects (n = 36) (mean 77.8, SD 14.5, P value of Scheffe's test = 0.005) or in stage II subjects (n = 53) (mean 79.2, SD 19.3, P value of Scheffe's test = 0.002). Thus, although the presence of emphysema was observed also in the subjects with mild COPD, this was more often associated with the more severe stage of COPD.
Unclassified participants with airway obstruction
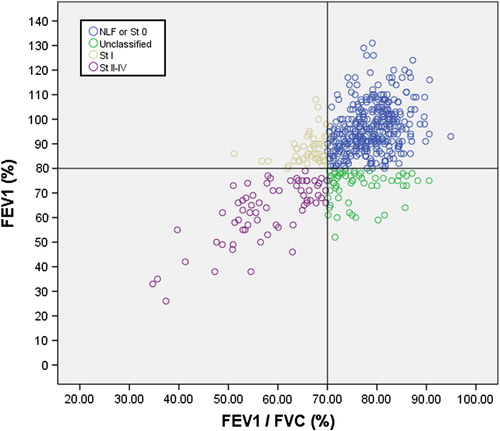
shows the scatterplot of the measured post-bronchodilator FEV1 (% of predicted) and FEV1/FVC (%) values. In this plot, the participants were assessed according to their GOLD 2007 COPD stages. The scatterplot clearly reveals that a total of 58 (11.3%) middle-aged subjects with airway limitation measured as FEV1 (%) < 80% but FEV1/FVC (%) ≥ 70% in spirometry were classified as healthy according to the GOLD 2007 criteria.
Figure 4. Scatterplot of forced expiratory volume in 1 second (FEV1) versus FEV1/FVC in spirometry with bronchodilatation test. COPD is classified by GOLD 2007 recommendations in the study with 513 participants. Stage 0 refers to cases with normal lung function and with chronic bronchitis. Unclassified COPD refers to FEV1 (%) < 80% predicted and FEV1/FVC (%) > 70% in BD spirometry.
The asthma–COPD overlap syndrome
Of the 117 GOLD 2007 COPD cases, 15 (12.8%) subjects were classified into the asthma–COPD overlap syndrome. Their clinical disease was COPD without any significant bronchodilator effects during their 2 weeks’ peak flow recordings.
Discussion
Our study confirmed that COPD is commonly encountered in a volunteer cohort of middle-aged ‘healthy’ smokers, but the clinical characteristics in all categories of COPD seem to change even during a relatively short follow-up of 3 years. Furthermore, health status and prevalence of chronic respiratory symptoms both in smokers with normal lung function and in the diagnosed COPD groups also varied over this time period. Most of the COPD cases were designated as mild-to-moderate according to the 2007 GOLD criteria or classified into grades A and B by GOLD 2011, thus exhibiting nearly normal lung function values but some degree of symptoms.
In our study, the distribution of COPD into a four-level stratification differed from that of the population-based distribution in an earlier study (Citation9). In that study, one-third of patients were assigned to two grades A and D, and fewer to grades B or C. In our study, the frequency distribution of GOLD 2011 grades differed: almost one-third of smokers were classified as grade A and nearly two-thirds as grade B. The observed proportion of subjects in grade A is as expected, because the participants were symptom-free smokers at baseline. However, the significant proportion of subjects in grade B may be an important prognostic finding. This is especially relevant because there is some evidence that mortality may be higher in grade B subjects than in their grade D or C counterparts (Citation6).
Earlier studies have suggested that persistent respiratory symptoms may be important indicators of all-cause mortality, independently of lung function, although the mechanisms behind this phenomenon are still far from clear (Citation23,Citation24). This is more likely to be the case if the smokers also exhibit other respiratory symptoms such as wheeze or dyspnoea (Citation25,Citation26). Both the CAT scores and the mMRC grades were used in grading the symptom severity in this study along with the 2011 GOLD recommendations. Interestingly, some ‘healthy smokers’ with normal lung function reported very high CAT scores, and this may indicate that smoking is exerting a major impact on their daily life and well-being. Thus, in this study, it seemed that inclusion of the CAT scores added more information to the spirometry results than was the case for the mMRC grade and thus they contribute relevant clinical values to the COPD classification.
It was also found here that the symptoms of chronic bronchitis may precede the development of airway limitation as has been postulated by others (Citation27,Citation28). Because of some previous controversial study results, in 2007 the so-called ‘at risk’ stage 0 COPD was deleted from the GOLD staging criteria (Citation5,Citation29). However, several studies have revealed that chronic bronchitis with or without other chronic pulmonary diseases increases the overall morbidity, i.e. these patients do require more medical care (Citation27,Citation29,Citation30).
It has been emphasized that not only the severity of COPD disease but also an assessment of health status, defining markers of progression, and prognosis of the disease is important when trying to understand the natural history of the disease (Citation31–34). The clinical characteristics and prognosis of patients in the asthma–COPD overlap syndrome (ACOS) are unclear, although these subjects may experience more significant health effects and increased disease severity (Citation35). In addition, it is still debated whether bronchodilator reversibility in spirometry, e.g. COPD–asthma overlap syndrome, represents a clinically important phenotype (Citation10,Citation36,Citation37). However, ACOS has been described recently as a chronic airway disease with not fully reversible airway limitation, accompanied by symptoms or signs typical of both asthma and COPD (Citation4,Citation38). In our study, the spirometric reversibility criteria for ACOS followed the international (Citation39) and the new national criteria of COPD guidelines (Citation40).
This study confirms that the emphysema component as measured by the transfer factor can be detected in mild COPD cases and not only in severe stages of GOLD 2007 COPD. The lowered values for the transfer factor and the transfer coefficient are found typically in COPD patients with advanced emphysema, as was found also in this study. It has been noted, that not only rapid decliners in FEV1 have been shown to be associated with the predominant emphysema subtype (Citation41), but also middle-aged smokers with normal lung function can have emphysema or other abnormalities when they are assessed by high-resolution computed tomography (HRCT) (Citation42,Citation43). We did not detect any rapid decliners in our study, but the severity of COPD with airway limitation did correlate with the emphysema stage.
The revised GOLD document emphasizes the importance of exacerbation risk in assessing COPD severity (44). In the present study, only some exacerbators and a few frequent exacerbators (two or more exacerbations per year before the third 6-year visit) were found. The reasons for the low exacerbation rate in this study are unknown. One explanation may be that these participants were ‘healthy’ at baseline. The low number of patients experiencing exacerbations may be due to the way in which the study participants were recruited in this population-based study. This study was not carried out in hospitalized patients. The strength of this prospective non-randomized longitudinal study is the carefully characterized cohort of middle-aged smokers with no diagnosed chronic diseases and no co-morbidities requiring daily medication at baseline.
In conclusion, variability in chronic symptoms or in health status was high in all airway obstruction severity stages. The results suggest that GOLD 2011 classification recognize the complex patient subgroups better than GOLD 2007. Furthermore, the clinical characteristics in all categories of COPD seem to change, even during a relatively short follow-up of 3 years.
Acknowledgements
We thank Ewen MacDonald for the linguistic revision of our manuscript and Mrs Maija-Liisa Tarkiainen for her secretarial assistance. We also acknowledge Professor Vuokko L. Kinnula (deceased 2012) for her valuable contribution to the design of this study.
Funding: This study was financially supported by a governmental subsidy from the Ministry of Social Affairs and Health (125/STA/TE/2006), a governmental subsidy for health science research (EVO) in Rovaniemi (86/23.4.2003). This study project received a clinical research grant via Lapland Central Hospital from the pharmaceutical company GlaxoSmithKline (CRT115378).
Declaration of interest: The authors report no conflicts of interest.
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). The global strategy for the diagnosis, management and prevention of COPD. 2011. Available at: http://www.goldcopd.org (accessed 30 May 2012).
- Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med. 2011;183:1129–37.
- Miravitlles M, Calle M, Soler-Cataluna JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48:86–98.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55.
- Johannessen A, Nilsen RM, Storebo M, Gulsvik A, Eagan T, Bakke P. Comparison of 2011 and 2007 Global Initiative for Chronic Obstructive Lung Disease guidelines for predicting mortality and hospitalization. Am J Respir Crit Care Med. 2013;188:51–9.
- Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186:975–81.
- Agusti A, Edwards LD, Celli B, Macnee W, Calverley PM, Mullerova H, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42:636–46.
- Soriano JB, Alfageme I, Almagro P, Casanova C, Esteban C, Soler-Cataluna JJ, et al. Distribution and prognostic validity of the new Global Initiative for Chronic Obstructive Lung Disease grading classification. Chest. 2013;143:694–702.
- Miravitlles M, Soriano JB, Ancochea J, Munoz L, Duran-Tauleria E, Sanchez G, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107:1053–60.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–57.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–31.
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186:155–61.
- Han MK, Muellerova H, Curran-Everett D, Dransfield MT, Washko GR, Regan EA, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1:43–50.
- Garcia-Rio F, Soriano JB, Miravitlles M, Munoz L, Duran-Tauleria E, Sanchez G, et al. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS One. 2014;9:e105220.
- Miravitlles M, Huerta A, Fernandez-Villar J, Alcazar B, Villa G, Forne C, et al. Generic utilities in chronic obstructive pulmonary disease patients stratified according to different staging systems. Health Qual Life Outcomes. 2014;12:120.
- Toljamo T, Kaukonen M, Nieminen P, Kinnula VL. Early detection of COPD combined with individualized counselling for smoking cessation: a two-year prospective study. Scand J Prim Health Care. 2010;28: 41–6.
- Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–6.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54.
- Jones PW. COPD assessment test—rationale, development, validation and performance. COPD. 2013;10:269–71.
- Sovijärvi A, Ahonen A, Hartiala J, L nsimies E, Savolainen S, Turjanmaa V, et al., editors. Kliinisen fysiologian perusteet. 1st ed. Otavan Kirjapaino Oy, Keuruu, Finland: Duodecim; 2012. [Finnish]
- Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130:1129–37.
- Pelkonen M. Smoking: relationship to chronic bronchitis, chronic obstructive pulmonary disease and mortality. Curr Opin Pulm Med. 2008;14:105–9.
- Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Nilsson PM, Lofdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res. 2005;6:98.
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530–5.
- Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329–32.
- de Marco R, Accordini S, Cerveri I, Corsico A, Anto JM, Kunzli N, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32–9.
- Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604.
- Kim V, Sternberg AL, Washko G, Make BJ, Han MK, Martinez F, et al. Severe chronic bronchitis in advanced emphysema increases mortality and hospitalizations. COPD. 2013;10:667–78.
- Renom F, Yanez A, Garau M, Rubi M, Centeno MJ, Gorriz MT, et al. Prognosis of COPD patients requiring frequent hospitalization: role of airway infection. Respir Med. 2010;104:840–8.
- Ong KC, Lu SJ, Soh CS. Does the multidimensional grading system (BODE) correspond to differences in health status of patients with COPD? Int J Chron Obstruct Pulmon Dis. 2006;1:91–6.
- Mannino DM, Diaz-Guzman E, Pospisil J. A new approach to classification of disease severity and progression of COPD. Chest. 2013;144:1179–85.
- Vanfleteren LE, Kocks JW, Stone IS, Breyer-Kohansal R, Greulich T, Lacedonia D, et al. Moving from the Oslerian paradigm to the post-genomic era: are asthma and COPD outdated terms? Thorax. 2014;69:72–9.
- Vestbo J, Agusti A, Wouters EF, Bakke P, Calverley PM, Celli B, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189:1022–30.
- Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44:341–50.
- Braman SS. The chronic obstructive pulmonary disease-asthma overlap syndrome. Allergy Asthma Proc. 2015;36:11–18.
- Global strategy for asthma management and prevention, global initiative for asthma (GINA) 2014. Available at: http://www.ginasthma.org/ (accessed 6 March 2015).
- Kankaanranta H, Harju T, Kilpelainen M, Mazur W, Lehto JT, Katajisto M, et al. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the Finnish guidelines. Basic Clin Pharmacol Toxicol. 2015;116:291–307.
- Cerveri I, Corsico AG, Grosso A, Albicini F, Ronzoni V, Tripon B, et al. The rapid FEV(1) decline in chronic obstructive pulmonary disease is associated with predominant emphysema: a longitudinal study. COPD. 2013;10:55–61.
- Stratelis G, Fransson SG, Schmekel B, Jakobsson P, Molstad S. High prevalence of emphysema and its association with BMI: a study of smokers with normal spirometry. Scand J Prim Health Care. 2008; 26:241–7.
- Xie X, Dijkstra AE, Vonk JM, Oudkerk M, Vliegenthart R, Groen HJ. Chronic respiratory symptoms associated with airway wall thickening measured by thin-slice low-dose CT. AJR Am J Roentgenol. 2014; 203:W383–90.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.