Abstract
Introduction. Undetermined strokes with an embolic pattern (USEP) represent a common phenotype. We assessed their frequency and compared USEP with cardioembolic stroke with a known source and non-cardioembolic stroke etiology.
Methods. Study patients were 540 consecutive ischemic stroke patients admitted to Helsinki University Hospital with primary end-point of recurrent stroke in a 21-month follow-up. Cox regression adjusting for CHA2DS2-VASc and anticoagulation estimated the risk of USEP on recurrent stroke.
Results. A total of 229 (42.4%) patients had a non-cardioembolic stroke etiology, 184 (34.1%) had a cardioembolic stroke with a known source, and 127 (23.5%) were classified as USEP. USEP patients had less diabetes and prior TIA, with more severe symptoms than the non-cardioembolic stroke cases. They were younger, had fewer comorbidities, and less severe symptoms than the cardioembolic stroke patients. Cumulative risk of recurrent stroke was 10.0% (95% CI 4.1%–15.9%) for USEP, 5.0% (1.1%–8.9%) for cardioembolic strokes, and 5.0% (3.0%–7.0%) for non- cardioembolic strokes (P = 0.089). USEP associated with a higher risk of recurrent stroke compared to non-cardioembolic strokes (hazard ratio 2.36, 95% CI 1.02–5.47; P = 0.046) and cardioembolic stroke with a known source (1.83, 1.07–3.14; P = 0.028).
Conclusions. Despite their younger age and more favorable risk factor profile compared with other phenotypes, USEP exhibited a high risk of stroke recurrence.
Key words: :
Undetermined strokes with an embolic pattern accounted for one-quarter of all cases and three-quarters of all cryptogenic strokes among 540 consecutive patients with ischemic stroke.
Patients with undetermined stroke with an embolic pattern were younger and had fewer comorbidities compared to those with cardioembolic stroke with a known source or non-cardioembolic stroke.
Undetermined stroke with an embolic pattern was independently associated with a higher risk of recurrent stroke in a 21-month follow-up.
Introduction
Of all ischemic strokes, etiology in 12% to 39% is classified as undetermined—i.e. a plausible causative mechanism for the stroke is not identified (Citation1). According to the literature, 60% to 87% of such strokes are non-lacunar, with embolic neuroimaging topography, appearing as superficial, cortical or cerebellar, large deep, or superficial and deep combined ischemic lesions (Citation2–4).
Criteria for undetermined stroke etiology, including the cases falling under the term ‘cryptogenic stroke’, are inconsistent, and only scarce data exist to guide secondary prevention after such events. These patients are generally treated with antiplatelet medication, possibly combined with statins and antihypertensive medication depending on patient characteristics and preferences. Various long-term (≥ 1 year of follow-up) rates of recurrent strokes after stroke of undetermined etiology have been reported, ranging from 1.3% to 4.5% per year in young patients (Citation5–11) and from 2.5% to 7.8% per year in older patients (Citation3,Citation4,Citation12–15). These rates suggest an active underlying pathology, such as occult atrial fibrillation (AF) as reported in recent AF detection trials (Citation16,Citation17).
A new concept, embolic stroke of undetermined source (ESUS), has been proposed as a subcategory for strokes of undetermined etiology for cases without a definitive source for embolism (Citation1). The classification seems relevant, as anticoagulation instead of an antiplatelet regimen could be superior with randomized trials ongoing. However, thus far only one study has systematically assessed the frequency of ESUS, finding a 10.0% prevalence, while 13.4% remained undetermined other than ESUS, mostly due to incomplete diagnostic work-up (Citation18). In clinical routine, full diagnostic examinations are not carried out in all consecutive patients due to e.g. patient non-compliance, limitation of care due to poor prognosis, or limited resources. Prognostic data on strokes of undetermined cause with embolic features are scarce, and comparisons of ESUS patients with other undetermined stroke patients have not been reported.
We thus pragmatically assessed the frequency, comorbidities, and risks of recurrent stroke and death in consecutive patients with undetermined stroke with an embolic pattern (USEP) sharing embolic lesion topography in neuroimaging irrespective of completeness of diagnostic evaluation. We compared established cardioembolic and non-cardioembolic strokes with USEP. Within USEP, we compared ESUS cases with those not meeting the ESUS criteria.
Methods
This retrospective observational cohort study was carried out at the Helsinki University Hospital and approved by the relevant institutional authorities. Informed consent was not required nor sought as the study setting was observational without direct patient contact. Our hospital serves as the only university teaching hospital in the province of Uusimaa, with a catchment population of 1.6 million. The hospital has the only neurological emergency room with 24/7 service in the province.
We identified all consecutive patients from the hospital's electronic discharge database from 1 January to 31 March 2010 and 1 January to 31 March 2012 with diagnosis of I63 according to 10th version of the International Classification of Diseases and resident in Finland. The patients were compiled into a single data set with uniform follow-up of 21 months (or until death) for all cases.
Subsequently, all electronic patient records, including ECGs, laboratory, radiological, and medication prescription database were retrieved for review from a province-wide electronic patient data archive. Pre-existing comorbidities recorded included hypertension (existing diagnosis or any blood pressure medication), diabetes (existing diagnosis or any antidiabetic medication), dyslipidemia (antilipemic medication or total cholesterol ≥ 5.0 mmol/L, low-density lipoprotein ≥ 3.0 mmol/L, high-density lipoprotein < 1.0 mmol/L, or triglycerides ≥ 2.0 mmol/L), smoking (current, smoking at least ≥ 1 cigarette/day within the last year; ex-smoker, quit > 1 year prior to stroke), prior history of coronary heart disease, congestive heart failure, myocardial infarction (MI), peripheral arterial disease, transient ischemic attack (TIA), stroke, AF, and cancer. Pre-stroke AF was classified as persistent/permanent or paroxysmal. Pre-stroke CHA2DS2-VASc score was calculated for each patient as follows: congestive heart failure (1 point), hypertension (1 point), age ≥ 75 years (2 points), diabetes (1 point), prior stroke or TIA (2 points), vascular disease (1 point), age 65–74 years (1 point), and female sex (1 point) (Citation19). If female sex was the only risk factor, it was not scored on CHA2DS2-VASc. Stroke severity on admission was assessed with the National Institutes of Health Stroke Scale (NIHSS). Acute stroke treatments and post-stroke antiplatelet and oral anticoagulation regimen was recorded.
All patients underwent brain scan at least once with CT or MRI, a chest X-ray, and a 12-lead ECG on admission and at least once during hospitalization. Imaging of extracranial cervical arteries was performed as judged by the treating physician either with Doppler ultrasound, CT angiography, or MR angiography, or combinations of these. Imaging of intracranial arteries was done with CT angiography or MR angiography. In patients with sinus rhythm and no history of paroxysmal AF, AF was screened either with: 1) continuous ECG monitoring during their stay in the emergency room, stroke unit, or intensive care unit; 2) repeated ECG; and/or 3) 48-hour Holter ECG. The main echocardiography parameters were recorded: left atrium diameter, left ventricle end-diastolic diameter, and left ventricle ejection fraction. Generally, the Finnish publicly funded health care system encourages performing ancillary diagnostic investigations only if their results potentially affect treatment decisions.
Board-certified neuroradiologists initially evaluated all brain and artery imaging scans. Lesions were defined as embolic if solitary or multiple superficial, cortical or cerebellar, large deep, or superficial and deep combined, in the absence of explanatory small-vessel disease or atherosclerotic stenosis in a relevant artery, or other uncommon cause such as dissection, vasospasm, vasculitis, or hemodynamic compromise. In case no embolic pattern was visible in baseline or follow-up brain imaging, embolism was supported by rapid recanalization of an occluded main brain-supplying artery or its branches or a computed tomography perfusion lesion compatible with embolic pattern. Stroke etiology was first classified according to Trial of Org in Acute Stroke Treatment (TOAST) (Citation20). Subsequently, according to clinicoradiological characteristics we classified patients as (A) non-cardioembolic stroke etiology (TOAST subgroups: 1, large-artery atherosclerosis; 3, small-vessel disease; 4, other determined etiology; and 5a, multiple concomitant causes), (B) cardioembolic stroke with a known high-risk source (TOAST subgroup 2, i.e. probable cardioembolism), and (C) USEP (TOAST subgroups 5b and 5c). Imaging-negative cases with undetermined etiology did not qualify for USEP and thus fell into the non-cardioembolism etiology group. High-risk sources for cardioembolism were those with arbitrary ≥ 2% primary risk for ischemic stroke, as previously described (Citation21), including AF or flutter, intracardiac thrombus, prosthetic cardiac valve, myxoma or other cardiac tumors, mitral stenosis, recent (< 4 weeks) MI, left ventricular ejection fraction < 30%, valvular vegetations, or infective endocarditis. All uncertain or low-risk sources for cardioembolism, such as patent foramen ovale, atrial septal aneurysm, or mitral valve calcification or prolapse, were classified as USEP in the presence of embolic lesion(s) in neuroimaging.
Among the USEP group, cases fulfilling the ESUS criteria according to NAVIGATE ESUS trial (https://clinicaltrials.gov/ct2/show/NCT02313909, accessed 7 January 2015) were identified as meeting the following criteria: non-lacunar stroke, absence of cervical carotid atherosclerotic stenosis ≥ 50% or occlusion, no AF in history or after ≥ 24-hour cardiac rhythm monitoring, no intracardiac thrombus on transthoracic echocardiography, and no other specific cause identified.
Since stroke patients are invariably hospitalized and treated in public hospitals in our country, we performed follow-up by reviewing all medical records in the province-wide electronic patient data archive. Mortality data came from Statistics Finland. Follow-up time was 21 months or until death. The primary end- point was non-fatal or fatal recurrent stroke. Secondary end-points were death from any cause and a composite of recurrent stroke or death, whichever occurred first.
Median and interquartile range (IQR) are reported for all continuous variables with non-normal distribution. Groups were compared with chi-square, Fisher's exact, Mann–Whitney U, and ANOVA tests where appropriate. Kaplan–Meier curves were plotted for end-points and groups defined by phenotypic classification compared with the log rank test. Life tables were used to calculate cumulative risks and their 95% confidence intervals (CI) of the end-points. Cox proportional hazards models were constructed to assess the impact of USEP on the end-points. All models were adjusted for pre-stroke CHA2DS2-VASc score and post-stroke anticoagulation use. CHA2DS2-VASc score was used as an ordinal variable. Models including mortality end-point were also adjusted for baseline NIHSS score as an ordinal variable. Since CHA2DS2-VASc score integrates the key parameters relevant to the studied end-points and has been shown to correlate accurately with the risk of stroke or death also in non-AF patients (Citation22), and to avoid over-adjustment, no further correction for comorbidities was made. Two-sided statistical significance was set at 0.05. All analyses used IBM SPSS 20 (IBM Corp, Armonk, NY, USA).
Results
We included 540 patients, of whom all underwent brain scan at least once: CT only (n = 407, 75.4%), MRI only (n = 20, 3.7%), or both CT and MRI (n = 113, 20.9%). Imaging of brain-supplying arteries was performed in 488 (90.4%) patients: intracranial arteries were imaged in 273 (50.6%). A total of 518 (95.9%) patients underwent repeated ECGs, continuous ECG monitoring was carried out in 237 (43.9%), and of these 71 (13.1%) patients underwent 48-h Holter recording. Echocardiography was performed in 156 (28.9%) patients (transesophageal investigation in 8 patients).
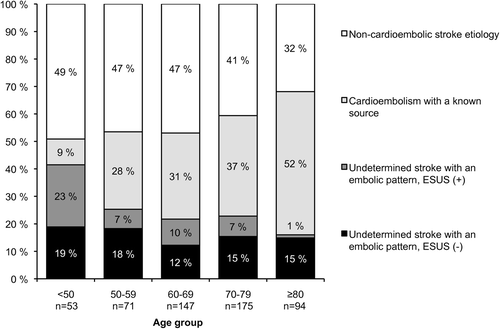
depicts the patient flow from admission to phenotypic classification. A total of 229 (42.4%) had non-cardioembolic stroke etiology, 184 (34.1%) had a cardioembolic stroke with a known source, and 127 (23.5%) were classified as USEP. USEP accounted for 72.2% of all strokes of undetermined cause (n = 176). Regarding phenotype change with age, the proportion of non-cardioembolic strokes and USEP declined, while the proportion of cardioembolic stroke with a known source increased with age ().
Figure 1. Flow chart of the study population from admission to phenotype with frequency of atrial fibrillation (AF) among the subgroups. * The dominant etiology was intracranial atherosclerosis in a relevant artery in the 2 patients with AF.
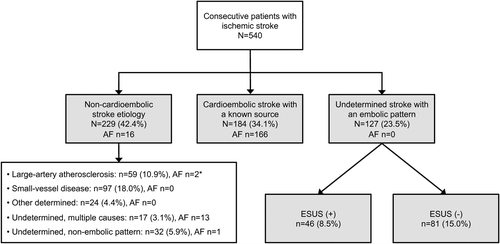
Figure 2. Change of the patient phenotype with age (chi-square P < 0.001). ESUS = embolic stroke of undetermined source.
Compared with non-cardioembolic strokes, USEP patients had less often diabetes mellitus (22% versus 13%) and prior TIA (8% versus 2%), but more severe stroke symptoms (median NIHSS score 2 versus 4). Compared with patients with cardioembolic stroke with a known source, USEP cases were younger (median age 72 versus 66 years), had less frequently heart failure (17% versus 5%) and lower CHA2DS2-VASc score (median 3 versus 2), and less severe stroke symptoms (median NIHSS score 5.5 versus 4). Antiplatelet medication was introduced in USEP patients as often as in non-cardioembolic stroke patients (85%) (). In transthoracic echocardiography, left ventricular ejection fraction was lower among those with cardioembolic stroke with a known source (55% versus 60%).
Table I. Baseline data of the study population stratified by patient phenotype.
Of the USEP patients, 46 (36.2%; 8.5% of all patients) fulfilled the diagnostic criteria for ESUS. Those not fulfilling the ESUS criteria were older, had more frequently hypertension and cancer, exhibited higher CHA2DS2-VASc scores, and were less often treated with intravenous thrombolysis. The main echocardiographic parameters were similar in both groups ().
Table II. Comparison of patients with undetermined stroke with an embolic pattern meeting the diagnostic criteria for embolic stroke of undetermined source (ESUS) (+), to those not meeting the criteria, ESUS (–).
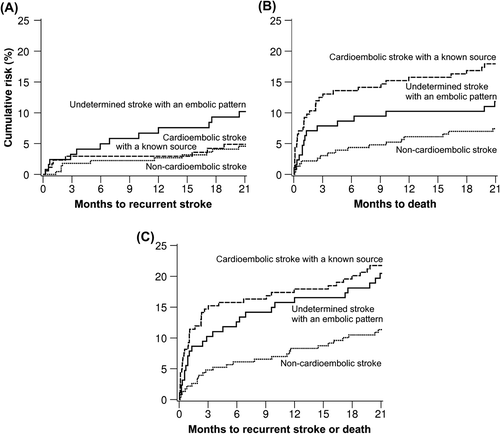
During the 21-month follow-up, a total of 65 deaths from any cause, 30 non-fatal or fatal recurrent strokes, and 92 composite events of recurrent stroke or death had occurred. Cumulative 21-month risk of recurrent stroke was doubled for USEP (10.0%, 95% confidence interval [CI] 4.1%–15.9%) as compared with the 5.0% (3.0%–7.0%) rate for non-embolic stroke and the 5.0% rate (1.1%–8.9%) for cardioembolic stroke with a known source group (; log rank P = 0.089). Compared with the group of non-embolic stroke, the 21-month cumulative risk of death was higher in the high-risk cardioembolism source group (7.0%, 95% CI 3.1%–10.9% versus 18.0%, 12.1%–23.9%), log rank P = 0.004 for overall comparison. Cumulative risk of death was 12.0% (6.1%–17.9%) for USEP (). Cumulative 21-month risk of the composite end-point of stroke or death was 11.0% (7.1%–14.9%) for non-embolic stroke, 22.0% (16.1%–27.9%) for cardioembolic stroke with a known source group, and 20.0% (12.2%–27.8%) for USEP, being thus significantly higher (P = 0.008) for the two last-mentioned compared with non-embolic stroke (). Cumulative rates of the end-points at 12 months appear in Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612.
Figure 3. Kaplan–Meier estimates of the risk of: A: recurrent stroke (log rank P = 0.089), B: death (P = 0.004), and C: composite of recurrent stroke or death (P = 0.008) in the 540 patients stratified by phenotype.
Among the 127 USEP patients, no difference emerged in the frequency of recurrent stroke between patients fulfilling the ESUS criteria versus those who did not (8.9% versus 10.9%, log rank P = 0.659) (Supplementary Figure 1A to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612). ESUS patients had lower mortality compared to non-ESUS cases (2.2% versus 17.3%, log rank P = 0.012) (Supplementary Figure 1B to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612). The risk of composite end-point of recurrent stroke or death was also lower in ESUS patients compared with non-ESUS patients (10.9% versus 25.9%, P = 0.037) (Supplementary Figure 1C to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612). There were no significant differences in the event risks between anticoagulation-treated USEP group versus those not treated with anticoagulation.
In the Cox regression adjusted for CHA2DS2-VASc score and anticoagulation use, USEP independently was associated with higher risk of recurrent stroke as compared with those with non-cardioembolic stroke (hazard ratio 2.36, 95% CI 1.02–5.47; P = 0.046). Compared with cardioembolic stroke with a known source, USEP was also associated with higher risk of recurrent stroke (1.83, 1.07–3.14; P = 0.028). Regarding secondary end-points, there were no statistical differences between USEP and non-cardioembolic stroke in the risks of death from any cause or composite of recurrent stroke or death. However, compared with cardioembolic stroke, risk of death was lower for the USEP group (0.33; 1.17–0.65; P = 0.001), with a similar trend for the composite end-point of recurrent stroke or death (0.60, 0.34–1.07; P = 0.083).
Discussion
The main result of our study was that non-lacunar strokes with an embolic imaging pattern without an identified cause accounted for about one-quarter of all the consecutive ischemic strokes and three-quarters of the undetermined strokes. Considering the various characterizations based on the lesion size and/or location, this proportion is in accordance with prior reports (60%–87%) (Citation2–4). In our study, USEP subtype was independently associated with a higher risk of recurrent stroke already in a 21-month follow-up in comparison to those with non-cardioembolic stroke or cardioembolic stroke with a known source.
Our proportion of ESUS, 8.5%, is in accordance with the recent Athens Stroke Registry data (10.0%) (Citation18) despite our under-utilization of continuous ECG monitoring. The NAVIGATE ESUS trial (Citation2) applied modified ESUS criteria, which we also used, and which do not necessitate intracranial artery imaging. In case of using the original diagnostic criteria (Citation2), the proportion of our ESUS patients falls to 5.4% mainly due to the under-utilization of intracranial artery imaging. The frequency of potential ESUS patients eligible for a prospective trial among all the USEP cases could thus be much higher than 1/10 with a more systematic screening.
With regard to comorbidities, notable differences emerged between USEP and patients with embolic stroke with known source. USEP patients were younger with less cardiovascular comorbidity using the CHA2DS2-VASc score. Compared with those with non-embolic stroke, USEP patients had less often diabetes mellitus and prior TIA. Stroke symptoms were more severe in USEP compared with non-cardioembolic stroke but less severe compared with cardioembolic stroke with a known source. These observations may at least partly point out that short paroxysms of AF may frequently underlie USEP, since strokes in the setting of persistent or permanent AF have been more severe compared with strokes due to paroxysmal AF (Citation23). Notably, the antithrombotic medication scheme was identical in non-cardioembolic strokes and USEP patients.
Among the USEP patients baseline differences emerged between those fulfilling the ESUS diagnostic criteria and those who did not. Since the cases not fulfilling the criteria were older with more comorbidities, the clinician may have judged further investigations unnecessary. As stroke severity was equivocal in both USEP subgroups, it is unlikely that this factor would have influenced the decision whether to carry out complete examinations. However, data on the specific reasons why complete investigations were not carried out by the individual clinician were not available.
Despite the more favorable clinical profile at baseline, USEP classification independently was associated with recurrent stroke in a relatively short follow-up of only 21 months. Our findings of the risk of recurrent stroke among the USEP cases (7.0% at 12 months) is within the limits of most earlier reports in patients with undetermined stroke, with annual rates ranging from 2.5% to 7.8% (Citation3,Citation4,Citation12–15). However, a Korean study described a 30% recurrence rate at 12 months for such patients (Citation24). Compared to our rate (16.0% at 12 months), a combined end-point of recurrent stroke or death occurred at a markedly lower rate (5.8% per year) in the subgroup of cryptogenic strokes in the prospective WARSS trial comparing aspirin with warfarin in secondary stroke prevention (Citation25). The relatively low number of end-points in our series precludes making firm conclusions regarding the risks stratified by the USEP subgroups. However, frequency of recurrent strokes appeared similar in the patients fulfilling the ESUS criteria and those not fulfilling them (Supplementary Figure 1A to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612).
High early mortality among our USEP patients not meeting the ESUS criteria may reflect their expected poor outcome and withdrawal of complete examinations (Supplementary Figure 1B to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612). Existing data on mortality in undetermined stroke are variable and sparse. The few reports showed 16.0% mortality at 3 months (Citation26), 17% at 6 months, 3.3% at 1 year (Citation3), and 8.0% at 2.1 years (Citation12). Notably, a population-based study conducted in the 1980s showed a 48.6% mortality rate for ischemic stroke of uncertain cause (Citation14). The rate variation probably reflects the varying criteria for defining undetermined or cryptogenic stroke as well as population and study period differences. Our findings suggest an approximately 10% mortality at 1 year in USEP patients, the rate being, however, considerably smaller in those fulfilling the ESUS criteria.
Based on the recent findings on AF detection trials, a large proportion of patients with cryptogenic stroke may have occult atrial fibrillation (Citation16,Citation17). In the CRYSTAL-AF trial, an implantable loop recorder detected annually ∼10% of new AF in a 36-month follow-up. Since our pragmatic USEP definition covered also patients with incomplete diagnostic evaluation— rather characterizing the neuroimaging presentation than underlying pathophysiology—it likely would capture some cases with a high-risk source for cardioembolism. Furthermore, e.g. intracranial atherosclerosis and subclinical malignancies may have gone unnoticed when not screened systematically (Citation27).
Our study has strengths and limitations. The main strengths are that it describes the phenotypic presentation in a real-life consecutive patient population without exclusions and documents the diagnostic work-up and follow-up in detail. The main limitations include its single-center setting and relatively low number of end-point events. Some selection and referral bias is also possible, since our center is a tertiary teaching hospital facility, and some non-acute and previously non-independent cases are treated in regional hospitals. Although we had access to province-wide comprehensive electronic patient data archives and all patients with stroke are generally hospitalized in our health care system, we might have missed a few events, for instance if treated outside the hospital catchment area or if neurological symptoms have gone unnoticed in severely disabled patients. Inappropriate ascertainment of end-point events seems rather unlikely, as we had access to province-wide electronic medical records, including imaging and laboratory records. Regarding fatal events, the quality of the data maintained by Statistics Finland has been assessed to be good (Citation28).
In conclusion, acute embolic strokes in neuroimaging without established source were common among consecutive ischemic stroke patients and associated with a high early risk of recurrent stroke. The number of patients fulfilling the ESUS criteria can be increased by a more thorough case screening by utilizing existing diagnostic methods, and our findings add to the rationale for testing whether anticoagulants are superior to antiplatelets in the ESUS patients. However, developing novel methods— e.g. continuous ECG monitoring devices, cardiac imaging, screening for thrombophilia, imaging-screening or biomarkers for malignancies—to discover the potential causes of stroke seems also justified (Citation27,Citation29). Finally, further work is needed to standardize the entity of undetermined stroke and establish prognosis in its subgroups.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612.
Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1057612.
iann_a_1057612_sm9500.pdf
Download PDF (558.9 KB)Funding: Helsinki University Hospital research funds (Grant No. TYH2014407).
Declaration of interest: J.P.: Research grant for related research: Finnish Medical Foundation, Helsinki University Hospital; honoraria for consultancy: Boehringer-Ingelheim, BMS-Pfizer, Medtronic; honoraria for lectures: Boehringer-Ingelheim, BMS-Pfizer, Orion Pharma, Bayer.
T.N.: Honoraria for consultancy: Boehringer-Ingelheim; honoraria for lectures: Boehringer-Ingelheim, Orion Pharma, Medtronic.
A.M.: Research grant for related research: Finnish Medical Foundation, Academy of Finland, National Health and Medical Research Council; honoraria for travel costs for a talk at the World Stroke Congress 2014; Siemens.
K.R.: Honoraria for lectures: Boehringer-Ingelheim.
M.L.: Honoraria for consultancy: Boehringer-Ingelheim, BMS-Pfizer, Bayer; honoraria for lectures: Boehringer-Ingelheim, BMS-Pfizer, Bayer.
T.T.: Scientific advisory board membership for Boehringer-Ingelheim, Bayer, and Pfizer; consultant to Boehringer-Ingelheim, Bayer, and Pfizer; research contracts with Boehringer-Ingelheim, Bayer, and Pfizer; speaker's honorarium from Boehringer-Ingelheim, Bayer, and Pfizer.
E.H., J.K., D.S., S.C., S.P. , N.H., S.M.: None.
References
- Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;1:429–38.
- Sacco RL, Prabhakaran S, Thompson JL, Murphy A, Sciacca RR, Levin B, et al. Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin-Aspirin Recurrent Stroke Study. Cerebrovasc Dis. 2006;2:4–12.
- Palomeras Soler E, Fossas Felip P, Cano Orgaz AT, Sanz Cartagena P. Cryptogenic infarct. A follow-up period of 1 year study. Neurologia. 2009;24:304–8.
- Soda T, Nakayasu H, Maeda M, Kusumi M, Kowa H, Awaki E, et al. Stroke recurrence within the first year following cerebral infarction—Tottori University Lacunar Infarction Prognosis Study (TULIPS). Acta Neurol Scand. 2004;110:343–9.
- Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–6.
- Putaala J, Haapaniemi E, Metso AJ, Metso TM, Artto V, Kaste M, et al. Recurrent ischemic events in young adults after first-ever ischemic stroke. Ann Neurol. 2010;68:661–71.
- Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–9.
- Homma S, DiTullio MR, Sacco RL, Sciacca RR, Mohr JP; PICSS Investigators. Age as a determinant of adverse events in medically treated cryptogenic stroke patients with patent foramen ovale. Stroke. 2004;3:2145–9.
- Arauz A, Murillo L, Marquez JM, Tamayo A, Cantu C, Roldan FJ, et al. Long-term risk of recurrent stroke in young cryptogenic stroke patients with and without patent foramen ovale. Int J Stroke. 2012;7:631–4.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology. 2009;73:89–97.
- De Castro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–13.
- Vallejos J, Jaramillo A, Reyes A, Illanes S, Orellana P, Manterola J, et al. Prognosis of cryptogenic ischemic stroke: a prospective single-center study in Chile. J Stroke Cerebrovasc Dis. 2012;21:621–8.
- Weber R, Goertler M, Benemann J, Diener HC, Weimar C;German Stroke Study Collaboration. Prognosis after cryptogenic cerebral ischemia in patients with coagulopathies. Cerebrovasc Dis. 2009;28:611–17.
- Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–8.
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–40.
- Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77.
- Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86.
- Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Manios E, Spengos K, et al. Embolic strokes of undetermined source in the athens stroke registry: a descriptive analysis. Stroke. 2015;46:176–81.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137: 263–72.
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
- Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–97.
- Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB, et al. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart. 2014;100:1524–30.
- Ntaios G, Vemmou A, Koroboki E, Savvari P, Makaritsis K, Saliaris M, et al. The type of atrial fibrillation is associated with long-term outcome in patients with acute ischemic stroke. Int J Cardiol. 2013;167: 1519–23.
- Bang OY, Lee PH, Joo SY, Lee JS, Joo IS, Huh K. Frequency and mechanisms of stroke recurrence after cryptogenic stroke. Ann Neurol. 2003;54:227–34.
- Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–51.
- Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–66.
- Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke. 2014;45:1186–94.
- Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–7.
- Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251–65.

