Abstract
Introduction. Our objective was to perform a meta-analysis examining the sensitivity of pulsatility index (PI) and various biomarkers and PI and mean arterial pressure (MAP) for the prediction of pre-eclampsia.
Material and methods. PubMed, CENTRAL, and Embase databases were searched from inception until 8 May 2014 using combinations of the search terms: pre-eclampsia, ultrasonography, pregnancy, biomarker, mean arterial pressure, placental protein 13, pregnancy-associated plasma protein-A, placental growth factor, activin A, inhibin A, pulsatility index. The pooled sensitivity of PI + biomarkers and PI + MAP were calculated, and reported with corresponding 95% confidence intervals (CIs).
Results. Fifteen studies were included in the meta-analysis. The pooled sensitivity of all biomarkers for the prediction of pre-eclampsia was 0.669 (95% CI 0.610–0.723), for the prediction of early-onset pre-eclampsia was 0.830 (95% CI 0.794–0.861), and for the prediction of late-onset pre-eclampsia was 0.564 (95% CI 0.499–0.627). Similarly, the predictive ability of PI + MAP for early-onset pre-eclampsia was good (sensitivity 0.894), while that for late-onset was poor (sensitivity 0.570).
Conclusion. The combination of PI and different biomarkers or MAP exhibits a good predictive ability for early-onset pre-eclampsia, and poor predictive ability for late-onset pre-eclampsia.
The predictive ability of PI and MAP for early-onset pre-eclampsia is good.
PI and different biomarkers or MAP exhibit good predictive ability for early-onset pre-eclampsia, but poor predictive ability for late-onset pre-eclampsia.
Introduction
Pre-eclampsia, new onset hypertension, and proteinuria occurring after 20 weeks’ gestation, or, in the absence of proteinuria, hypertension together with evidence of systemic disease (e.g. thrombocytopenia or elevated liver transaminases), is an important cause of maternal and fetal morbidity and mortality (Citation1). The condition is estimated to affect 2%–8% of pregnancies worldwide (Citation2–5). Current evidence suggests that pre-eclampsia is a result of impaired placentation, and the condition is divided into early-onset pre-eclampsia (occurring before 34 weeks’ gestation) and late-onset pre-eclampsia (occurring at or after 34 weeks’ gestation) (Citation1,Citation6,Citation7). The distinction is important as early-onset pre- eclampsia is associated with a markedly higher incidence of adverse maternal and fetal outcomes than the late-onset form (Citation6,Citation8). As a result, considerable research has gone into developing methods to identify patients at risk of developing pre-eclampsia such that increased surveillance and early preventative treatment may be provided.
A number of biomarkers have been examined for identifying pregnancies at risk for developing pre-eclampsia, either alone or in combination (Citation9–12). Some biomarkers which have shown promise for the prediction of pre-eclampsia are activin A, inhibin A, pregnancy-associated plasma protein-A (PAPP-A), placental protein 13 (PP13), and placental growth factor (PGF) (Citation9–12). Activin A is a dimeric protein that is a member of the transforming growth factor (TGF)-β superfamily with numerous biological functions, and placental and serum levels are elevated in patients with pre-eclampsia (Citation13). Inhibin A is also a member of the TGF-β superfamily with a β subunit similar to that of activin A, and study has also shown levels to be increased in patients with pre-eclampsia (Citation14). Low levels of PAPP-A, used in screening for fetal chromosomal aneuploidies (Citation9), PP13, a glycan-binding protein expressed in the placenta and thought to be involved with immune tolerance (Citation15), and PGF, expressed in trophoblasts and important for angiogenesis and vasculogenesis during embryo development (Citation16), have been shown to be significantly associated with the development of pre-eclampsia and may have value in identifying patients at risk of developing pre-eclampsia (Citation9,Citation12,Citation15). Other methods for identifying patients at risk for developing pre-eclampsia include measurement of maternal mean arterial pressure (MAP) (Citation17), and uterine artery pulsatility index (PI) determined by Doppler ultrasound (Citation15).
As there are many studies in the literature that have examined the aforementioned biomarkers and other methods for identifying patients at risk of developing pre-eclampsia, the purpose of this study was to perform a systematic review of the literature and meta-analysis examining the sensitivity of PI and various biomarkers and PI and MAP for the prediction of pre-eclampsia.
Material and methods
Literature search strategy
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines (Citation18). PubMed, CENTRAL, and Embase databases were searched from inception until 8 May 2014 using combinations of the search terms: pre-eclampsia, ultrasonography, pregnancy, biomarker, mean arterial pressure, placental protein 13, pregnancy-associated plasma protein-A, placental growth factor, activin, inhibin, pulsatility index. Examples of keyword combinations used are: pre-eclampsia AND biomarkers AND ultrasonography; (pre-eclampsia OR pregnancy) AND (ultrasonographic OR ultrasonography) AND pregnancy-associated plasma protein-A. Filters used were abstract available, human, and English. Reference lists of relevant studies (e.g. other meta-analysis) were also hand-searched.
To identify relevant studies, a two-step search process was used after duplicate citations were identified and discarded. In the first step, the title and abstracts of all citations identified in the search were screened against the inclusion and exclusion criteria. In the second step, the full texts of the remaining articles were obtained and reviewed. Studies meeting all of the inclusion criteria and none of the exclusion criteria were included in the analysis.
Selection criteria and data extraction
Inclusion criteria were: 1) Prospective, retrospective, case-controlled, or cohort studies; 2) Patients with a diagnosis of pre-eclampsia; 3) Uterine artery Doppler ultrasound PI and serum biomarker(s) or MAP were used for the prediction of pre-eclampsia; 4) Quantitative outcomes included sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC). Letters, comments, editorials, case reports, proceedings, personal communications, and non-English articles were excluded. Studies that did not provide quantitative outcome data were also excluded. Studies were identified by the search strategy by two independent reviewers, and a third reviewer was consulted when disagreement arose.
Data extracted from studies that met the inclusion criteria were name of the first author and year of publication, study design, type and number of patients and demographic data, biomarker(s) examined and/or MAP and time of testing, maternal age and body mass index (BMI), gestational age at delivery, and infant birth weight (sensitivity, specificity, and AUC). Data extraction was performed by two independent reviewers, and a third reviewer was consulted for any uncertainties. Plot forms were not used.
Quality assessment
The quality of included studies was assessed according to QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) criteria (Citation19). This tool uses signaling questions in each of four risk-of-bias domains and three applicability domains. For example, to assess the risk of bias under the patient selection domain, questions include: ‘Was a consecutive or random sample of patients enrolled?’, ‘Was case-control study avoided’, and ‘Did the study avoid inappropriate exclusions?’ The index test domain questions include: ‘Were the index test results interpreted without knowledge of the results of the reference standard?’, and ‘If a threshold was used, was it pre-specified?’ To assess applicability, under the patient selection domain the question asked is: ‘Are there concerns that the included patients do not match the review question?’ Under the reference standard domain, the question asked is: ‘Are there concerns that the target condition as defined by the reference standard does not match the review question?’ Data extraction and quality assessment was carried out independently by the same two investigators, and disagreements were resolved by consensus.
Outcome measures and data analysis
The sensitivity of the PI and different biomarkers (i.e. PI + activin A, PI + inhibin A, PI + PAPP-A, PI + PGF, PI + PP13) and PI + MAP for the prediction of PE were investigated in this meta-analysis.
Heterogeneity among the studies was assessed by the Cochran Q and the I2 statistics. For the Cochran Q statistic, P < 0.10 is considered to indicate statistically significant heterogeneity; for the I2 statistic, which indicates the percentage of the observed between-study variability due to heterogeneity rather than chance, an I2 = 0%–25% indicates no heterogeneity, I2 = 25%–50% indicates moderate heterogeneity, I2 = 50%–75% indicates large heterogeneity, and I2 = 75%–100% indicates extreme heterogeneity. Random-effects models of analysis were used if heterogeneity was detected (Cochran Q, P < 0.10; I2 > 50%); otherwise, fixed-effect models were used. The pooled sensitivity of the biomarkers in combination with PI (PI + PAPP-A, PI + activin A, PI + inhibin A, PI + PGF, PI + PP13) and PI + MAP were calculated and reported with corresponding 95% confidence intervals (CIs). In all analysis, a two-sided value of P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Comprehensive Meta-Analysis, version 2.0 software (Biostat, Englewood, NJ, USA).
Results
Literature search
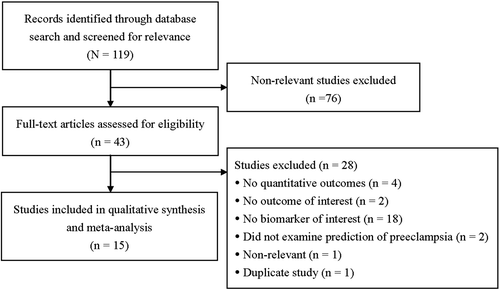
A flow diagram of study selection is shown in . After initially identifying 119 studies in the literature searches, 66 non-relevant studies were excluded and 43 full-text articles were assessed for eligibility. Subsequently, 28 studies were excluded, the reasons for which are shown in , and thus 15 studies were included in the qualitative synthesis and meta-analysis (Citation20–34).
Study characteristics
The basic characteristics of the 15 studies are summarized in . The total number of participants in the studies ranged from 10 to 1,426 in the pre-eclampsia groups, and from 73 to 57,458 in the control groups. The mean maternal age ranged from 28 to 33 years in the pre-eclampsia groups, and from 30 to 33 years in the control groups. Five of the 15 studies provided results of patients with pre-eclampsia overall, 10 studies the results of patients with early-onset pre-eclampsia, and eight studies results of patients with late-onset pre-eclampsia. In 14 of the 15 studies, the diagnosis of pre-eclampsia was based on the International Society for the Study of Hypertension in Pregnancy guidelines, and one study (Citation21) used the American College of Obstetricians and Gynecology guidelines Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1059483.
Table I. Summary of studies included in the meta-analysis.
In the majority of the studies, biomarker and PI testing was done in the late first trimester. In studies examining PI + PAPP-A, testing was done at 11–13 weeks; however, PI testing in three studies (Citation29,Citation31,Citation34) was done at 24 weeks. In two studies examining PI + activing A (Citation22,Citation29), activin A was tested at 11–13 weeks and PI at 22–24 weeks, and in one study (Citation32) both were examined at 22–24 weeks. Of the studies examining PI + inhibin A, in one study inhibin A was examined at 12–16 weeks and PI at 22–24 weeks (Citation22), in one inhibin A was tested at 11–13 weeks and PI at 22–24 weeks (Citation29), and in one both were examined at 22–24 weeks (Citation32). In studies examining PI + PGF, two performed testing at 11–13 weeks (Citation20,Citation23), one testing at 24 weeks (Citation28), and in one PGF was tested at 12–16 weeks and PI at 22–24 weeks (Citation22). In studies examining PI + PP13, two studies performed testing at 11–13 weeks (Citation21,Citation33), one at 22–24 weeks (Citation30), and in one study PP13 was tested at 11–13 weeks and PI at 22–24 weeks (Citation31). All studies examining PI + MAP performed both tests at 11–13 weeks (Citation20,Citation24).
The outcomes of the 15 studies are summarized in . In general, the specificity of PI plus the various biomarkers and PI + MAP for the prediction of pre-eclampsia ranged from 80% to 95%. The sensitivities, however, varied greatly with a sensitivity as low as 35% for PI + PAPP-A for the prediction of pre-eclampsia (Citation26) to as high as 90% for PI + PP13 for the prediction of pre-eclampsia (Citation33). The results between studies for specific biomarkers also varied greatly. For example, Spencer et al. (Citation30) reported PI + PP13 exhibited a sensitivity of 100% for the prediction of early-onset pre-eclampsia, whereas Odibo et al. (Citation21) reported the combination had a sensitivity of only 45%.
Table II. Summary of outcomes.
Pooled sensitivities of biomarkers for predicting pre-eclampsia
All pre-eclampsia
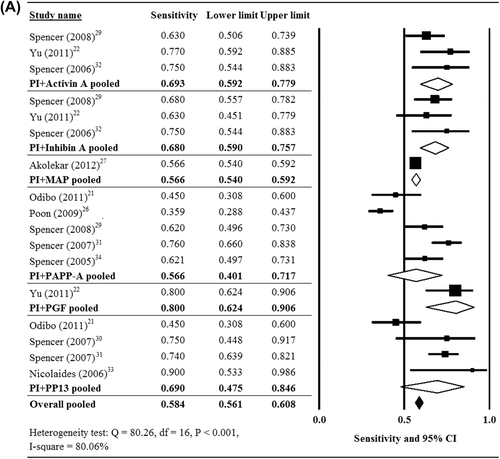
There was significant heterogeneity when data from the 15 studies were pooled (Cochran Q = 80.26, df = 16, P < 0.001, I2 = 80.06%); thus, a random-effects model of analysis was used. The pooled sensitivity of all biomarkers for the prediction of pre-eclampsia was 0.584 (95% CI 0.561–0.608) (). The pooled sensitivity of PI + activin A (three studies (Citation22,Citation29,Citation32)) = 0.693 (95% CI 0.592–0.779); of PI + inhibin A (three studies (Citation22,Citation29,Citation32)) = 0.680 (95% CI 0.590–0.757); of PI + PAPP-A (five studies (Citation21,Citation26,Citation29,Citation31,Citation34)) = 0.566 (95% CI 0.401–0.717); and of PI + PP13 (four studies (Citation21,Citation30,Citation31,Citation33)) = 0.690 (95% CI 0.475–0.846).
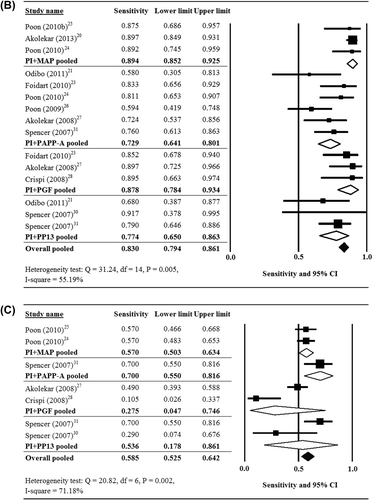
Figure 2. Forest plots showing results of the meta-analysis for the sensitivity of the biomarkers for the prediction of (A) all pre-eclampsia, (B) early-onset pre-eclampsia, and (C) late-onset pre-eclampsia. CI = confidence interval.
Early-onset pre-eclampsia
There was significant heterogeneity when data from the 15 studies were pooled (Cochran Q = 31.24, df = 14, P = 0.005, I2 = 55.19%); thus, a random-effects model of analysis was used. The pooled sensitivity of all biomarkers for the prediction of early-onset pre-eclampsia was 0.830 (95% CI 0.794–0.861) (). The pooled sensitivity of PI + MAP (three studies (Citation20,Citation24,Citation25)) = 0.894 (95% CI 0.852–0.925); of PI + PAPP-A (six studies (Citation21,Citation23,Citation24,Citation26,Citation27,Citation31)) = 0.729 (95% CI 0.641–0.801); of PI + PGF (three studies (Citation23,Citation27,Citation28)) = 0.878 (95% CI 0.784–0.934); and of PI + PP13 (three studies (Citation21,Citation30,Citation31)) = 0.774 (95% CI 0.650–0.863).
Late-onset pre-eclampsia
There was significant heterogeneity when data from the six studies that provided data with respect to late-onset pre-eclampsia were pooled (Cochran Q = 20.82, df = 6, P = 0.002, I2 = 71.18%); thus, a random-effects model of analysis was used. The pooled sensitivity of all biomarkers for the prediction of late-onset pre-eclampsia was 0.585 (95% CI 0.525–0.642) (). The pooled sensitivity of PI + MAP (two studies (Citation24,Citation25)) = 0.570 (95% CI 0.503–0.634); of PI + PGF (two studies (Citation27,Citation28)) = 0.275 (95% CI 0.047–0.746); and of PI + PP13 (two studies (Citation30,Citation31)) = 0.536 (95% CI 0.178–0.861).
Quality assessment
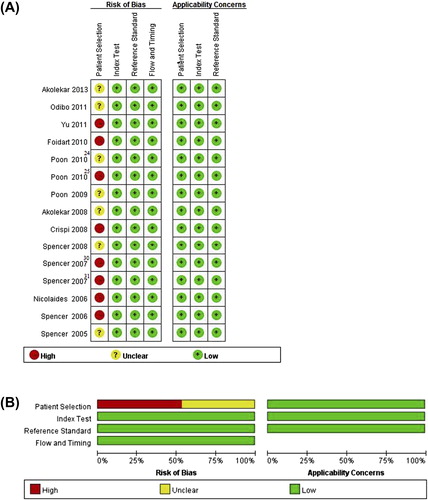
Results of the quality assessment of the included studies are shown in . Three items were used to assess the risk of bias for patient selection for each of the included studies: 1) Was a consecutive or random sample of patients enrolled? 2) Was a case-control design avoided? 3) Did the study avoid inappropriate exclusions? A considerable proportion of studies were considered to be at high risk of bias with respect to patient selection. Eight of the 15 studies (53%) did not avoid using a case-control design, and seven of 15 studies (47%) did not select patients randomly or consecutively. The domains of index test and reference standard, both in risks of bias and applicability concerns, were designated ‘low risk and low concerns’. In addition, the flow and timing domain was designated ‘low risk’.
Discussion
The results of this meta-analysis indicate that uterine artery PI plus various biomarkers exhibit modest sensitivity for the prediction of pre-eclampsia. More specifically, while an overall good predictive ability for early-onset pre-eclampsia was observed, the predictive ability for late-onset pre-eclampsia was poor. Similarly, the predictive ability of PI + MAP for early-onset pre-eclampsia was good, while that for late-onset was poor.
As pre-eclampsia, especially early-onset pre-eclampsia, is associated with significant maternal and fetal morbidity and mortality, considerable attention has been given to developing methods to detect the disease early and predict those patients at risk of being affected. To this end, a relatively large number of biomolecules have been identified which may be involved in the pathogenesis of pre-eclampsia and have been shown to have potential value with respect to early diagnosis and prediction of the development of pre-eclampsia (Citation11,Citation35,Citation36). These biomarkers can be broadly categorized as angiogenic (pro- and anti-angiogenic) (vascular endothelial growth factor (VEGF), PGF, soluble fms-like tyrosine kinase 1 (sFlt-1), soluble endoglin (sEng)), renin-angiotensin system related (autoantibodies against angiotensin II type 1 receptor), immunological (PP13, PAPP-A), metabolic (visfatin), and endocrine (activin A, inhibin A) (Citation11,Citation35).
Low levels of PP13, PGF, and PAPP-A and elevated level of inhibin A have been found to be significantly associated with the development of pre-eclampsia later in pregnancy (Citation11,Citation15,Citation35). The 2010 systematic review by Giguère et al. (Citation12) examined 37 studies that assessed 71 different combinations of biomarkers and found that in general combinations resulted in a higher sensitivity for predicting pre-eclampsia than single biomarkers. In low-risk populations, combinations including PP13, PAPP-A, activin A, or inhibin A measured in first or early second trimester and uterine artery Doppler measurement (pulsatility index or resistance index and/or presence of a notch) in the second trimester exhibited a sensitivity of 60%–80% and a specificity of 80%). In one study limited to a diagnosis of severe pre-eclampsia, PP13 + PI measured in the first trimester had a sensitivity of 90% and specificity of 90%. A subsequent review by Kuc et al. (Citation15) evaluated seven biomarkers (ADAM12, f-hCG, inhibin A, activin A, PP13, PGF, and PAPP-A) and uterine artery Doppler ultrasound performed in the first trimester. The results showed that the detection rates of single markers, fixed at 10% false-positive rate, for the prediction of early-onset pre-eclampsia ranged from 22% to 83%, while the detection rates for combinations of multiple markers ranged from 38% to 100%. A recent meta-analysis by Allen et al. (Citation37) examined the association of biomarkers and pre-eclampsia. The analysis included 30 studies comprising 65,538 women, and 24 studies assessed pre-eclampsia of any onset, 10 early-onset pre-eclampsia, and seven late-onset. The odds ratio (OR) of PAPP-A, PP13, and inhibin A for any pre-eclampsia were 2.1, 4.4, and 3.6, respectively, and for early-onset pre-eclampsia were 4.8, 7.5, and 4.1, respectively. PGF was also associated with early-onset disease (OR 3.4). A review by Poon et al. (Citation9) in 2014 concluded that first trimester screening with a combination of maternal risk factors, uterine artery Doppler, MAP, PAPP-A, and PGF can identify about 95% of cases of early-onset PE with a false-positive rate of 10%.
In this study, PI + PAPP-A, PI + PGF, PI + PP13 showed good sensitivity for the prediction of the early-onset pre-eclampsia, and the timed expression of these markers and the gestational age at which they are measured may explain these findings (Citation10). The results also showed that PI + MAP exhibited good sensitivity, and again the timing and method of determining MAP are important. A recent study showed that screening for pre-eclampsia by MAP has the best performance when MAP is determined at both 11–13 and 20–24 weeks’ gestation rather than at only one of these gestational age ranges (Citation17).
Variation in the sensitivities of PI and certain biomarkers was noted when individual studies were compared. Crispi et al. (Citation28) reported a very low detection rate for PI + PGF, and this may be because risk factors for cardiovascular disease in southern European countries such as black ethnicity, BMI, atherogenic lipid profile, and vitamin C and E dietary intake are different than in other populations with a higher prevalence of pre-eclampsia. In the 2007 studies by Spencer et al. (Citation30,Citation31) the detection rate of PI + PP13 for pre-eclampsia overall was similar (75% and 74%, respectively); however, the detection rates for early-onset and late-onset were markedly different (100% versus 79% and 29% versus 70%, respectively). This may be due to the timing of testing as in the first study screening was only done at 22–24 weeks’ gestation whereas in the second study PP13 was measured at 11–13 weeks and PI at 22–24 weeks.
There are a number of limitations of this analysis that should be considered. Because of the small number of studies included and the significant heterogeneity between the studies the results should be interpreted with caution. Importantly, the time of testing varied between the studies. Studies that examined the predictive ability of PI + activin A and PI + inhibin A only classified patients as having pre-eclampsia, and did not distinguish between early-onset and late-onset forms. Combinations of biomarkers were not assessed as there were not enough studies with the same combinations of biomarkers to perform an analysis (i.e. there were fewer than three studies with the same combinations of biomarkers). However, a study has shown that using various combinations of biomarkers increases their predictive value (Citation9). Maternal characteristics have been shown to be predictive of the development of pre-eclampsia (Citation38), and risk-factors for early- and late-onset pre-eclampsia are different (Citation6,Citation8); these factors were not considered in this study. There are a number of other biomarkers (e.g. sFlt-1, sEng, VEGF) that may have predictive value for the development of pre-eclampsia; however, these were not examined because the number of studies was not sufficient to perform an analysis (i.e. there were fewer than three studies). Most of the included studies did not report specific cut-off values of the biomarkers, and AUC data were extracted from the studies. Because the included studies did not provide false-positive, false-negative, true-positive, and true-negative rates, it was not possible to analyze the AUC data.
In conclusion, combination of uterine artery PI and biomarkers/MAP has good detection rate for early-onset of pre-eclampsia, but a low detection rate for late-onset disease. More clinical studies that assess the prediction value of these biomarkers and explore more prediction methods for late-onset pre-eclampsia are needed.
Supplementary material available online
iann_a_1059483_sm4586.pdf
Download PDF (39.3 KB)Acknowledgements
Xiao-Lu Zhu and Juan Wang are co-first authors who contributed equally to the work.
Declaration of interest: The authors report no conflicts of interest.
References
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–80.
- Steegers EAP, Von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44.
- Hutcheon JA, Lisonkova S, Joseph KS. The epidemiology of preeclampsia and the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–306.
- Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following pre-eclampsia. JAMA. 2006;296:1357–62.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–12.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. Am J Obstet Gynecol.2013;208:476.e1–5.
- Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66:497–506.
- Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int. 2014;2014:297397.
- Müller-Deile J, Schiffer M. Preeclampsia from a renal point of view: insides into disease models, biomarkers and therapy. World J Nephrol. 2014;3:169–81.
- Kar M. Role of biomarkers in early detection of preeclampsia. J Clin Diagn Res. 2014;8:BE01–4.
- Giguère Y, Charland M, Bujold E, Bernard N, Grenier S, Rousseau F, et al. Combining biochemical and ultrasonographic markers in predicting preeclampsia: a systematic review. Clin Chem. 2010; 56:361–75.
- Williamson RD, O’Keeffe GW, Kenny LC. Activin signalling and pre-eclampsia: from genetic risk to pre-symptomatic biomarker. Cytokine. 2015;71:360–5.
- Ree PH, Hahn WB, Chang SW, Jung SH, Kang JH, Cha DH, et al. Early detection of preeclampsia using inhibin a and other second-trimester serum markers. Fetal Diagn Ther. 2011;29:280–6.
- Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GH, Schielen PC. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv. 2011;66:225–39.
- Than NG, Balogh A, Romero R, Kárpáti E, Erez O, Szilágyi A, et al. Placental protein 13 (PP13) - a placental immunoregulatory galectin protecting pregnancy. Front Immunol. 2014;5:348.
- Gallo D, Poon LC, Fernandez M, Wright D, Nicolaides KH. Prediction of preeclampsia by mean arterial pressure at 11–13 and 20–24 weeks’ gestation. Fetal Diagn Ther. 2014;36:28–37.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–94.
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15.
- Odibo AO, Zhong Y, Goetzinger KR, Odibo L, Bick JL, Bower CR, et al. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta. 2011;32:598–602.
- Yu J, Shixia CZ, Wu Y, Duan T. Inhibin A, activin A, placental growth factor and uterine artery Doppler pulsatility index in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2011;37:528–33.
- Foidart JM, Munaut C, Chantraine F, Akolekar R, Nicolaides KH. Maternal plasma soluble endoglin at 11–13 weeks’ gestation in pre-eclampsia. Ultrasound Obstet Gynecol. 2010;35:680–7.
- Poon LC, Stratieva V, Piras S, Piri S, Nicolaides KH. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11–13 weeks. Prenat Diagn. 2010; 30:216–23.
- Poon LC, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemicalmarkers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35:662–70.
- Poon LC, Maiz N, Valencia C, Plasencia W, Nicolaides KH. First- trimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound Obstet Gynecol. 2009;33:23–33.
- Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:732–9.
- Crispi F, Llurba E, Domínguez C, Martín-Gallán P, Cabero L, Gratacós E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–9.
- Spencer K, Cowans NJ, Nicolaides KH. Maternal serum inhibin-A and activin-A levels in the first trimester of pregnancies developing pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:622–6.
- Spencer K, Cowans NJ, Chefetz I, Tal J, Kuhnreich I, Meiri H. Second-trimester uterine artery Doppler pulsatility index and maternal serum PP13 as markers of pre-eclampsia. Prenat Diagn. 2007;27: 258–63.
- Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–34.
- Spencer K, Yu CK, Savvidou M, Papageorghiou AT, Nicolaides KH. Prediction of pre-eclampsia by uterine artery Doppler ultrasonography and maternal serum pregnancy-associated plasma protein-A, free beta-human chorionic gonadotropin, activin A and inhibin A at 22 + 0 to 24 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27:658–63.
- Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17.
- Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25:949–53.
- Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10:531–40.
- Adekola H, Romero R, Chaemsaithong P, Korzeniewski SJ, Dong Z, Yeo L, et al. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. J Matern Fetal Neonatal Med. 2014 Oct 28:1–12. [Epub ahead of print]
- Allen RE, Rogozinska E, Cleverly K, Aquilina J, Thangaratinam S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:194–201.
- Kuc S, Koster MP, Franx A, Schielen PC, Visser GH. Maternal characteristics, mean arterial pressure and serum markers in early prediction of preeclampsia. PLoS One. 2013;8:e63546.