Abstract
Background. Telomeres are causally involved in senescence. Senescence is a potential factor in the pathogenesis and progression of heart failure. In heart failure telomeres are shorter, but the prognostic value associated with telomere length has not been defined.
Methods. Telomere length was prospectively determined by quantitative polymerase chain reaction in 890 patients with New York Heart Association (NYHA) functional class II to IV heart failure. After 18 months, we examined the association between telomere length and the predefined primary end-point: time to death or hospitalization for heart failure.
Results. Mean age of the patients was 71 years, 39% were women, 51% were in NYHA class II, and 49% were in class III/IV. A total of 344 patients reached the primary end-point (130 deaths and 214 hospitalizations). Patients with shorter telomeres were at an increased risk of reaching the primary end-point (hazard ratio 1.79; 95% confidence interval (CI) 1.21–2.63). In multivariate analysis shorter telomere length remained associated with a higher risk for death or hospitalization (hazard ratio, 1.74; 95% CI 1.07–2.95) after adjustment for age of heart failure onset, gender, hemoglobin, renal function, and N-terminal pro-B-type natriuretic peptide level, a history of stroke, atrial fibrillation, and diabetes.
Conclusions. Shorter length of telomeres predicts the occurrence of death or hospitalization in patients with chronic heart failure.
Key words::
Key messages
Shorter telomere length predicts the occurrence of death or hospitalization in patients with chronic heart failure.
| Abbreviations | ||
| CAD | = | coronary artery disease |
| CHF | = | chronic heart failure |
| COACH | = | Co-ordinating study evaluating outcomes of Advising and Counseling in Heart failure |
| NYHA | = | New York Heart Association |
| T/S | = | ratio telomere repeat copy number to single-copy gene copy number ratio |
Introduction
Chronic heart failure (CHF) is a major epidemiological problem with an estimated 5.2 million Americans suffering from this condition. Some 30%–40% of patients die from CHF within 1 year after diagnosis. As the population ages, it is expected that the prevalence of CHF will increase further, imposing a major social and economic burden on society (Citation1). Even with optimal therapy CHF is associated with a high annual mortality (Citation2,Citation3). Genetic predisposition, coronary artery disease, hypertension, cardiomyopathy, and diabetes are common etiologic factors for CHF. Nevertheless, our understanding of the exact triggers of CHF is limited (Citation1,Citation4,Citation5). Environmental, genetic, and epigenetic factors are thought to contribute to the susceptibility to develop CHF and to progress to end stage CHF.
Telomeres are specialized functional deoxyribonucleic acid (DNA)-protein complexes located on the extreme ends of chromosomes (Citation6). Telomeres play a critical role in cell-cycling and chromosomal stability. In somatic tissue, telomeres will erode due to replicative and environmental stressors, and the length is therefore considered a marker of biological age (Citation7). Cells in which the length of telomeres reach a critical threshold will become genomically unstable, senescent, or apoptotic. CHF can be considered a condition that is associated with, and perhaps even partially triggered by, the processes related to cellular ageing (Citation8). Only recently, reduced telomere length has been implicated as a potential factor in the pathogenesis and progression of cardiovascular disease, including atherosclerosis and CHF (Citation9–14). In CHF systemic abnormalities occur, including release of mediators that can cause telomere shortening, such as oxidative stress (Citation15), proinflammatory cytokines, and activation of the renin-angiotensin system (Citation12). Although leukocyte telomere length has been reported to be reduced in CHF, the relation with clinical outcome is unknown (Citation8). We therefore examined telomere length in a large cohort of patients with CHF who were part of a randomized trial assessing the value of a CHF management program (Citation16). The primary objective of the present study was to examine the relation between telomere length and clinical outcome in patients with CHF.
Material and methods
This sub-study was prospectively planned in the development of the Co-ordinating study evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) (Citation16,Citation17). In brief, in the COACH study patients there were two types of interventions and a control treatment. All patients received he usual routine management by their cardiologist. Patients in the two intervention groups received either basic support or intensive support by a registered heart failure nurse. This support consisted of additional education, behavioral strategies to improve adherence, and in the intensive support group it also included routine contact with the heart failure nurse on a monthly basis. The medical ethics committee of each participating center approved both the main COACH study as well as this DNA sub-study. All patients provided written informed consent. A total of 133 patients participated in COACH, but did not participate in the present DNA sub-study, leaving 890 (87%) subjects from whom DNA was collected. The investigators who initiated the present sub-study had full access to and analyzed the data and wrote the manuscript.
Patients
Eligible patients were 18 years of age or older and had evidence of structural underlying heart disease, as evidenced by cardiovascular imaging. Both patients with impaired and those with preserved left ventricular ejection fraction could participate. The diagnosis was made by a combination of typical signs and symptoms, for which hospitalization was considered necessary, including the need of intravenous medication. Before discharge from the hospital (i.e. before inclusion into the study), patients had to be stable on oral CHF medication.
Follow-up
The first out-patient visit was within 2 months after discharge, and all patients were studied for a median duration of 18 months (Citation16,Citation17). Data on mortality and number of readmissions were collected from medical records and patient interviews.
End-points
The original COACH study had two co-primary end-points (Citation16) The first co-primary end-point was a composite of death or CHF hospitalization and was a priori considered the only primary end-point for this sub-study. A hospitalization for CHF was defined as an unplanned overnight stay in a hospital due to progression of CHF or directly related to CHF. The second co-primary end-point of COACH was the number of unfavorable days. To decrease the risk of a type 1 error, this end-point was a priori defined as secondary for this sub-study. Unfavorable days were defined as days lost to death or hospitalization during 18 months of follow-up. Other secondary end-points were death from any cause and hospitalization for heart failure. All reported deaths and hospital admissions were referred to and adjudicated in a blinded fashion by a central and independent clinical event committee (Citation17).
Telomere length
Whole blood was collected during the first out-patient clinic visit, and leukocyte DNA was extracted according to standard procedures. Mean telomere length was measured from DNA by a quantitative polymerase-chain-reaction-based assay performed on the 384-well ABI7900HT TaqMan platform as described previously (Citation14). We determined the relative ratio of telomere repeat copy number (T) to single-copy gene copy number (36B4 gene, encoding ribosomal phosphoprotein PO, located on chromosome 12; S) with all samples being compared to the same reference DNA (Citation9,Citation14). T/S ratios have been confirmed previously to be correlated with the classical Southern blot on terminal restriction fragments (correlation coefficient of around 0.81) (Citation9,Citation15). All DNA samples were assayed in triplicate on separate plates, but in the same well positions. The mean ± SD coefficient of variation was 7% ± 5% for the T and 6% ± 4% for the S assay, respectively. Determination of T and S quantities was performed using standardized thresholds and without knowledge of clinical data.
Statistical analysis
Because the distribution of telomere length was skewed, statistical analyses were performed on natural log-transformed data. Base-line data among quartiles of telomere length were tested by one-way analysis of variance, Kruskal-Wallis, or chi-square test when appropriate. One primary end-point was defined: time to first major event (CHF hospitalization or death). Time to event between quartiles of telomere length was compared with the use of Kaplan-Meier curves, and statistical significance was evaluated with the use of the log-rank test. The Cox proportional hazard model was used to calculate the hazard ratios and 95% confidence intervals (CI) for natural log-transformed telomere length data. Sequential models were fitted with the first model including no covariates (unadjusted) and the second model adjusted for age, age of onset of CHF, and gender. Next all univariable predictors of outcome were identified, and predictors related to outcome (P < 0.1) were incorporated in the multivariable Cox proportional hazard model. Interactions between telomere length and variables were analyzed using bivariable Cox regression including interactions between the two co-variables; however, no significant interactions were identified. We constructed the multivariable models using both forward and backward stepwise conditional Cox regression. At each step, all excluded covariates were re-evaluated, and if they then did reach the inclusion threshold they were re-included into the model. For inclusion of factors in the model a probability of P < 0.05 was used, and for exclusion a probability of P > 0.1 was used. We also studied telomere length using a multivariable fractional polynominal modeling approach (mfp) using both the default closed-test algorithm and the Royston and Altman model selection algorithm, both with a nominal P-value set at 0.1 for variable selection by backward elimination. We studied the stability of our models by performing bootstrapping (1000 repetitions) and jack-knife techniques. The assumptions underlying the proportional hazards model were tested and found valid. The numbers of days lost to death or hospitalization during the 18-month follow-up period were non-normally distributed due to a large number of zeros. Therefore we used Nptrend to test differences among quartiles and standard logistic regression (0 versus all other values) analysis. Standard linear regression techniques were used to associate telomere length with individual factors and to adjust for age, age of CHF onset, and gender. All analyses were performed using STATA version 10.0 for Windows software (StataCorp LP, College Station, TX, USA), and a two-sided P-value of 0.05 or less was interpreted to indicate statistical significance.
Results
A total of 890 patients were studied. The duration of follow-up was 18 months. By the end of the study, the survival status of all patients was known, and 344 (39%) had reached the primary end-point.
Study population
Base-line characteristics of patients according to quartiles of telomere length are presented in Table I. Patients had moderate to severe heart failure and received standard medication. As expected, age was highly significantly different among quartiles of telomere length. Telomere length decreased with age at a mean yearly rate of 0.005 ± 0.001 in T/S ratio (P = 2.21 × 10−9) (). Addition of squared or cubed age terms did not have an effect on this relationship (P = 0.895 and P = 0.902, respectively), suggesting a linear relationship between telomere length and age. Use of renin-angiotensin system inhibitors differed significantly among groups, and there were small differences in history of myocardial infarction, hemoglobin levels, estimated renal function, and (non-significantly) left ventricular ejection fraction among the quartiles of telomere length (). Median telomere length in NYHA II at discharge was 0.70 (interquartile range 0.60–0.85), 0.69 (0.59–0.8) for NYHA III, and 0.64 (0.54–0.77) for NYHA IV (Kruskal-Wallis P = 0.24). In correlations of telomere length with clinical and biochemical characteristics are presented. Shorter telomere length was associated with lower hemoglobin levels and lower estimated renal function after adjusting for age, age of CHF onset, and gender.
Figure 1. Telomere length, expressed as the telomere-to-single-reference-gene (T/S) ratio, is plotted as a function of age. Telomere length decreases with age at a mean yearly rate of 0.005 ± 0.001 in T/S ratio (P = 2.21 × 10−9). Shaded is the 95% confidence interval of the mean.
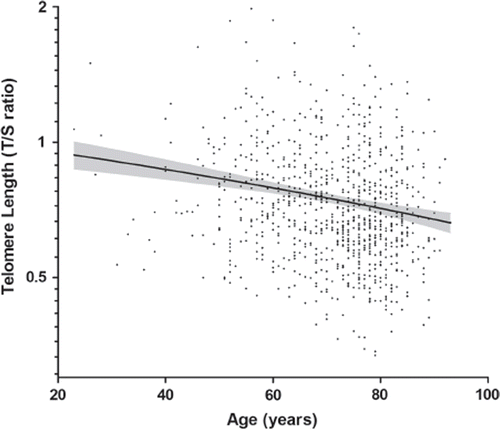
Table I. Base-line characteristics.
Table II. Clinical and biochemical associations with telomere length.
Primary outcome
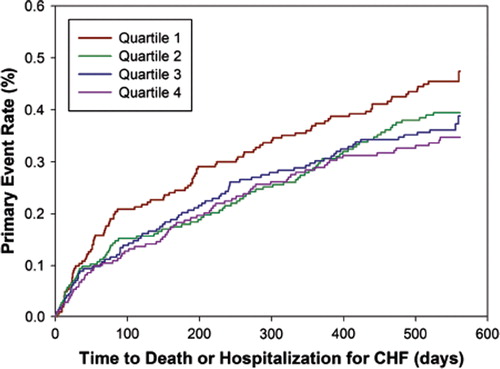
By the end of the study, death was the primary event in 130 (15%) patients, and hospitalization for CHF in 214 (24%). Kaplan-Meier estimates of the time to first event (primary outcome) for all subjects according to quartiles of telomere length are shown in . In Cox regression analysis with telomere length as a categorical variable, the unadjusted hazard ratios for the primary event in patients with telomere length in the second, third, and fourth quartiles, as compared with those in the first quartile, were 1.25 (95% CI 0.94–1.67; P = 0.12), 1.33 (95% CI 1.00–1.79; P = 0.052), and 1.46 (95% CI 1.08–1.97; P = 0.014), respectively. When telomere length was analyzed as a continuous variable in Cox regression analysis, a decrease of one (natural log-transformed) unit of telomere length ratio was associated with an unadjusted hazard ratio of 1.79 (95% CI 1.21–2.63; P = 0.003).
Figure 2. Kaplan-Meier curves for outcome according to quartile of telomere length. The telomere length quartiles were as follows: first quartile, less than 0.59; second quartile 0.59 to 0.69; third quartile 0.69 to 0.86; and fourth quartile, more than 0.86. P = 0.009 by the log-rank test for the overall comparison among groups.
Age, age of heart failure onset, a history of myocardial infarction, atrial fibrillation, or cerebrovascular event, diabetes, diastolic blood pressure, N-terminal pro-B-type natriuretic peptide, hemoglobin, use of renin-angiotensin system inhibitors, use of nitrates, and estimated renal function were related to outcome (P < 0.1) and were considered as confounders for multivariable modeling. Using both forward and backward stepwise conditional Cox regression led to the inclusion of sex, age of heart failure onset, estimated renal function, N-terminal pro-B-type natriuretic peptide, hemoglobin, stroke, atrial fibrillation, and diabetes in the model. The hazard ratio of telomere length was 1.74 (95% CI 1.07–2.82; P = 0.025). Forcing in the model a history of myocardial infarction and/or left ventricular ejection fraction and/or the use of renin-angiotensin system inhibitors did not affect the point estimate or confidence interval of telomere length for outcome (data not shown). The estimated hazard ratio with bootstrapping was 1.74 (95% CI 1.02–2.95; P = 0.040) and with jack-knife 1.74 (95% CI 1.02–2.95; P = 0.041), suggesting our model was stable. To exclude potential confounding due to curve-shaped relationships we also evaluated all covariates with multivariable fractional polynominal modeling using two different algorithms. The point estimate of the hazard ratio of telomere length was comparable for both algorithms (). represents the distribution of (natural log-transformed) telomere length ratio and the associated hazard ratio for the final multivariable model.
Figure 3. Hazard ratios per unit decrease in telomere length ratio for outcome. The (adjusted) hazard ratio and 95% confidence intervals (CI) are shown for the primary outcome for telomere length ratio univariately (model 1), after adjustment for age, age of CHF onset, and gender (model 2), the model after adjustment for the covariates selected by forward and backward stepwise conditional selection (model 3), the model after adjustment for the covariates selected by the multivariable fractional polynominal closed-test algorithm (model 4), and the model after adjustment for the covariates selected by the Royston and Altman model selection algorithm (model 5). CHF = chronic heart failure; NT-proBNP = N-terminal pro-B-type natriuretic peptide; Hb = hemoglobin; EGFR = estimated renal function; CVA = history of cerebrovascular accident.
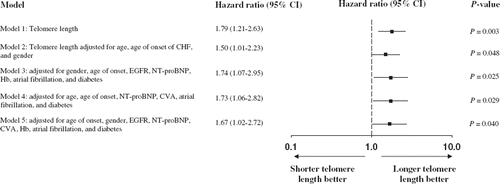
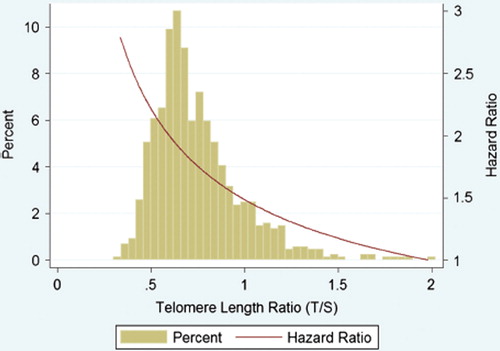
Figure 4. Distribution of telomere length and associated hazard ratio. The green bars represent the histogram of the untransformed telomere length ratio (horizontal axis versus percentage on left vertical axis). The red line represents the estimated hazard ratio for the primary end-point (total mortality and hospitalization for heart failure) after multivariable adjustment for age of onset, gender, estimated renal function, N-terminal pro-B-type natriuretic peptide, hemoglobin, a history of atrial fibrillation, and diabetes (model 3).
Death and other secondary end-points
Of patients with hospitalization as first event, 93 died during further follow-up resulting in a total of 223 deaths (25%). We observed a trend towards patients with shorter telomeres being more likely to die (hazard ratio 1.59; 95% CI 0.99–2.55; P = 0.057). After adjustment for age, age of CHF onset, and gender this trend was lost (hazard ratio 1.21; 95% CI 0.75–1.97; P = 0.43).
During the study 214 patients had at least 1 hospitalization for CHF. Patients with shorter telomeres were more likely to be hospitalized (hazard ratio 1.84; 95% CI 1.12–3.00; P = 0.016). This finding remained significant after adjusting for age, age of CHF onset, and gender (hazard ratio 1.68; 95% CI 1.01–2.78; P = 0.044), but lost its significance after additionally adjusting for estimated renal function, N-terminal pro-B-type natriuretic peptide, hemoglobin, stroke, atrial fibrillation, and diabetes in the model (hazard ratio 1.66; 95% CI 0.89–3.08; P = 0.11).
The total number of unfavorable days (days lost to death or all-cause hospitalization during the 18-month study) was 88,730 days. The number of unfavorable days was 25,618 days, 22,004 days, 22,426 days, and 18,682 days, respectively, from the shortest to the longest quartile of telomere length (P for trend 0.015). Per patient the median days lost were 12 (interquartile range 0–133), 8 (interquartile range 0–96), 11 (interquartile range 0–118), and 5 (interquartile range 0–43). In logistic regression, patients with longer telomeres were less likely to experience unfavorable days (coefficient 0.70, 95% CI 0.22–1.17; P = 0.004), but only a trend remained after adjusting for age, age of CHF onset, and gender (coefficient 0.45, 95% CI -0.04–0.95; P = 0.07).
Discussion
We prospectively studied the association between leukocyte telomere length and outcome in patients with CHF who were receiving optimal standard medical therapy. Our study provides evidence that shorter telomere length is associated with increased occurrence of death and hospitalization for CHF also after adjusting for potential confounders.
We and others have recently demonstrated that telomere length is shorter in patients with CHF compared to healthy age-matched controls (Citation13,Citation14). We estimated a linear yearly loss of telomere length ratio of 0.005 which is in good accordance with previous reports on telomere length loss in heart failure (Citation14). Whether causal or not, our study supports the hypothesis that telomere length may not only be related to the development of CHF, but to CHF progression as well. Previously, only retrospective studies have evaluated the value of telomere length in predicting outcome. In stroke survivors, a doubled risk for death was observed in patients with shorter telomeres (Citation18). In presumably healthy elderly subjects the results have been more inconsistent (Citation19–21).
The existence of a perfect biomarker of ageing is thought to be unlikely. However, telomere length has been suggested as a marker of an organism's biological age for several reasons (Citation7). Telomere length is heritable, reduces progressively with increasing chronological age, it varies significantly among individuals, it is linked to the biology of ageing as it triggers cellular senescence, and it is influenced by the balance of oxidative stress and anti-oxidative defense mechanisms and possible pharmacological therapy (Citation22,Citation23). Interestingly, the lowest quartile deviated most from the other three quartiles of telomere length in our study. This is in accordance with the observation that telomeres reaching a critical threshold cause genomic instability, senescence, or apoptosis (Citation6,Citation7). In our study, as well as in all other studies with markers of biological age, telomere length was strongly affected by chronological age as defined by date of birth. In contrast to many other markers, however, telomere length provides an attractive mechanism to bring together different strands in the etiology of CHF (Citation7,Citation24).
The current study does not allow us to draw conclusions concerning the cause or consequence of the association between telomere length and heart failure, and this will remain difficult with any future studies as well. However, several recent experimental and clinical studies have suggested an emerging role for telomere biology in CHF. In mice in which telomerase was genetically knocked out, telomere shortening in subsequent generations was associated with the development of overt CHF (Citation25). In observational studies, reduced circulating leukocyte telomere length has been associated with factors commonly present in CHF, including oxidative stress, activation of the renin-angiotensin system, diabetes, smoking, sedentary life-style, psychological stress, coronary artery disease, and a reduced left ventricular ejection fraction (Citation8,Citation10,Citation12,Citation15,Citation26). Telomere length can therefore be considered a marker of the global burden of stress which could explain its association with CHF, coronary artery disease (CAD), and other age-related diseases. In addition, leukocyte telomere length of circulating cells is also shorter in offspring of patients with CAD compared to offspring from healthy parents (Citation27). We now add important clinical data by demonstrating the relevance of telomere length for risk of death and hospitalization for CHF. Interestingly, telomere stabilization in neutrophils by reactivation of telomerase in the microenvironment of the atherosclerotic plaques might lead to coronary instability (Citation28). Shorter telomere length in patients with CHF is not limited to their circulating leukocytes but correlates well with telomere length of and functionality of their bone-marrow (Citation29,Citation30). Thus, it can be speculated that shorter circulating leukocyte telomere length might mark exhaustion of the proliferative capacity of bone-marrow stem cells. Since progenitor cells have been attributed a role in neoangiogenesis, rejuvenation of the endothelial monolayer, and are considered for cell-based therapy, exhaustion of these cells might affect outcome (Citation31). Lower hemoglobin levels and higher red cell distribution was found to be a very strong predictor of outcome in CHF (Citation32). Erythropoietin treatment is even considered as a new treatment for CHF (Citation33,Citation34). Our finding that reduced telomere length is correlated with lower hemoglobin levels might be another indicator of reduced bone-marrow functionality. Furthermore, telomere length of circulating leukocytes correlates well with vascular telomere content and is indicative of changes in vascular tissue (Citation35).
Renal dysfunction is also a frequent co-morbidity and powerful predictor of mortality in patients with CHF (Citation36). Earlier attempts to relate renal function directly to vascular dysfunction, inflammation, or systemic parameters of the renin-angiotensin system have failed (Citation37). The current study confirms previous cross-sectional data association reduced telomere length and with reduced kidney function in patients with CHF (Citation38). We hypothesize that at some point, a functionally challenged nephron is irreversibly shut down, but what abnormality triggers this event and if telomeres are causally involved remain to be elucidated.
We have not tested the practicability or cost-effectiveness of telomere measurements as potential routine measurements in clinical practice. Currently, studies are on-going to determine longitudinal changes and the effect of pharmacotherapy on telomere attrition rate. These findings will help us to further consider telomere length measurements as potential clinical biomarkers. With the understanding of the complexity of telomere biology in CHF, it is interesting to note that our simple quantitative polymerase-chain-reaction-based method of determining leukocyte telomere length was associated with different outcomes in patients with CHF. Our results suggest that telomere length measurements could be a new prognostic marker, but more importantly it suggests involvement of a new potential genetic mechanism in the pathophysiology of CHF which could have significant implications for both our understanding and the identification of novel therapeutic targets.
Acknowledgements
We like to thank all the patients, investigators, heart failure nurses, and the committees of the COACH study (for names see Jaarsma et al. (Citation17)). We are indebted to Germaine Benus for her excellent technical support. This work was supported by Netherlands Heart Foundation (Grant 2000Z003 and Grant 2006B140) and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VENI, grant 916.76.170 to P. van der Harst). P. van der Harst and R. A. de Boer are research fellows of the Netherlands Heart Foundation (grant 2006T003 and 2004T004, respectively) and the Interuniversitair Cardiologisch Instituut Nederland (ICIN). N. J. Samani holds a British Heart Foundation Chair. D. J. van Veldhuisen and A. A. Voors are Established Investigators of the Netherlands Heart Foundation (grant D97-017 and 2006T037, respectively).
Declaration of interest: The authors report no conflicts of interest.
References
- Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007; 356:1140–51.
- Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, . Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007; 357:2248–61.
- van der Harst P, Voors AA, van Gilst WH, Bohm M, van Veldhuisen DJ. Statins in the treatment of chronic heart failure: a systematic review. PLoS Med. 2006; 3:e333.
- Taha M, Lopaschuk GD. Alterations in energy metabolism in cardiomyopathies. Ann Med. 2007; 39:594–607.
- Karkkainen S, Peuhkurinen K. Genetics of dilated cardiomyopathy. Ann Med. 2007; 39:91–107.
- Blackburn EH. Telomere states and cell fates. Nature. 2000; 408:53–6.
- Samani NJ, van der Harst P. Biological ageing and cardiovascular disease. Heart. 2008; 94:537–9.
- Wong LS, de Boer RA, Samani NJ, van Veldhuisen DJ, van der Harst P. Telomere biology in heart failure. Eur J Heart Fail. 2008; 10:1049–56.
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, . Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007; 369:107–14.
- Collerton J, Martin-Ruiz C, Kenny A, Barrass K, von Zglinicki T, Kirkwood T, . Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. Eur Heart J. 2007; 28:172–6.
- Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, . Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003; 22:131–9.
- Vasan RS, Demissie S, Kimura M, Cupples LA, Rifai N, White C, . Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system. The Framingham Heart Study. Circulation. 2008; 117:1138–44.
- Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, . Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003; 93:604–13.
- van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, . Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007; 49:1459–64.
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, . Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004; 101:17312–5.
- Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, . Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating study evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med. 2008; 168:316–24.
- Jaarsma T, van der Wal MH, Hogenhuis J, Lesman I, Luttik ML, Veeger NJ, . Design and methodology of the COACH study: a multicenter randomised Coordinating study evaluating Outcomes of Advising and Counselling in Heart failure. Eur J Heart Fail. 2004; 6:227–33.
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006; 60:174–80.
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003; 361:393–5.
- Martin-Ruiz CM, Gussekloo J, van Heemst D, Von Zglinicki T, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005; 4:287–90.
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, . Telomere length predicts survival independent of genetic influences. Aging Cell. 2007; 6:769–74.
- Von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005; 5:197–203.
- Spyridopoulos I, Haendeler J, Urbich C, Brummendorf TH, Oh H, Schneider MD, . Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 2004; 110:3136–42.
- Patel JV, Sosin M, Gunarathne A, Hussain I, Davis RC, Hughes EA, . Elevated angiogenin levels in chronic heart failure. Ann Med. 2008; 40:474–9.
- Wong LS, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P. Telomere biology in cardiovascular disease: the TERC-/- mouse as a model for heart failure and ageing. Cardiovasc Res. 2009; 81:244–52.
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, . The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008; 168:154–8.
- Brouilette SW, Whittaker A, Stevens SE, van der Harst P, Goodall AH, Samani NJ. Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart. 2008; 94:422–5.
- Narducci ML, Grasselli A, Biasucci LM, Farsetti A, Mule A, Liuzzo G, . High telomerase activity in neutrophils from unstable coronary plaques. J Am Coll Cardiol. 2007; 50:2369–74.
- Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, . Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007; 49:2341–9.
- Spyridopoulos I, Erben Y, Brummendorf TH, Haendeler J, Dietz K, Seeger F, . Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol. 2008; 28:968–74.
- Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008; 28:208–16.
- Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, . Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007; 50:40–7.
- Lipsic E, Westenbrink BD, van der Meer P, van der Harst P, Voors AA, van Veldhuisen DJ, . Low-dose erythropoietin improves cardiac function in experimental heart failure without increasing haematocrit. Eur J Heart Fail. 2008; 10:22–9.
- Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, . Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007; 28:2018–27.
- Wilson WR, Herbert KE, Mistry Y, Stevens SE, Patel HR, Hastings RA, . Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J. 2008; 29:2689–94.
- Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006; 113:671–8.
- van der Harst P, Smilde TD, Buikema H, Voors AA, Navis G, van Veldhuisen DJ, . Vascular function and mild renal impairment in stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2006; 26:379–84.
- van der Harst P, Wong LS, de Boer RA, Brouilette S, van der Steege G, Voors AA, . Possible association between telomere length and renal dysfunction in patients with chronic heart failure. Am J Cardiol. 2008; 102:207–10.
