Abstract
Background. A low adiponectin level is associated with high blood pressure which, in turn, often results in left ventricular hypertrophy. We evaluated the association between plasma adiponectin concentrations and echocardiographic measurements, including left ventricular mass index (LVMI), in 933 middle-aged subjects consisting of 453 hypertensives and 480 controls.
Methods. Plasma adiponectin concentrations were measured with an enzyme-linked immunosorbent assay (ELISA) method. One experienced cardiologist performed echocardiographic examinations, and LVMI was calculated according to Devereux’s method.
Results. Low plasma adiponectin levels were independently associated with increased intraventricular septum thickness, posterior ventricular wall thickness, and left ventricular mass index (P<0.001) in the whole cohort. In the subgroup analysis, the association between these echocardiographic parameters and adiponectin concentrations was observed only in the hypertensive cohort although fractional shortening revealed an association with adiponectin levels also in the control cohort (P=0.021). Findings remained significant after adjustment for the major risk factors for LVMI, such as age, sex, smoking, and systolic blood pressure.
Conclusions. This study in a large population sample detected an association between low plasma adiponectin concentration and LVMI, a marker of left ventricular hypertrophy. This association may be one of the factors that could explain the reported increased cardiovascular risk in subjects with low adiponectin levels.
Key messages
Adiponectin is negatively associated to left ventricular mass index and it protects from the development of left ventricular hypertrophy.
| Abbreviations | ||
| AMP | = | adenosine monophosphate |
| ANOVA | = | analysis of variance |
| BMI | = | body mass index |
| BP | = | blood pressure |
| BSA | = | body surface area |
| ELISA | = | enzyme-linked immunosorbent assay |
| GLM | = | general linear model |
| LV | = | left ventricular |
| LVH | = | left ventricular hypertrophy |
| LVM | = | left ventricular mass |
| LVMI | = | left ventricular mass index |
| NO | = | nitrogen oxide |
| OPERA | = | Oulu Project Elucidating Risk of Atherosclerosis |
Introduction
It is now widely recognized that adipose tissue is hormonally active, secreting a large number of proteins, such as a peptide-hormone adiponectin (Citation1,Citation2). In contrast to the other products of adipose tissue, the plasma adiponectin concentrations are much lower in obese than in non-obese subjects (Citation2). Furthermore, low plasma adiponectin concentrations have been associated with insulin resistance, type 2 diabetes, and metabolic syndrome (Citation3–6).
It has been shown that a low plasma adiponectin concentration is an independent risk factor for hypertension (Citation7,Citation8). Adiponectin has also a protective effect against atherogenesis, acting on the endothelium (Citation8) and smooth muscle cells, elevating NO secretion, and inhibiting the production of adhesion factors (Citation9). In the heart, adiponectin prevents cardiomyocyte hypertrophy and myocardial fibrosis (Citation10,Citation11), though the mechanisms involved are poorly understood. The circulating adiponectin levels have also been shown to have prognostic significance in evaluating the development of cardiovascular diseases in human patients (Citation11).
Left ventricular hypertrophy (LVH) is a common complication in patients with high blood pressure. LVH is an independent risk factor for cardiovascular morbidity and mortality in both hypertensive patients (Citation12,Citation13) and the general population (Citation14). It is known that a low plasma adiponectin concentration is an independent predictor of the presence of moderate to severe left ventricular dysfunction in a population of patients referred for coronary angiography (Citation15). A few studies have indicated that there is a negative correlation between plasma adiponectin and left ventricular mass or mass index (Citation16–19). However, the number of subjects in these studies has been low.
The purpose of our study was to further clarify the correlation between plasma adiponectin levels and echocardiographic measurements, such as left ventricular mass index, in a study population consisting of 993 middle-aged subjects of the hypertensive (453 subjects) and control (480 subjects) cohorts of the Oulu Project Elucidating Risk of Atherosclerosis (OPERA). One strength of the present study is its large sample size.
Methods
Subjects
This study is a part of the OPERA project, which is a population-based, epidemiological study designed to address the risk factors and disease end-points of atherosclerotic cardiovascular diseases. The study population and selection criteria have been previously described in detail (Citation20). The study population consists of a hypertensive cohort (300 men and 300 women) and a control cohort (300 men and 300 women) living in the city of Oulu and who were 40–59 years old at the time of recruitment. The hypertensive cohort was randomly selected by age stratification (15 men and 15 women per year) from the Social Insurance Institute register for reimbursement of anti-hypertensive medication. According to the register they were entitled to a special refund (higher reimbursement class) of antihypertensive medication. For each hypertensive subject, an age- and sex-matched control was randomly selected from the national health register (including all inhabitants) excluding any subjects with the right to reimbursement for hypertension medication. The study was approved by the Ethical Committee of the University of Oulu, and all the subjects volunteered to participate.
Clinical measurements
At the visit, anthropometric measurements (weight, height, waist, hip) and blood pressure (BP) measurements were carried out. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Body surface area was determined by the Dubois equation (Citation21). All blood pressure measurements were recorded with an automatic oscillometric blood pressure recorder (Dinamap, Critikon Ltd, Ascot, UK), and the procedure of measurement was according to the recommendations of the American Society of Hypertension. The resting blood pressure was measured a total of three times at 1-minute intervals on the right arm after the subject had been seated for a minimum of 5 minutes. The blood pressure value used in the analyses is the mean value of the second and the third blood pressure measurement.
Laboratory analyses were acquired after an overnight fast. Plasma was separated by centrifugation and stored at −20°C. Plasma adiponectin concentrations were measured with an enzyme-linked immunosorbent assay (ELISA) with a method devised in the Laboratory of the Department of Internal Medicine in Oulu (Citation6).
Echocardiographic methods
A Hewlett-Packard ultrasound colour system, Sonos 500 (Hewlett-Packard Company, Massachusetts, USA), was used for the echocardiographic examinations. All procedures were performed by one experienced cardiologist (M.I.), who was blinded to the other data and grouping of the study subjects. M-mode measurements were obtained under 2-D guidance according to the recommendations of the American Society of Echocardiography (Citation22). The left ventricular mass (LVM) was calculated using the formula of Devereux et al. (Citation23) and the left ventricular mass index (LVMI) by dividing the difference between the LVM values by body surface area. Fractional shortening was calculated by dividing the difference between the left ventricular internal dimensions in diastole and systole by the diastolic dimension and then multiplying that value by 100. Transmitral flow velocities were measured by pulsed Doppler at the tips of the mitralleaflets from an apical transducer position, and the ratio of maximum early and late diastolic filling was calculated.
Statistical methods
The data were analysed with SPSS version 15.0. The data are presented as mean (standard deviation (SD)) unless otherwise stated. Analysis of variance (ANOVA) was used to test the differences in variable means between the hypertensive and control cohorts. The adjustment for confounding factors was performed using the analysis of covariance in the general linear model (GLM). P-values <0.05 were considered statistically significant.
Results
shows the general characteristics of the subjects in the hypertensive and control cohorts. Hypertensive subjects were heavier, more often smokers, had larger body surface area, higher systolic and diastolic blood pressure, and lower plasma adiponectin concentrations in comparison with the control subjects. The types of antihypertensive therapy are given in .
Table I. General characteristics of study groups.
Table II. Use of selected medications among the hypertensive and control cohorts by gender.
The echocardiographic measurements were performed in both hypertensive and control cohorts, and they are shown in . The hypertensive subjects displayed higher results (P from <0.001 to 0.035) in all measurements, except for the left ventricle internal dimension, compared to the control subjects.
Table III. The echocardiographic measurements in relation to study group.
In order to analyse the association between plasma adiponectin levels and the echocardiographic measurements, the cohort was subdivided into adiponectin quartiles. The cardiac ultrasound indexes in relation to adiponectin quartiles in all subjects before and after adjustments are presented in . The plasma adiponectin concentrations were inversely associated with all the echocardiographic measurements. The highest adiponectin quartile always showed the lowest value (P<0.001), except for fractional shortening which did not exhibit any significant association. Even after adjustment for age, sex, pack-years, and systolic blood pressure, the association persisted between adiponectin quartiles and intraventricular septum thickness (P=0.009), posterior ventricular wall thickness (P=0.003), left ventricular (LV) mass (P=0.001) and LV mass index (P=0.028) ().
Table IV. The echocardiographic measurements in the four adiponectin quartiles in all of the subjects.
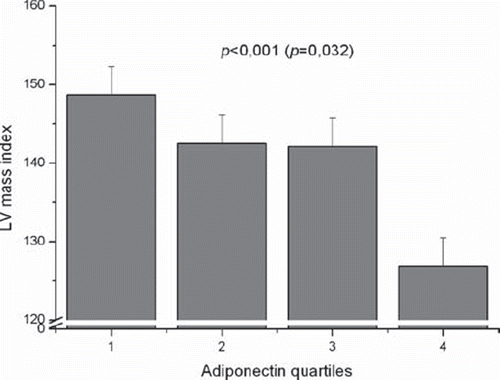
The hypertensive () and control cohorts were analysed separately. The results demonstrate that among the hypertensive cohort all the unadjusted echo measurements (P<0.001), with the exception of fractional shortening, were inversely associated with plasma adiponectin quartiles. The association between adiponectin quartiles and intraventricular septum thickness (P=0.025), posterior ventricular wall thickness (P=0.013), LV mass (0.003), and LV mass index (P=0.032) remained statistically significant after adjustment for age, sex, pack-years, and systolic blood pressure.
Table V. The echocardiographic measurements in the four adiponectin quartiles in the hypertensive cohort.
The association between echo measurements and adiponectin quartiles observed in the hypertensive cohort was also observed in the control cohort before adjustment for confounding variables. However, after adjustments, only the association between adiponectin quartiles and fractional shortening persisted (P=0.021) (data not shown).
All the association analyses were also performed with further adjustment for BMI. These results suggested that adjustment for total adiposity converted all of the associations to a non-significant level. However, LV mass index was not adjusted for BMI since body weight is already included in the Devereux formula, but adjustment for other conventional risk factors did not alter the association to any remarkable extent (P=0.028 for the whole study group, P=0.032 for the hypertensive cohort, and P=0.112 for the control cohort) ( and ).
Figure 1. Left ventricular (LV) mass index in the four adiponectin quartiles in the whole study group. P<0.001 before and P=0.028 (in parentheses) after adjustment for age, sex, pack-years, and systolic blood pressure. 1=the lowest quartile, 4=the highest quartile.
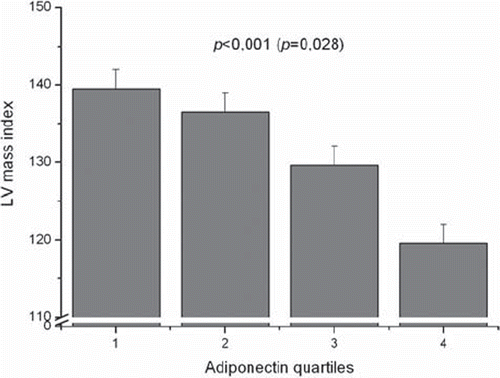
Figure 2. Left ventricular (LV) mass index in the four adiponectin quartiles in the hypertensive cohort. P<0.001 before and P=0.032 (in parentheses) after adjustment for age, sex, pack-years, and systolic blood pressure.
We also divided hypertensive patients according to LV geometric pattern (data not shown). When LVMI quartiles were considered, plasma adiponectin levels (adjusted for age, sex, pack-years, and systolic blood pressure) were the lowest among those belonging to the highest LVMI quartile, and the trend was significant in the whole cohort (P=0.002).
Discussion
The major finding of the present study was that low plasma adiponectin levels were associated with the values of several echocardiographic parameters, such as increased intraventricular septum thickness, posterior ventricular wall thickness, and left ventricular mass index (LVMI). In the subgroup analysis, the association between these echocardiographic parameters and adiponectin concentrations was observed only in the hypertensive cohort, although fractional shortening showed an association with adiponectin levels also in the control cohort. Our findings remained statistically significant after adjustments for the major commonly recognized risk factors for LVMI, such as age, sex, smoking, and systolic BP. As far as we are aware, this is the first study reporting an association between plasma adiponectin concentrations with echocardiographic parameters estimated with such a large sample size.
LVMI is associated with left ventricular hyper-trophy and therefore with cardiovascular morbidity and mortality (Citation12–14). Some small previous studies have indicated that low plasma adiponectin levels are correlated with increased LVMI (Citation16–19), though the mechanisms are poorly understood (Citation11). An accumulating number of previous studies, including ours (Citation6), have reported that low adiponectin concentrations are linked to elevated blood pressure (Citation24–27) which is one of the most important factors influencing the development of LVH. In the present study, the association between plasma adiponectin concentrations and left ventricular mass index in the general linear model remained significant after adjustment for systolic blood pressure in the hypertensive cohort. Plasma adiponectin levels are also negatively related to obesity and associated co-morbidities (Citation3–5). Obesity increases left ventricular mass and is a considerable confounding factor. However, body weight is already included in the formula of left ventricular mass index, and total adiposity has thus already been taken into account in the definition of LVMI according to Devereux’s method. Thus it appears that conventional risk factors do not explain the association between low plasma adiponectin and elevated LVMI. Therefore, some additional mechanisms need to be considered. Cardioprotective effects of adiponectin have been demonstrated in animal studies. It has been speculated that these are mediated by the activation of adenosine monophosphate (AMP)-activated protein kinase (Citation10,Citation28). In addition, adiponectin has beneficial effects on endothelium (Citation8,Citation9) and cardiomyocytes, preventing their hypertrophy and fibrosis. It has been suggested that adiponectin may act as a suppressor of angiotensin II-stimulated myocyte hypertrophy (Citation19). Adiponectin could also play a role in left ventricular remodelling, but this has not yet been investigated in humans (Citation17). A low level of adiponectin may be an indicator of LVH acting through a common cardiovascular pathway involving haemodynamic, paracrine/endocrine, and/or genetic mechanisms (Citation29).
The negative association between adiponectin and echocardiographic measurements was seen only in hypertensive patients who also had lower plasma adiponectin levels than the control subjects. There are several potential explanations to account for this marked difference between cohorts. First, it is possible that adiponectin levels are down-regulated only in the advanced state of left ventricular hypertrophy, which could explain the lack of association in controls, in whom the LVMI was generally lower than in hypertensives. Second, hypertensive subjects had lower plasma adiponectin concentrations compared to controls. These reduced levels seem to be suppressed even more, by some unknown mechanism, if there is some type of end-organ damage, such as left ventricular hypertrophy.
It is now known that there are different isoforms of adiponectin: e.g. low- and high-molecular weight. The high-molecular weight form of adiponectin is known to be the biologically active form. In a previous study (Citation17), the level of high-molecular weight adiponectin, but not the low-molecular weight version, was associated with ventricular wall thickness. Unfortunately, in our study the different adiponectin isoforms were not measured.
In conclusion, we have shown in our large population-based study that total adiponectin concentrations and LVMI are negatively associated even after adjustment for the commonly recognized risk factors for left ventricular hypertrophy. In stratified analyses, this association was seen only in the hypertensive subjects. Experimental and prospective studies are warranted to elucidate the role of adiponectin in left ventricular hypertrophy.
Acknowledgements
This study was supported by the Medical Council of the Academy of Finland and the Finnish Foundation for Cardiovascular Research. We acknowledge the excellent technical assistance of Ms Helena Kalliokoski, Ms Saija Kortetjärvi, Ms Sirpa Rannikko, and Ms Liisa Mannermaa.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, . Paradoxical decrease of an adiposespecific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83.
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–12.
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, . Plasma concentrations of a novel, adiposespecific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9.
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, . Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5.
- Stefan N, Stumvoll M. Adiponectin—its role in metabolism and beyond. Horm Metab Res. 2002;34:469–74.
- Santaniemi M, Kesäniemi YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006;155:745–50.
- Sung S, Chuang S, Sheu W, Lee W, Chu P, Chen C. Adiponectin, but not leptin or high-sensitivity C-reactive protein, is associated with blood pressure independently of general and abdominal adiposity. Hypertens Res. 2008;31:633–40.
- Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14.
- Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27–35.
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, . Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9.
- Maia-Fernandes T, Roncon-Albuquerque R Jr, Leite-Moreira AF. Cardiovascular actions of adiponectin: pathophysiologic implications. Rev Port Cardiol. 2008;27:1431–49.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;323:1706–7.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101–7.
- Cavusoglu E, Chopra V, Battala V, Ruwende C, Yanamadala S, Eng C, . Baseline plasma adiponectin levels as a predictor of left ventricular systolic dysfunction in patients referred for coronary angiography. Am J Cardiol. 2008;101:1073–8.
- Ebinç H, Ebinç FA, Ozkurt ZN, Doğru MT, Tulmaç M, Yilmaz M, . Impact of adiponectin on left ventricular mass index in non-complicated obese subjects. Endocr J. 2008;55:523–8.
- Kozakova M, Muscelli E, Flyvbjerg A, Frystyk J, Morizzo C, Palombo C, . Adiponectin and left ventricular structure and function in healthy adults. J Clin Endocrinol Metab. 2008;93:2811–8.
- Top C, Sahan B, Onde ME. The relationship between left ventricular mass index and insulin sensitivity, postprandial glycaemia, and fasting serum triglyceride and adiponection levels in patients with type 2 diabetes. J Int Med Res. 2007;35:909–16.
- Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–42.
- Rantala AO, Kauma H, Lilja M, Savolainen MJ, Reunanen A, Kesäniemi YA. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Intern Med. 1999;245:163–74.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. The committee on M-mode standardization of the American Society of Echocardiography: recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83.
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Gampo E, Sachs I, . Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8.
- Della Mea P, Lupia M, Bandolin V, Guzzon S, Sonino N, Vettor R, . Adiponectin, insulin resistance, and left ventricular structure in dipper and nondipper essential hyper-tensive patients. Am J Hypertens. 2005;18:30–5.
- Matsuzawa Y. Adiponectin: Identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler Suppl. 2005;6:7–14.
- Bełtowski J, Jamroz-Wiśniewska A, Widomska S. Adiponectin and its role in cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets. 2008;8:7–46.
- Nishida M, Funahashi T, Shimomura I. Pathophysiological significance of adiponectin. Med Mol Morphol. 2007;40:55–67.
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–17.
- Blake J, Devereux RB, Herrold EM, Jason M, Fisher J, Borer JS, . Relation of concentric left ventricular hypertrophy and extracardiac target organ damage to supranormal left ventricular performance in established essential hypertension. Am J Cardiol. 1988;62:246–52.
