Abstract
Aims. We examined the prevalence and prognostic impact of poor R-wave progression (PRWP) in a standard electrocardiogram (ECG) in a general population.
Methods. Data and standard resting ECG recording were collected from a large nationally representative (random sample) health examination survey conducted in Finland in 2000–2001. The final study population consisted of 5613 individuals.
Results. The prevalence of PRWP (defined as RV3 ≤ 3 mm and RV2 ≤ RV3) was 7.0% in women and 2.7% in men (P≤ 0.001 for difference). During follow-up of 70 ± 9 months (mean ± SD), 317 patients died (5.6%). Both all-cause and cardiovascular mortality was higher in the group with PRWP than in those without PRWP in both women and men. In Cox regression analysis after adjustment for age, hypertension, diabetes, previous myocardial infarction, and coronary heart disease, the relative risk for all-cause mortality for PRWP was 1.69 (95% CI 0.89-3.22, P=0.112) for men and 2.00 (95% CI 1.28-3.13, P=0.002) for women. For cardiovascular mortality the relative risk for individuals with PRWP was 1.85 (0.74-4.65, P=0.19) for men and 3.02 (1.54-5.93, P=0.001) for women.
Conclusions. PRWP is a common ECG finding and predicts risk for total and cardiovascular mortality in women in a general population.
| Abbreviations | ||
| AP | = | angina pectoris |
| BMI | = | body mass index |
| BP | = | blood pressure |
| CHD | = | coronary heart disease |
| ECG | = | electrocardiogram |
| HDL | = | high-density lipoprotein |
| IL | = | Illinois |
| LAH | = | left anterior hemiblock |
| LBBB | = | left bundle branch block |
| LDL | = | low-density lipoprotein |
| LVH | = | left ventricular hypertrophy |
| MI | = | myocardial infarction |
| mRNA | = | messenger ribonucleic acid |
| NC | = | North Carolina |
| PRWP | = | poor R-wave progression |
| RBBB | = | right bundle branch block |
| RRWP | = | reverse R-wave progression |
| WHO | = | World Health Organization |
| WI | = | Wisconsin |
Key messages
Poor R-wave progression is a common electrocardiogram finding and predicts risk for total and cardiovascular mortality in women in a general population.
Introduction
Electrocardiogram (ECG) changes in the acute setting of a myocardial infarction (MI) are usually characteristic enough to allow confirmation of the diagnosis together with elevated biochemical markers of myocardial injury. Recognition of healed MI is more difficult. Once the ST-T changes stabilize or resolve, only abnormal Q-waves remain (Citation1,Citation2) . In some patients, MI-related Q-waves may regress or disappear in 4 weeks and in 10%–20% of patients over a 1–2 year period (Citation2–4). Moreover, loss of anterior depolarization forces due to anterior myocardial infarction has long been established clinically and experimentally to produce the abnormally low R-wave amplitude extending from the right into the mid or left precordial leads (Citation5–7) . This ECG phenomenon, termed poor R-wave progression (PRWP), is a troublesome clinical finding. Although in many cases indicating myocardial infarction of the anterior wall, the finding is often seen in patients with a variety of cardiac disorders and not infrequently in apparently normal subjects. PRWP or reverse R-wave progression (RRWP) may appear in the presence of incomplete or complete left bundle branch block (LBBB), right bundle branch block (RBBB), left ventricular hypertrophy (LVH), left anterior hemiblock (LAH), pseudo-Q-wave caused by perpendicular orientation of the initial QRS deflection to the lead axis, mitral valve prolapse, and abnormally low diaphragm position in pulmonary emphysema (Citation8). In normal subjects without evident cardiac or pulmonary disease, the ECG pattern may be caused by a shift of the transitional zone to the left or by an abnormally high placement of the mid-precordial chest leads.
PRWP was observed in 19% of women and 11% of men who were hospitalized adult patients (Citation9) . In a university hospital setting with adult patients, the prevalence of PRWP and RRWP was 7%–10% and 1%–2%, respectively (Citation10) . PRWP is a frequent abnormal ECG pattern faced in insurance medicine (Citation11) . In 1250 symptomatic patients who were evaluated for suspected angina pectoris, PRWP was present in 8%, with equal distribution in both sexes (Citation5) . However, the prevalence and clinical significance of the ECG finding in the general population is not well known (Citation12).
Therefore, the aim of the present study was to examine the prevalence and prognostic impact of PRWP in standard resting ECG in a general population. In addition, we tested separately for men and women whether PRWP increases the risk of all-cause and cardiovascular mortality.
Materials and methods
This study is based on the Health 2000 Survey, a major Finnish population study. It was carried out in 2000-2001, and a representative stratified random cluster sample of the Finnish population was examined. For the population aged S=80 years, the sampling probability was twice as high as among those <80. After a home interview, a comprehensive health examination, including questionnaires, measurements (e.g. blood pressure, resting ECG), and physician's physical examination, was performed. The implementation of the survey is described in detail elsewhere (Citation13). One of the goals of the Health 2000 Survey was to obtain contemporary information about major diseases in Finland.
The Health 2000 sample comprised 8028 individuals (3637 men and 4391 women) aged 30 + , of whom 79% (6354 individuals; 2876 men and 3478 women) participated in the health examination. The National Hospital Discharge Register and the national register on rights to reimbursements for medication costs were linked to the Health 2000 Survey data. The study protocol of the Health 2000 survey was approved by the Epidemiology Ethics Committee of the Helsinki and Uusimaa Hospital District. The participants in the survey signed an informed consent both before the health interview and at the beginning of the health examination.
Laboratory tests
Venous blood samples were drawn from the ante-cubital vein. High-density lipoprotein (HDL) cholesterol, total cholesterol, triglyceride, and plasma glucose concentrations were determined enzymatically (Roche Diagnostics, GmbH, Mannheim, Germany for HDL; Olympus System Reagent, Hamburg, Germany for total cholesterol, triglycerides, and glucose) with a clinical chemistry analyzer (Olympus, AU400, Hamburg, Germany). Low-density lipo-protein (LDL) cholesterol was calculated with the Friedewald formula.
ECG registration and analysis
Standard 12-lead ECGs were recorded in the resting supine position using recommended standardized procedures and MAC 5000 recorder (by Marquette Hellige, Freiburg, Germany and Milwaukee, WI, USA). ECG was recorded and printed using the paper speed of 50 mm/sec. The maximal filter setting of the system (150 Hz) was used. The ECG analyses were performed by a Health 2000 investigator blinded to the clinical data of the patient. The Minnesota coding was performed at the Institute of Cardiology, Kaunas Medical Academy, Lithuania by two investigators, who also were blinded to the clinical data of the patient. ECGs were obtained successfully in 6318 individuals (99%) who attended the health examination. ECG data were stored electronically and transmitted in dispatches of approximately 100 ECGs per transmission to the National Institute for Health and Welfare for further analysis. Abnormalities identified visually in the ECG strips were coded in accordance with the Minnesota coding scheme (Citation14). The electrical recordings were analyzed by means of Magellan software program (Marquette Electronics Inc., Milwaukee, WI, USA). At this phase, the measurement points were checked and corrected if needed. Nineteen ECGs were rejected owing to data lost in further process, leaving 6299 ECGs for analysis.
Definition of coronary heart disease
The examining physicians followed detailed written instructions and applied uniform diagnostic criteria in accordance with good clinical practice. The examining physician critically assessed history and available documents and performed a structured physical examination. Diagnostic assessments were recorded on structured forms. Information on the rights for drug reimbursements was obtained from the national register. All persons with coronary heart disease (CHD) in Finland are entitled to special reimbursement for medication costs. To obtain that right, they have to apply for it and append a medical certificate by their physician to show that the objective criteria of CHD are fulfilled. The study participants were asked whether they used any medications, and the names and doses of these medications were recorded. Persons with typical angina pectoris (AP) symptoms were identified by the World Health Organization (WHO) chest pain questionnaire. Also, history of coronary by-pass surgery or percutaneous coronary intervention was checked during the interview.
Information on previous hospitalization for MI or CHD was obtained from hospital discharge summaries that study participants brought along or from the National Hospital Discharge Register. The Finnish hospital discharge register has been shown to be valid in identifying major CHD events (Citation15).
Classification as CHD required at least one of the following: diagnosis of MI and/or AP during the field health examination by a physician, large Q-waves in resting ECG, hospitalization for CHD (International Classification of Diseases (ICD)-8 or ICD-9 codes 410–414, or ICD-10 codes I20–I25), a history of coronary revascularization procedure, the right to drug reimbursements for CHD, or the use of nitroglycerine combined with an anticoagulant, acetyl salicylic acid, or beta-blocker. Typical AP symptoms identified by the WHO chest pain questionnaire only were not considered to be an indicator of CHD.
Myocardial infarction (MI)
Classification for MI required either a clinical diagnosis of old MI by the examining physician, large Q-waves in resting ECG, or a previous discharge diagnosis of MI (ICD-8 or ICD-9 code 410, or ICD-10 codes I21–I22). MI was defined as a positive history of the condition in the medical records or old MI on ECG or typical self-reported history of MI treated in hospital. Large Q-waves indicating probable previous MI included Minnesota codes 1.1-1.3.
Other measurements and definitions
Height and weight were measured and body mass index (BMI) calculated. Blood pressure was measured with a mercury sphygmomanometer (Mercuro 300, Speidel & Keller, Juningen, Germany) from the right arm. The first measurement was carried out after at least 5 minutes of rest in the sitting position. Korotkoff's first phase was used as the sign of systolic blood pressure, and the fifth phase as the sign of diastolic pressure. The measurement was repeated 2 minutes after the first measurement. The average of the two measurements was used in the analysis. Clinic hypertension was defined as a clinic blood pressure (BP) ≥ 140/90 mmHg. Diabetes mellitus was defined as a serum glucose level of 7.0 mmol/L or greater or a history of the use of oral hypoglycemic agents or insulin injections. Smoking was defined as the daily use of tobacco products.
Exclusion criteria
Patients with suspected pathological Q/QS in ECG were excluded using Minnesota codes (MC) 1.1-1.3. All the patients with ventricular conduction defects, mainly LBBB or right bundle branch block (RBBB) or LAH, were excluded (MC 7). Also, one individual with ECG signs of the Wolff-Parkinson-White syndrome was excluded. Finally, 5613 ECGs were used for analysis, 3151 from female and 2462 from male individuals.
Definition of PRWP
We defined PRWP as an R-wave in the precordial lead V3 ≤ 3 mm and R in lead V2 ≤ R in lead V3 (Citation16) () . This criterion was first adopted by Zema et al. (in 1980) and has been used commonly in studies concerning PRWP (5,12,16,17) . We did not include RRWP, used in some studies (Citation5), in our definition.
Follow-up
The information about deaths was obtained from the Statistic Finland after follow-up of 70 ± 9 months.
Statistical analyses
For the analyses of prevalence, the data were weighted to reduce the bias due to non-response and to correct for the over-sampling in the age group of 80 years and older. The prevalence of PRWP was determined within the sexes and various age groups. The complex sampling design was taken into account by using SUDAAN procedures version 10.0 (SUDAAN Language Manual; RTI International, Research Triangle Park, NC). The rest of the statistical analyses were performed with the SPSS release 15.0 for Windows (SPSS Inc., Chicago, IL) and SAS version 9.1 (SAS Institute, Inc., Cary, NC). The difference in PRWP prevalence between sexes was determined with logistic regression using PRWP as a dependent and sex as an independent variable. Thereafter, women and men were analyzed in their own groups. Continuous subject characteristics were compared between those with and without PRWP using the t test for independent samples and the chi-square test for dichotomous variables, including mortality. The relative risks of PRWP for all-cause and cardiovascular death were estimated with a Cox proportional hazards model using the following covariates: age, hypertension, diabetes, previous MI, and CHD. P<0.05 was considered statistically significant in all the analyses.
Power calculation was performed for Cox regression separately for men and women. Given two-sided alpha of 0.05, standard deviation of PRWP as well as the number of subjects and cardiovascular deaths, the power to reach clinically meaningful relative risk of 1.5 is 90% for men and 100% for women.
Results
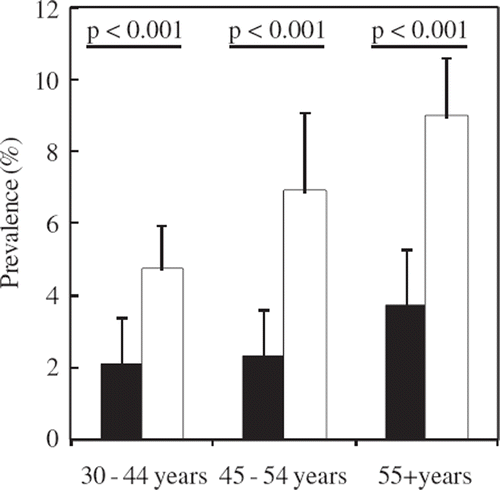
The prevalence of PRWP for three age groups among men and women is shown in . The ECG finding was more frequent in women than in men for all the age groups. Altogether there were 287 subjects with PRWP in their resting ECG (), 2.7% of men and 7.0% of women (P <0.001 for difference between men and women). Women were significantly more prone to have PRWP than men (odds ratio 2.58, P<0.001 in logistic regression).
Table I. Baseline characteristics of The Health 2000 Survey participants.
Figure 2. Prevalence of poor R-wave progression for the three age groups among men (black bars) and women (white bars); the upper 95% confidence interval limits and the significances of the difference between the sexes are shown (chi-square test).
Men and women with PRWP were older, had more diabetes, CHD, and previous MIs, than did those without PRWP, while for hypertension there was a difference between the groups that only concerned females ( and ).
Table II. Clinical characteristics and mortality of the study population.
During follow-up of 70 ± 9 months (mean ± SD), 317 patients died (5.6%); 120 (2.1%) were cardiovascular deaths. Both all-cause and cardiovascular mortality were higher in the group with PRWP than in those without PRWP in both women and men ().
A total of 787 persons (14.0%) fulfilled Minnesota criteria for left or right ventricular hypertrophy (Minnesota code 3.1 and/or 3.3). Thirty-three (4%) individuals with and 254 (5.3%) without ECG markers of ventricular hypertrophy fulfilled criteria for PRWP. A negative P-wave in leads V2 and/or V3 was observed in 65 individuals (1.2%), 36 women and 29 men.
In Cox regression analysis after adjustment for age, the relative risk for all-cause mortality for PRWP was 1.89 (95% CI 1.00-3.59, P=0.051) for men and 2.22 (95% CI 1.42-3.46, P<0.001) for women. For cardiovascular mortality, the relative risk for individuals with PRWP was 2.28 (0.91-5.68, P=0.08) for men and 3.47 (1.78-6.76, P<0.001) for women. When individuals with previous MI (n =166) were excluded, the results remained essentially similar: the relative risk for all-cause mortality for PRWP was 1.78 (95% CI 0.83-3.80, P=0.14) for men and 2.44 (95% CI 1.54-3.89, P<0.001) for women; for cardiovascular mortality the relative risk was 1.33 (0.32-5.49, P=0.69) for men and 3.57 (95% CI 1.71-7.42, P=0.001) for women. When individuals with ventricular hypertrophy were excluded from the analyses, the relative risk for all-cause mortality was 1.78 (95% CI 0.87-3.64, P=0.12) for men and 2.31 (1.42-3.76, P<0.001) for women.
In Cox regression analysis after adjustment for age, hypertension, diabetes, previous MI, and CHD, the relative risk for all-cause mortality for PRWP was 1.69 (95% CI 0.89-3.22, P=0.112) for men and 2.00 (95% CI 1.28-3.13, P=0.002) for women, respectively. For cardiovascular mortality, the relative risk for individuals with PRWP was 1.85 (0.74-4.65, P=0.19) for men and 3.02 (1.54-5.93, P=0.001) for women. When also subjects with inferior and anterior Q-waves were included in the analyses, the relative risk for all-cause mortality for PRWP was 1.86 (95% CI 1.12-3.09, P=0.017) for men and 2.17 (95% CI 1.45-3.24, P<0.001) for women.
For cardiovascular mortality, the relative risk for individuals with PRWP was 2.09 (95% CI 1.02–4.26, P =0.043) for men and 2.88 (95% CI 1.56-5.30, P=0.001) for women.
Discussion
This is, to our knowledge, the first study to show the prevalence of PRWP in a general adult population. In our study population, PRWP proved to be a common ECG finding. Additionally, this ECG phenomenon was more frequent in women than in men in three age groups 30 years or older. Importantly, PRWP predicted risk for total and cardiovascular mortality in women. The findings of the present study could be helpful in screening general populations for risk of total and cardiovascular mortality.
Differences in the reported prevalence of PRWP in non-population-based materials are partly explained by different criteria for the ECG finding. DePace et al. reviewed the resting ECGs in 1250 consecutive patients who underwent thallium-201 scintigraphy (Citation5). Using the same inclusion criteria but slightly different exclusion criteria as in the present study, they reported an 8% prevalence of PRWP in their patients, who all had chest pain or were evaluated for suspected AP At Glasgow Royal Infirmary, the prevalence of PRWP was estimated by reviewing all electrocardiograms (n =1315) recorded over a 2-week period. As in the present study, PRWP was more frequent in women (19% versus 11%) than in men (Citation9) . The authors also found that the positioning of electrodes beneath rather than above the breast was not responsible for the increased prevalence of poor R-wave progression in women with a variety of clinical problems. Negative P-waves in leads V2 and/or V3, a possible sign of high electrode placement (Citation18), was found with a very low prevalence (n =65, 1.2%) and without major impact on the study results.
In the present study, both men and women with PRWP had increased unadjusted total and cardiovascular mortality during an average follow-up of 5.8 years. In Cox regression analysis after adjustment for age, hypertension, diabetes, previous MI, and CHD, PRWP was an independent determinant of both all-cause and cardiovascular mortality in women, but not in men (for cardiovascular mortality the relative risk was 3.02 (P =0.001) for women and 1.85 (P =0.19) for men). When also subjects with inferior and anterior Q-waves were included in the analysis, PRWP independently predicted all-cause and cardiovascular mortality in both sexes. This is not surprising as Q-wave MI is a well documented etiology of PRWP.
Interestingly, the risk increase is in the same range as that reported with the presence of major or minor Q-waves, and higher than the risk associated with minor ST-segment and/or T-wave abnormalities (Citation19,20). The explanation for the finding that PRWP predicted mortality independently of CHD and MI in women but not in men is not fully evident from the present findings, but an explanation could be provided by the fact that PRWP was more often associated with CHD and MI in men than in women (). In accordance, PRWP strongly predicted mortality in women after excluding subjects with previous myocardial infarction from the analysis in the present study. Also, earlier reports support the view that PRWP is strongly associated with CHD and MI and that this association appears to be more often visible in men than in women: in a retrospective study, men with PRWP had a higher probability than did women for anterior wall motion abnormality on echocardiography (Citation21). In the study by DePace et al. (Citation5), men with PRWP had a higher probability for anterior scar than did women by thallium scintigraphy. Zema et al. showed that the relative risk of autopsy-documented anterior MI was 6-fold increased in patients meeting PRWP criteria (Citation22). In another study, wall motion abnormalities were associated with PRWP in patients with left ventricular end-diastolic diameters 5 cm or more, but not in patients with smaller ventricular diameters (Citation23). Another explanation for CHD- and MI-independent predictive power of PRWP in women could be that women had more undiagnosed CHD or MI than did men in the present general population. Altogether, these findings support the view that PRWP could be a useful screening tool for cardiovascular morbidity and mortality in general populations of women.
In our study, men with PRWP had more than two times higher probability for CHD or MI than did women. However, in the vast majority of individuals from both sexes, PRWP was not associated with known CHD or a history of MI. In post-MI left ventricular remodeling, the infarct area undergoes proliferation and differentiation of fibroblasts and other interstitial cells and the elaboration of bioactive molecules which contribute to a robust synthesis of extracellular matrix (ECM) for the purposes of scar formation (Citation24). Reappearance of R-waves in anterior MI was associated with a larger extent of stunned but viable myocardium and a trend towards a smaller amount of necrotic myocardium, compared to patients with persisting Q-waves (Citation25). Magnetic resonance imaging has shown that MI size is associated with Q-waves after ST-elevation MI (Citation26). The fact that we excluded individuals with pathological Q-waves may have resulted in the selection of a relatively low-risk post-MI sub-population of individuals in our study. This in turn could be one explanation for the ECG phenomenon not being associated with poor outcome in males.
It is well known that patients with LVH represent a subgroup of PRWP. To make our study results applicable to a large patient group, we decided not to exclude patients with ECG criteria for LVH from our main analyses. However, excluding individuals with LVH had no major impact on the study results. The prevalence of LVH in the ECG depends on which criteria are chosen for analysis. In this study, Minnesota criteria (including clinically widely used Sokolow-Lyon criteria) were used to define LVH, which may be considered as a study limitation. Another limitation is the fact that echocardiographic data on left ventricular function or LVH were not available in the present study.
The validity of the results of our study is dependent on the representativeness of the study sample. The study was based on a large nationally representative health examination survey conducted in Finland. Participation rate in the health examination survey was high (79%), especially when the home interviews were taken into account (91%). Since non-participation is selective with regard to morbidity and disability, high participation rates are essential in examining the prevalence of a chronic disabling disease. It is possible that persons with the most severe disease were not selected for the health examinations.
In conclusion, PRWP is a common ECG finding and predicts risk for total and cardiovascular mortality in women in a general population. This finding could aid in screening general populations for risk of total and cardiovascular mortality.
Acknowledgements
We thank the personnel in the field and support organizations of the Health 2000 Surveys.
Declaration of interest: no conflicts of interest declared.
References
- Asch FM, Shah S, Rattin C, Swaminathan S, Fuisz A, Lindsay J. Lack of sensitivity of the electrocardiogram for detection of old myocardial infarction: a cardiac magnetic resonance imaging study. Am Heart J. 2006;152:742–8.
- Isobe S, Okada M, Ando A, Nanasato M, Nonokawa M, Izawa H, . Clinical significance of changes in electrocardiographic R-wave voltage on chest leads in patients with acute anterior myocardial infarction. J Electrocardiol. 2002;35:173–80.
- Dwyer EM. The predictive accuracy of the electrocardiogram in identifying the presence and location of myocardial infarction and coronary artery disease. Ann N Y Acad Sci. 1990;601:67–76.
- Okada M, Isobe S, Tanahashi Y, Saka Y. Comparison between changes of R wave on electrocardiogram and myocardial SPECT images in patients with acute myocardial infarction. J Nucl Card. 1999;43:S78.
- DePace NL, Colby J, Hakki AH, Manno B, Horowitz LN, Iskandrian AS. Poor R wave progression in the precordial leads: clinical implications for the diagnosis of myocardial infarction. J Am Coll Cardiol. 1983;2:1073–9.
- Zema MJ, Kligfield P. Electrocardiographic poor R wave progression. I: correlation with the Frank vectorcardiogram. J Electrocardiol. 1979;12:3–10.
- Zema MJ, Kligfield P. Electrocardiographic poor R wave progression. II: correlation with angiography. J Electrocardiol. 1979;12:11–5.
- Surawicz B, Knilans TK. Chou's Electrocardiography In Clinical Practice. 6th. Philadelphia, PA, USA: Saunders Elsevier; 2008. 193–4.
- Colaco R, Reay P, Beckett C, Aitchison TC, Mcfarlane PW. False positive ECG reports of anterior myocardial infarction in women. J Electrocardiol. 2000;33 Suppl:239–44.
- Zema MJ, Kligfield P. ECG poor R-wave progression: review and synthesis. Arch Intern Med. 1982;142:1145–8.
- MacKenzie R. Poor R-wave progression. J Insur Med. 2005;37:58–62.
- Gami AS, Holly TA, Rosenthal JE. Electrocardiographic poor R-wave progression: analysis of multiple criteria reveals little usefulness. Am Heart J. 2004;148:80–5.
- Kattainen A, Salomaa V, Harkanen T, Jula A, Kaaja R, Kesaniemi YA, . Coronary heart disease: from a disease of middle-aged men in the late 1970s to a disease of elderly women in the 2000s. Eur Heart J. 2006;27:296–301.
- Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA: John Wright-PSG, Inc.; 1982.
- Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, . The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–7.
- Zema MJ, Luminais SK, Chiaramida S, Goldman M, Kligfield P. Electrocardiographic poor R wave progression III. The normal variant. J Electrocardiol. 1980;13:135–42.
- Mittal SR, Srivastava P. Differentiation of poor R wave progression of old anteroseptal myocardial infarction from that due to emphysema. Int J Cardiol. 1986;13:92–4.
- Garcia-Niebla J. Comparison of p-wave patterns derived from correct and incorrect placement of V1-V2 electrodes. J Cardiovasc Nurs. 2009;24:156–61.
- Ashley EA, Raxwal VK, Froelicher VF. The prevalence and prognostic significance of electrocardiographic abnormalities. Curr Probl Cardiol. 2000;25:1–72.
- Greenland P, Xie X, Liu K, Colangelo L, Liao Y, Daviglus ML, . Impact of minor electrocardiographic ST-segment and/or T-wave abnormalities on cardiovascular mortality during long-term follow-up. Am J Cardiol. 2003;91:1068–74.
- Stuglin C, DeVilliers S, Akhtar J, Blair T. The relationship of poor R wave progression in precordial leads on 12-lead electrocardiogram and anterior wall motion abnormality on echocardiography. J Am Coll Cardiol. 2004;43:A120.
- Zema MJ, Collins M, Alonso DR, Kligfield P. Electrocardiographic poor R-wave progression. Correlation with postmortem findings. Chest. 1981;79:195–200.
- Yape IMP, Chua WT, Calleja HB, Cabahung RF. Poor R wave progression (PRWP) by elecrocardiography: 2-D echocardiographic (2-D echo) correlation. Circ J. 2002;66 Suppl 1:570.
- Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342.
- Nagase K, Tamura A, Mikuriya Y, Nasu M. Significance of Q-wave regression after anterior wall acute myocardial infarction. Eur Heart J. 1998;19:742–6.
- Moon JC, De Arenaza DP, Elkington AG, Taneja AK, John AS, Wang D, . The pathologic basis of Q-wave and non-Q-wave myocardial infarction: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2004;44:554–60.