Abstract
Background. Several traditional cardiovascular risk factors assessed in the middle-aged are associated with the risk of dementia, but they are known to lose much of their prognostic value when measured in the elderly. The aim of the study was to compare B-type natriuretic peptide (BNP) with previously known risk markers for dementia in their association with cognitive decline and dementia during a follow-up.
Methods. A total of 464 subjects free of dementia aged 75 years or more were examined and followed up for 5 years in a prospective population-based stratified cohort study. The association of clinical variables to base-line Mini Mental State Examination score (MMSE), the decline of MMSE, and onset of dementia during the follow-up were examined.
Results. The only variable to significantly associate with the decline of MMSE was BNP (beta 0.140; P = 0.019). A total of 59 new cases of dementia were diagnosed after the follow-up. Significant predictors of the occurrence of dementia over the study period were BNP (adjusted odds ratio (OR) 1.53; 95% confidence interval (CI) 1.09–2.16; P = 0.013), length of education (OR 0.50; 95% CI 0.33–0.77; P = 0.001), and diagnosis of hypertension (OR 0.53; 95% CI 0.27–0.95; P = 0.036). BNP remained as a significant predictor of dementia and the decline of MMSE even after adjustment to the base-line MMSE.
Conclusions. BNP is an independent harbinger of the cognitive decline and incidence of new onset of dementia in an elderly general population. This is a ground for testing the impact of antihypertensive treatment in the prevention of cognitive impairment in those with elevated BNP.
Key messages
Elevated B-type natriuretic peptide is associated with the higher occurrence of dementia in an elderly general population.
Although traditional cardiovascular risk markers have been showing mixed results in their association with the decline of cognitive function when measured in the elderly, cardiovascular burden remains as a predictor of dementia also in late life.
Introduction
The lifetime risk of developing dementia is 20% for men and 33% for women (Citation1). Due to aging of the population, the incidence and prevalence of various types of dementia are expected to double within the next 50 years among those over 75 years of age (Citation1). Hence, finding clinically usable factors associative with future dementia has been a topic of wide-spread epidemiological and clinical research.
Low level of education, high total cholesterol, obesity, hypertension, diabetes, and smoking in middle age are associated with increased incidence of dementia and cognitive impairment at older age (Citation2–5). However, the link between these risk markers and cognitive dysfunction is less clear in studies conducted in aged populations. Typically, these markers seem to lose their prognostic value in elderly populations and, in some studies, even inverse relations of cholesterol, hypertension, and obesity to dementia have been reported (Citation3,Citation6). Of the traditional cardiovascular risk factors observed in the elderly, only smoking and diabetes have been more invariably connected with the onset of dementia; though, occasionally, these variables have also failed to show an association (Citation2,Citation6).
Observations from small cross-sectional studies have suggested that high levels of B-type natriuretic peptide (BNP) are associated with cognitive impairment in patients with cardiovascular disease (Citation7), and that BNP is associated with the Mini Mental State Examination score (MMSE) among in-hospital heart failure patients (Citation8). Elevated levels of mid-regional pro-atrial natriuretic peptide (MR-proANP), a functionally inactive surrogate of atrial natriuretic peptide, have been associated with Alzheimer's disease in one cross-sectional study (Citation9).
To the best of our knowledge, no previous study has examined whether base-line BNP is a predictor of future decline in MMSE and occurrence of dementia. Neither has BNP been studied for its cross-sectional association with cognitive dysfunction in the general population. The aim of the present study was to compare BNP with other cardiovascular risk markers for their value to predict the decline of cognitive function and new cases of dementia in an elderly general population free of dementia.
Material and methods
Study population
This study is a part of a larger population-based, multidisciplinary Kuopio 75+ health study focusing on the clinical epidemiology of diseases, medication, and functional capacity in elderly persons aged 75 or older. The target population was a stratified random sample (n = 700) of all the inhabitants of the city of Kuopio (eastern Finland), who were aged 75 or older on 1 January 1998 (n = 4518).
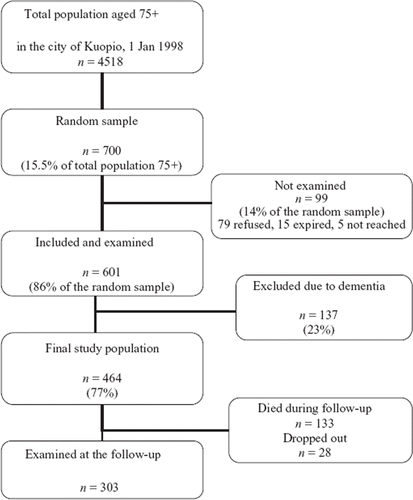
The cohort included 700 participants (). A total of 79 persons refused to take part in the study, 5 could not be contacted, and 15 expired before the examination could take place. These 601 participants attended a structured clinical examination and an interview conducted by a geriatrician and trained nurse. Participants with diagnosed dementia (n = 137) were excluded from this substudy, and the final study population was 464 attendants. A total of 303 participants attended the follow-up visit after 5 years. Of the 161 participants missing, 133 had expired during the study period and 28 either refused to continue the study or could not be contacted.
A geriatrician and trained nurse examined the participants at the out-patient clinic of the municipal hospital and interviewed them regarding their medical history and the use of medicines; medicines in current use were recorded. If a participant was unable to visit the study site, the nurse and the geriatrician visited the home to perform the interview and examination. Medical records from the municipal health centre, home nursing service, local hospitals, and the Kuopio University Hospital were also available. Base-line clinical and demographic data were also recorded. Diabetes was defined as a diagnosis of diabetes in medical records or fasting plasma glucose 7.0 mmol/L or more. Other cardiovascular conditions were recorded from medical records. Blood systolic and diastolic pressures were measured twice, and the average of the measurements was recorded. Depression was screened using Zung's self-rating Depression Scale (Citation10).
Written informed consent was obtained from the study participants or their relatives as stipulated in the Declaration of Helsinki. The study was approved by the ethics committees of the Hospital District of Northern Savo and the Kuopio University Hospital.
Main outcome measures
A history of cognitive decline was obtained by interviewing the subject and relatives, and by examining the medical records. Behavioural and psychiatric symptoms of demented patients were obtained from the relatives or care-giving personnel whenever possible, as well as from the interview and examination. MMSE was used to screen cognitive capacity. As a part of a diagnostic process, brain imaging either by computer tomography (CT) or magnetic resonance imaging (MRI) was carried out for all participants with the suspicion of dementive illness and no brain imaging in medical history. Dementia was diagnosed as Alzheimer's disease, vascular dementia, dementia with Lewy bodies, or dementia due to other medical conditions according to the DSM-IV criteria (Citation11) and the consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (Citation12) by an experienced neurogeriatrician (author RS). The clinical diagnosis of dementia was established, and the type of the dementia determined in consensus meetings, using all the data available.
Laboratory analysis
Basic blood count, creatinine, cholesterol profile, and blood glucose were measured once in the Kuopio University Hospital after 12 hours of fasting. All serum total cholesterol assays were analysed in the Kuopio University Hospital laboratory using standard enzymatic techniques. Creatinine clearance was calculated using creatinine, age, and body-weight according to Cockcroft-Gault's formula. The estimation of low-density lipoprotein (LDL) cholesterol was calculated using Friedewald's formula. The blood samples for natriuretic peptide analysis were withdrawn similarly with other blood samples into chilled tubes containing 1.5 mg K2-EDTA (ethylenediaminetetraacetic acid) per mL blood after the patient had been in a supine position for 30 min at 8 a.m. The whole blood was centrifuged and plasma immediately frozen and stored at −70°C. BNP was extracted from plasma using Sep-Pak C18 cartridges. The radioimmunoassay used for BNP has been previously described (Citation13). The sensitivity of the BNP assays is 0.5 pmol/L. The within and between assay coefficients of variation are <10% and <15%, respectively. With this method, BNP plasma levels of 6.25 ± 2.12 pmol/L (mean ± SD) have been detected in healthy adults aged 20–55. All laboratory data were analysed in random order and blind to clinical data.
Statistical analysis
Base-line characteristics are presented for each BNP tertile (). The differences between the groups were tested with ANOVA-test for independent samples for continuous variables; a prior logarithmic transformation was performed for non-normally distributed variables. The categorical variables were compared using the chi-square test. MMSE at the base-line and the change in MMSE between the two visits (1998 and 2003) were studied as continuous variables. Univariable linear regression analysis was used to determine the impact of various base-line variables on initial MMSE and on the change of MMSE over the study period ().
Table I. Base-line characteristics and drug treatment in all participants (n = 464) according to their B-type natriuretic peptide (BNP) tertiles.
Table II. Associations of clinical variables with base-line MMSE, decline of MMSE and new cases of dementia in 5-year follow-up.
Binary logistic regression models were applied to determine the impact of each base-line variable on the onset of dementia during the follow-up. An additional logistic regression model was constructed for new dementia cases using all the independent variables with significant association in univariable models (). A separate regression model was adjusted to base- line MMSE to determine the value of the variables on the prediction of dementia independent of MMSE ().
Table III. Multivariable logistic regression model presenting adjusted odds ratios and 95% confidence intervals per 1 SD increase of clinical variables for the association with future dementia. The model on the left includes the variables significant in Table II. The model on the right is similar but also adjusted with the base-line MMSE.
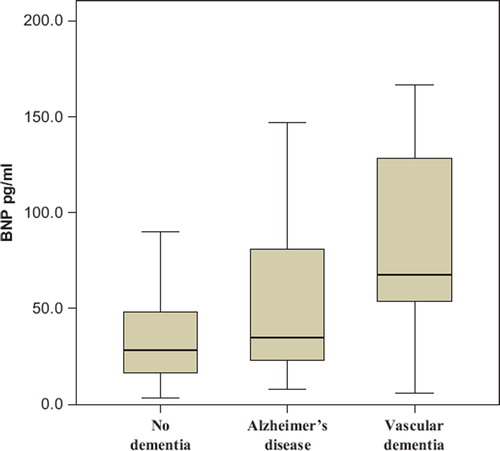
BNP levels at the base-line visit in participants according to their status of dementive illness after the follow-up are illustrated using box plots () with the differences between the groups assessed with ANOVA. The data were analysed using SPSS release 15.0 for Windows (Chicago, Illinois). Statistical differences were considered significant when P < 0.05.
Figure 2. Box plots for B-type natriuretic peptide of the participants. Participants divided to groups according to their follow-up visit dementia status. Participants with no dementia (n = 230) BNP = mean 39.3 (SD 37.2) pg/mL, Alzheimer's disease (n = 42) BNP = 56.9 (57.6) pg/mL, vascular dementia (n = 10) BNP = 79.6 (56.7) pg/mL. ANOVA P = 0.001 in between the groups.
Results
Base-line characteristics
Base-line data according to BNP tertiles are shown in . The participants with a higher level of BNP were more likely to be older and to have a history of heart failure, atrial fibrillation, or stroke than were those with lower level of BNP. Systolic and diastolic blood pressures were lower among the participants with high BNP.
Main outcome measures
The impact of the clinical variables on base-line MMSE as well as on the decline of MMSE and incidence of dementia during the follow-up is presented in . Of the traditional cardiovascular risk factors and illnesses, previously diagnosed heart failure (standardized regression coefficient (beta) 20.141; P = 0.002) and stroke (beta 20.149; P = 0.001), New York Heart Association (NYHA) class (beta −0.177; P < 0.001), resting heart rate (beta −0.127; P = 0.006), haemoglobin (beta 0.125; P = 0.007), creatinine clearance (beta 0.182; P < 0.001), high-density lipoprotein (HDL)-cholesterol (beta 0.174; P = 0.001), and BNP (beta −0.151; P = 0.001 were significantly linked with MMSE. In addition, MMSE at the base-line was associated with age (beta −0.335; P < 0.0001) and length of education (beta 0.392; P < 0.001). No significant association was found between base-line MMSE and total cholesterol, smoking, diagnosis of hypertension, sex, diabetes, BMI, systolic or diastolic blood pressure ().
The change of MMSE during the follow-up of 5 years was, on average, −1.43 (SD 4.95) points. The only variable to predict the decline of MMSE during the follow-up in univariable analysis was BNP (beta 0.140; P = 0.019). When adjusted with the level of base-line MMSE, BNP continued to predict the decline of MMSE (beta 0.143; P = 0.017). The traditional risk factors for cognitive decline such as age, education, smoking, base-line MMSE, or cardiovascular risk markers or illnesses showed no association with the decline of MMSE ().
During the follow-up visit, 59 new cases of dementia were detected. Dementias were further classified as Alzheimer's disease (n = 42), vascular dementia (n = 10), dementia with Lewy bodies (n = 3), and other dementias (n = 4). Using univariable logistic regression (), the variables to predict the incidence of dementia during the follow-up were age (odds ratio (OR) 1.51; 95% confidence interval (CI) 1.14–2.11; P = 0.006), education (OR 0.50; 95% CI 0.32–0.75; P = 0.001), diagnosis of hypertension (OR 0.53; 95% CI 0.27–0.95; P = 0.036), base-line MMSE (OR 0.34; 95% CI 0.23–0.89; P < 0.001), and BNP (OR 1.55; 95% CI 1.13–2.12; P = 0.007). A multivariable regression model was developed for the above-mentioned significant variables. Length of education (OR 0.50; 95% CI 0.33–0.77; P = 0.001), hypertension (OR 0.53; 95% CI 0.27–0.95; P = 0.036), and BNP (OR 1.53; 95% CI 1.09–2.16; P = 0.013) were independent predictors of the new cases of dementia, while age (OR 1.23; 95% CI 0.89–1.88; P = 0.169) lost its significance in the multivariable model. The multivariable model was additionally adjusted to base-line MMSE (for results see ).
Testing was still extended to subgroups of participants without previously diagnosed heart failure (n = 346) and without stroke history (n = 413). In a subgroup without previous heart failure BNP was significantly associated with new dementia cases when tested both alone (OR 1.49; 95% CI 1.07–2.09; P = 0.020) and when tested in multivariable model with age, length of education, and diagnosed hypertension (OR 1.51; 95% CI 1.05–2.18; P = 0.026). In a subgroup free of stroke BNP remained as a significant predictor of future dementia without any material change in results (data not shown). Furthermore, occurrence of stroke was not prognostic about the decline of MMSE or new onset of dementia among all patients.
The BNP levels of the participants with no dementia, Alzheimer's disease, and vascular dementia at the follow-up using box-plot are presented in . When studied separately in a univariable model according to the type of dementia, base-line BNP was predictive of both Alzheimer's disease (OR 1.59; 95% CI 1.09–2.30; P = 0.015) and vascular dementia (OR 2.71; 95% CI 1.31–5.60; P = 0.007).
Discussion
The main finding of our study was an association of BNP with all the end-points: base-line MMSE, the decline of the MMSE during the follow-up, and, most importantly, new diagnoses of demented illnesses during the follow-up. Several previously known risk markers for cognitive impairment such as low educational level, age, and cardiovascular illnesses and risk markers were associated with base-line MMSE, but none of these were linked to the further decline of MMSE over the follow-up in this elderly population free of dementia at the base-line; remarkably, BNP was the only variable to be connected with this outcome. The association between the decline of MMSE and BNP was not explained by the commonly known confounding factors—age, sex, total years of education, depression, or base-line MMSE.
The prevalence of dementia at the base-line in the present study population was 22.8%, being in concordance with previous data (Citation14). Similarly, the high annual mortality of 8.1% during the follow-up was expected in this elderly population. High levels of natriuretic peptides and dementia are both known to associate with excess mortality in the elderly (Citation15,Citation16). The use of diuretics and agents affecting the renin-angiotensin system, known also to lower the level of BNP, was more common among participants with a higher level of BNP in the present study. Hence, high mortality and the use of medication affecting the BNP level might even have attenuated the ability of the BNP to predict cognitive decline.
HDL (but not total) cholesterol was associated with low MMSE score at the base-line, but it did not predict further decline of cognitive function in our study. This is in concordance with earlier studies where lipid levels in middle age but not in later years were associated with future cognitive impairment (Citation6,Citation17). The existing literature supports the notion that the relation between blood pressure and cognitive level is age-dependent. In studies conducted in middle-aged populations, hypertension has predicted cognitive impairment (Citation18,Citation19), but the role of the blood pressure in cognitive decline in the elderly is less clear: some studies have (Citation20) but most have not (Citation6,Citation21) reported an association between blood pressures and cognitive decline. On the contrary, in late-life cross-sectional studies low blood pressure has been constantly associated with poor cognitive function (Citation6,Citation22). In our study, there was a trend towards association between low diastolic pressure and new onset of dementias. Interestingly, diagnosis of hypertension was associated with lower incidence of dementia in the follow-up independently of previously known risk factors of dementia. This may be explained by more common use of antihypertensive medication among participants who have been diagnosed with hypertension (P < 0.001 for all classes of antihypertensive medication, separately, data not shown). This is backed by earlier studies in which the treatment of hypertension lowered the incidence of cognitive decline in the elderly (Citation23,Citation24).
Blood pressure amongst the aged may not be as good a marker of cardiovascular morbidity as in younger populations, since it is attenuated by many factors common in the elderly population, such as dehydration, heart failure, atrial fibrillation, and aortic stenosis. Therefore, it is possible that the heart and cardiovascular system are under stress and predispose to cognitive impairment even if blood pressure is not elevated. This is a postulated mechanism why antihypertensive medication would protect against dementia even in the presence of lower blood pressures. It is also possible that elevated blood pressure in the elderly is an indicator of robust cardiac pump function, which in turn is required for adequate perfusion through aged vasculature to various organ systems, including the brain.
BNP is a marker of cardiac—especially left ventricular—pump function and has been linked to both cardiovascular and total mortality in the general elderly population (Citation15). In heart failure patients, high levels of BNP have been linked to cognitive dysfunction in a small (n = 60) cross-sectional trial (Citation8). Heart failure, linked to BNP, did not predict cognitive decline or dementia in the present data. The explanation for the ability of BNP to predict forthcoming cognitive impairment is not clear. In our study, the history of stroke was taken into account, and it did not predict cognitive decline as BNP did (). The predictive value of BNP was further tested in subgroup analyses in participants without history of heart failure or stroke, separately, without any material change in results. High levels of BNP are associated with endothelial dysfunction, and this phenomenon has recently been linked to cognitive function (Citation25).
Blood-based biomarkers of vascular pathology in Alzheimer's disease have been an issue of intensive research during recent years (Citation26). The vascular adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), regulators of transcapillary permeability, have been studied for their ability to predict Alzheimer's disease with conflicting results (Citation27,Citation28). The study with negative result concerning adhesion molecules (Citation28) found peripheral inflammatory markers, C-reactive protein, and interleukin 6 to be associated with an increased risk of Alzheimer's disease replicating the results about the positive association of inflammatory markers and risk of Alzheimer's disease (Citation29). Regulators of vasodilatation, MR-proANP, endothelin-1 (ET-1), and adrenomedullin (ADM), were studied in a recent cross-sectional study by Buerger et al. (Citation9). Alzheimer's disease patients were found to have elevated levels of vasodilatators, MR-proANP, and ADM, and lower levels of vasoconstrictor ET-1, compared to healthy controls.
As expected, the majority of the participants with dementia in our study population were diagnosed to have Alzheimer's disease. BNP was associated with multiple base-line parameters, such as age and low BMI, previously found to be associated with Alzheimer's disease. In line with the study by Buerger et al. (Citation9), BNP, a potent vasodilatator, was significantly associated with both new cases of Alzheimer's disease and vascular dementia in a subgroup analysis. The association with vascular dementia appeared to be even stronger than with Alzheimer's disease, but the results with a small number of vascular dementia cases (n = 10) should be interpreted with caution.
A recent analysis from the Cardiovascular Health Study indicated that the use of angiotensin-converting enzyme (ACE) inhibitors crossing the blood—brain barrier might be associated with better outcome in terms of cognitive function (Citation30). ACE inhibitors are known to lower the circulating levels of BNP, but this is probably secondary to decreased cardiac afterload (Citation31). BNP was originally isolated from porcine brain extracts (hence the other name, brain natriuretic peptide), but its concentrations in the brain are actually very low or, in some species such as in the rat, even undetectable (Citation32). Therefore it would seem unlikely that the positive effects of centrally active ACE inhibitors on cognition were mediated by locally produced BNP. In our study population 59 participants used ACE inhibitors, and there was a trend towards lower frequency of diagnosed dementia among ACE inhibitor-users (OR 0.476; P = 0.100). The data are too limited for further analysis between different types of ACE inhibitors.
Our finding about the connection between cognitive dysfunction and BNP indicates that cardiovascular morbidity and stress also significantly affect cognitive decline in the elderly population. Future studies investigating the effect of antihypertensive therapy on cognitive function in the elderly should clarify whether the concomitant decrease in BNP stratifies the risk of cognitive impairment. If it does, BNP determination might discriminate which patients would potentially benefit from antihypertensive therapy in the prevention of dementia.
As a study limitation, it is possible that factors found insignificant in our study would associate with cognitive impairment in a larger study sample. Unfortunately, MMSE was measured only at the base-line and during the follow up visit at 5 years. Repeated measurements of MMSE would enable monitoring changes in cognitive function more reliably. Our study was carried out in an almost exclusively white Caucasian population which limits generalizability to other ethnic groups. The study population was an elderly general population with high prevalence of cardiovascular disease but without dementia at the base-line. The study results cannot be directly extrapolated to younger populations or to elderly with lower prevalence of cardiovascular morbidities. Strengths of our study were the population-based approach, good characterization of the study population, and the small drop-out rate during follow-up. Diagnosis and classification of dementing illnesses were made by an experienced geriatrician (author RS), and brain imaging by CT or MRI was routinely used. The consistent association of BNP to separate end-points measuring cognitive impairment—the decline of MMSE and new cases of dementia—further adds to the strength of our findings.
In conclusion, this cohort study conducted in an elderly population suggests that BNP is an independent harbinger of the decline of the MMSE and forthcoming dementia. This finding serves as ground for testing the impact of antihypertensive treatment in the prevention of cognitive impairment in those with elevated BNP.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60: 1119–22.
- Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166: 367–78.
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT Jr, . Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–42.
- Fratiglioni L, Grut M, Forsell Y, Viitanen M, Grafström M, Holmén K, . Prevalence of Alzheimer's disease and other dementias in an elderly urban population: relationship with age, sex, and education. Neurology. 1993;43:13–20.
- Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, . Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–3.
- Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585:97–108.
- Gunstad J, Poppas A, Smeal S, Paul RH, Tate DF, Jefferson AL, . Relation of brain natriuretic peptide levels to cognitive dysfunction in adults > 55 years of age with cardiovascular disease. Am J Cardiol. 2006;98:538–40.
- Feola M, Rosso GL, Peano M, Agostini M, Aspromonte N, Carena G, . Correlation between cognitive impairment and prognostic parameters in patients with congestive heart failure. Arch Med Res. 2007;38:234–9.
- Buerger K, Ernst A, Ewers M, Uspenskaya O, Omerovic M, Morgenthaler NG, . Blood-based microcirculation markers in Alzheimer's disease-diagnostic value of midregional pro-atrial natriuretic peptide/C-terminal endothelin-1 precursor fragment ratio. Biol Psychiatry. 2009;65:979–84.
- Zung WW. A self-rating Depression Scale. Arch Gen Psychiatry. 1965;12:63–70.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994. 143–7.
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, . Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB international workshop. Neurology. 1996;47:1113–24.
- Ala-Kopsala M, Magga J, Peuhkurinen K, Leipala J, Ruskoaho H, Leppaluoto J, . Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A- and B-type natriuretic peptides. Clin Chem. 2004;50:1576–88.
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, . Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–6.
- Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–16.
- Jagger C, Andersen K, Breteler MM, Copeland JR, Helmer C, Baldereschi M, . Prognosis with dementia in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000; 54:16–20.
- Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, . Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14–20.
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, . Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21: 49–55.
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, . Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60.
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, . Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–8.
- Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–81.
- Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ. 1996;312:805–8.
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, . Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–51.
- Veld BA, Ruitenberg A, Hofman A, Stricker BH, Breteler MM. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol Aging. 2001;22:407–12.
- Chong AY, Blann AD, Patel J, Freestone B, Hughes E, Lip GY. Endothelial dysfunction and damage in congestive heart failure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 2004; 110:1794–8.
- Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer's disease. Exp Gerontol. 2010;45:75–9.
- Zuliani G, Cavalieri M, Galvani M, Passaro A, Munari MR, Bosi C, . Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J Neurol Sci. 2008;272:164–70.
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, . Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch Neurol. 2004;61:668–72.
- Dziedzic T. Systemic inflammatory markers and risk of dementia. Am J Alzheimers Dis Other Demen. 2006;21: 258–62.
- Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, . Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch Intern Med. 2009;169: 1195–202.
- Vuolteenaho O, Ala-Kopsala M, Ruskoaho H. BNP as a biomarker in heart disease. Adv Clin Chem. 2005;40:1–36.
- Ogawa Y, Nakao K, Mukoyama M, Shirakami G, Itoh H, Hosoda K, . Rat brain natriuretic peptide—tissue distribution and molecular form. Endocrinology. 1990;126:2225–7.