Abstract
Background. Menopausal hot flushes may be a marker for a difference in vascular function. We studied the associations between hot flushes of varying severity and ambulatory blood pressure (BP) and heart rate (HR).
Methods. A total of 147 women with onset of menopause within the preceding 6–36 months reported no hot flushes (n = 23) or mild (n = 33), moderate (n = 30), or severe (n = 61). Ambulatory BP and HR were registered for 24 hours. The variables, analyzed separately for day-time and night-time, were compared among the four study groups.
Results. Hot flushes failed to show any relationship to mean day- or night-time BP, nocturnal dipping of BP, or HR. However, severe night-time hot flushes were accompanied by elevations in systolic BP (4.1 ± 10.5 mmHg, P = 0.061), diastolic BP (3.1 ± 6.8 mmHg, P = 0.032), and heart rate (3.0 ± 7.2 beats/minute, P = 0.043).
Conclusion. Hot flushes are not associated with ambulatory BP or heart rate in normotensive, recently post-menopausal women, although severe night-time hot flush episodes are followed by significant elevations in BP and heart rate. The latter may be of clinical significance.
Key messages
Hot flushes are not associated with 24-h ambulatory blood pressure or heart rate in healthy, recently post-menopausal women with no, mild, moderate, or severe hot flushes.
In women with severe hot flushes transient increases in systolic and diastolic blood pressure and heart rate in relation to nighttime hot flush episodes were detected.
| Abbreviations | ||
| ABP | = | ambulatory blood pressure |
| ANOVA | = | analysis of variance |
| BP | = | blood pressure |
| CVD | = | cardiovascular disease |
| DBP | = | diastolic blood pressure |
| E1 | = | estrone |
| E2 | = | estradiol |
| FEI | = | free estradiol index |
| FSH | = | follicle-stimulating hormone |
| HFWWS | = | Shot flush weekly weighted score |
| HR | = | heart rate |
| HT | = | hormone therapy |
| SBP | = | systolic blood pressure |
Introduction
Blood pressure (BP) increases in many women after menopause (Citation1), but the mechanisms behind this change are poorly understood (Citation2,Citation3). The increase in BP may not occur concomitantly with the onset of menopause, but rather over a number of years, and may also be influenced by other factors, such as an increased body mass index with advancing age (Citation2,Citation4,Citation5). Blood pressure is one of the most accurate determinants of cardiovascular risk in women (Citation3), and it is therefore important to determine the factors that might predict these changes. It has been postulated that women with menopausal vasomotor symptoms may differ from those without these symptoms in regard to cardiovascular risk factors, although the exact etiology and mechanisms behind hot flushes are unknown (Citation6–9). However, sympathetic activation is involved in the regulation of both menopausal hot flushes (Citation10–12) and cardiovascular function (Citation2). Thus, the presence of hot flushes in recently menopausal women could indicate a difference in BP and other vascular risk factors.
Less than a decade ago post-menopausal hormone therapy (HT) was commonly recommended for the prevention of cardiovascular disease (CVD) on the basis of results from numerous observational HT studies (Citation13–15). However, HT was shown to fail as regards both primary (Citation16,Citation17) and secondary (Citation18,Citation19) prevention of CVD in randomized, placebo-controlled trials (Citation20,Citation21). These conflicting data may be explained in part by the differences in the study populations, such as age at the start of HT (Citation22–24) or vasomotor symptom status (Citation6–9). Indeed, in non-randomized studies women with disturbing hot flushes were likely to have started the use of HT (Citation13,Citation14), whereas such women were not included into randomized HT trials (Citation16–19).
Previous population-based data on the association between retrospectively recorded hot flushes and BP are conflicting, showing either higher BP in women with hot flushes (Citation25,Citation26) or no association between hot flush status and BP (Citation27). We therefore studied the impact of prospectively assessed hot flushes of different degrees of severity on ambulatory blood pressure (ABP) in normotensive, recently post-menopausal, healthy women. In addition, we evaluated the responses in BP to severe night-time hot flush episodes.
Subjects and methods
A total of 150 women were included in this study. Inclusion criteria were onset of menopause within the preceding 6–36 months, serum follicle-stimulating hormone (FSH) level ≥30 IU/L, age 48–55 years, and no previous use of HT. To be able to determine the possible independent impact of hot flushes on BP and heart rate (HR), women with known cardiovascular factors (smoking, BP >140/90 mmHg, body mass index >30 kg/m2, or any clinically significant disease or drug treatment) were excluded from this study. Hot flushes were assessed prospectively via a structured questionnaire over a period of 2 weeks. Vasomotor symptoms were rated with a hot flush weekly weighted score (HFWWS) (Citation28–31). The women reported either mild (n = 34, HFWWS 0.5–9.5), moderate (n = 30, HFWWS 10.0–99.5) or severe (n = 63, HFWWS 100.0–452.5) hot flushes, or no hot flushes at all (n = 23, HFWWS 0.0). This study was conducted according to principles of good clinical practice and the Declaration of Helsinki, and it was approved by the Helsinki University Women’s Hospital Ethics Committee (no. 329/E8/03). Written informed consent was obtained from all participants.
Measurement of 24-h ABP
The participants wore calibrated oscillometric ABP monitors (SpaceLabs 90217, SpaceLabs Healthcare, Washington, USA) on their non-dominant arm for 24 hours. The ABP measurements were initiated by trained personnel at the Division of Clinical Physiology, Helsinki University Central Hospital, and accuracy of the monitors and cuff placement were confirmed (difference between manual and ABP measurement <6 mmHg) before each recording by simultaneous control measurements against a calibrated mercury sphygmomanometer using a T-connector. The majority of recordings were performed during normal working days, and the monitor was programmed to take readings at 20-minute intervals during periods of wakefulness and at 30-minute intervals during sleep, according to each participant's estimated times of going to bed and getting up. The subjects were instructed to maintain their normal daily activities during the ABP recordings, but to keep their arms extended and immobile during the measurements. During the ABP recordings the subjects noted their activities and vasomotor symptoms in a diary. All valid measurements of systolic (SBP) and diastolic (DBP) BP, together with simultaneous HR and the exact time of day of the measurement were transferred from the ABP system to a spreadsheet for further analysis. Times of wakefulness and sleep were defined according to diary entries.
Absolute dipping, referring to the drop in BP that normally occurs during sleep, was defined as the difference between average SBPs when asleep and awake. Relative dipping was calculated as absolute dipping as a percentage of SBP when awake. Relative dipping of at least 10% was considered normal (Citation32). Pulse pressure (PP) was calculated as SBP minus DBP.
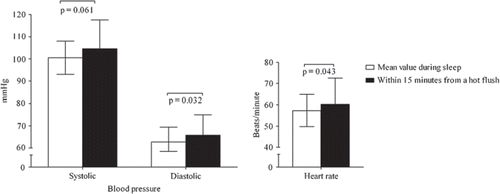
We further assessed the ABP variables in relation to hot flushes in women with severe vasomotor symptoms. To avoid any interference in ABP from daily activities, only recordings during sleep were used. The diaries were used to identify the exact time of the hot flush, and the recording occurring within 15 minutes after a hot flush episode was defined as representing BP and HR during and after a hot flush. The data were compared with average values during sleep. In this manner we were able to identify 44 hot flush episodes among 26 women. When any one woman experienced multiple hot flushes the mean value per subject was included.
Laboratory measurements
Serum levels of estrone (E1) were assayed as dansyl derivatives by liquid chromatography-tandem mass spectrometry, as described before (Citation31). The intra- and interassay coefficients of variation were between and 7.8% and 12.0%. The levels of estradiol (E2) were determined by radioimmunoassay, using established, commercially available kits (Estradiol-2, Diasorin Inc., Stillwater, MN, USA), and the intra- and interassay coefficients of variation were less than 6.1%. The serum samples were also employed for assay of sex hormone-binding globulin, FSH, and thyroid stimulating-hormone, using routine laboratory methods. The free estradiol index (FEI) was calculated as the ratio: E2 (nmol/L) × 100 / sex hormone-binding globulin (nmol/L).
Statistical analyses
Normality was assessed with the Shapiro–Wilk test. To achieve normal distributions logarithmic transformations were carried out as regards the FEI and FSH data and inverse transformation for the E1 and E2 data. One-way between-groups univariate analysis of variance (ANOVA) was used to investigate the impact of hot flush status on ABP, with the ABP variables as dependent variables and the hot flush status (no hot flushes, mild, moderate, severe) as the fixed factor. Due to its skewed distribution, time since menopause was converted to ranked cases, and thereafter used as a covariate in analyses of covariance, as were the normally distributed variables age, body mass index, and transformed levels of E1, E2, and FEI data. Data with non-normal distribution were compared by using the Kruskal–Wallis test, and post hoc analyses were carried out with the Mann–Whitney U test with Bonferroni corrections. To further analyze the association between hot flush status and ABP, separate linear regression analyses were carried out with mean day-time and night-time SBP, DBP, PP, and HR as the dependent variables, and hot flush status (Model 1) and E1, E2, and FEI (Model 2, including hot flush status) as the independent variables. Repeated measures ANOVA was used to assess the contours of the dependent variables during the 24-hour ABP registration in all patients and the changes in BP and HR in women with severe night-time hot flushes. Independence between hot flush and dipping status was studied by using the chi-square test. Relationships between variables were investigated by using Spearman's non-parametric correlation coefficient. The analyses were done with SPSS software for Windows version 14.0, 2005 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± SD for normally distributed variables and median and range for variables with non-normal distribution. A two-tailed P-value <0.05 was considered statistically significant.
Results
All women were truly post-menopausal as demonstrated by the FSH levels being ≥30 IU/L (). In spite of initially normal office BP values, two women with severe hot flushes and one woman without hot flushes had hypertension (average day-time SBP 141–165 mmHg and DBP 95–98 mmHg) in ABP measurement, and they were excluded from further analysis of the data. Thus, the final study group consisted of 147 women (23 without hot flushes, 33 with mild, 30 with moderate, and 61 with severe hot flushes). These groups differed only in HFWWS, but they were fully comparable as regards other pertinent variables ().
Table I. Base-line characteristics (mean ± SD or median and range) of recently post-menopausal women. The free estradiol index was calculated as the ratio: estradiol (nmol/L) × 100 / sex hormone-binding globulin (nmol/L).
The average number of accepted measurements over 24 hours was 62.2 ± 5.5 (SD). Mean SBP, DBP, PP, and HR (awake and sleep) showed no differences between the groups. Absolute and relative dipping patterns were also comparable between the groups (). The contours of SBP, DBP, and HR over 24 hours failed to show any dependence on hot flush status (). No dependence between these variables and the levels of E1 and E2, the FEI, or time since menopause was found. The HFWWS was not correlated to any of the variables derived from the ABP measurements.
Figure 1. Mean contours of ambulatory systolic and diastolic blood pressure and heart rate over 24 hours in women with no (n = 23), mild (n = 33), moderate (n = 30), or severe (n = 61) hot flushes. There were no differences between groups (P > 0.05).
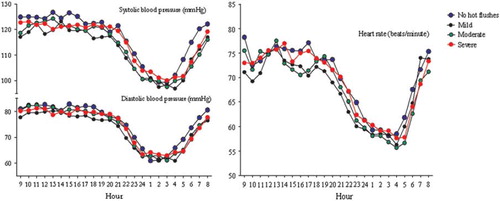
Table II. Average ambulatory blood pressure, heart rate and nocturnal dipping. Data presented as mean ± SD, number of subjects, or percentages of group totals. There were no differences between groups (P > 0.05).
Severe night-time hot flushes were accompanied by increases in SBP (4.1±10.5 mmHg (SD), P = 0061), DBP (3.1 ± 6.8 mmHg (SD), P = 0.032), and HR (3.0 ± 7.2 beats/minute (SD), P = 0.043), compared with control values (). The responses showed great individual variation, but approximately half of the hot flush episodes were associated with marked rises of up to 35 mmHg in SBP, 18 mmHg in DBP, and 21 beats/minute in HR.
Discussion
The etiology of menopausal vasomotor symptoms is unknown, but hypoestrogenism is a key factor (Citation11,Citation12). Vasomotor hot flushes are associated with alterations in vascular function (Citation11,Citation12,Citation27,Citation33) and may therefore contribute to the conflicting data between observational (hot flushes present) and randomized HT trials (hot flushes absent) (Citation7–9). In the present study we assessed the associations between hot flushes of varying degrees of severity and ABP variables. In many studies hot flushes have been recorded retrospectively, which is inaccurate and does not allow reliable grading of the severity of the flushes (Citation9). Moreover, it is known that women showing cardiovascular risk factors, such as obesity, smoking, and a low level of education, are more likely to report hot flushes than are women without these risk factors (Citation34). Therefore, it was vital for our study that hot flush status was prospectively recorded and that the volunteers were healthy, and, most importantly, recently post-menopausal, since in clinical practice these women make the decision of whether or not to initiate the use of HT.
Our results show comparable day- and night-time ABP and HR in recently post-menopausal women with and without hot flushes. These findings are partly in line with previous data showing comparable DBP during both day (Citation25,Citation35) and night (Citation25). Systolic BP during sleep was also comparable among women with and without hot flushes in one study (Citation36). However, differences in DBP (Citation36) and SBP at night-time (Citation25,Citation35), and in SBP specifically during working hours, have been reported (Citation36). It should be noted that in all of the previous studies hot flushes were retrospectively recorded, and most often on an absent–present basis (Citation25,Citation27,Citation35,Citation36). Several studies regarding BP and hot flushes have also included women with hypertension (Citation25,Citation26), smokers (Citation25–27,Citation35,Citation36), or women using HT (Citation26,Citation27), and therefore the results may not be applicable to a healthy and HT-naive population such as we studied, and who represent the majority of women considering the use of HT.
We found that severe night-time hot flush episodes were followed by significant rises in DBP and HR and a tendency towards a rise in SBP. These findings are partly in line with those in some previous studies in which day-time hot flush episodes have been assessed (Citation10,Citation37). However, decreases in BP during hot flush episodes have also been reported (Citation37,Citation38). Hot flush-associated elevations in BP and HR may be a consequence of a sympathetic surge, perhaps with concomitant withdrawal of parasympathetic activity, although the mechanisms are uncertain (Citation39). Menopause and the subsequent decline in endogenous estrogens confer alterations in autonomic nervous system functioning that may contribute to an increase in BP in women with severe hot flushes (Citation3,Citation39). Furthermore, endogenous estrogen is a potent vasodilator (Citation40), and menopause-associated hypoestrogenism may lead to vasoconstriction and thus contribute to the rise in BP (Citation39).
Our current data may indicate that women experiencing severe hot flushes could have a more receptive vasculature compared with women without hot flushes, and this is also supported by previous findings (Citation33). We cannot draw conclusions concerning the duration of the hot flush-associated elevations in BP and HR, but evidently they were relatively short in duration, because the mean night-time variables showed no elevations in women with severe hot flushes. The elevations in BP that are associated with hot flushes are detrimental to blood vessels (Citation41,Citation42), but the transient character of the BP elevation may negate the possible unfavorable vascular influence. There is a possibility that elevations in BP and HR following night-time hot flushes may be related, and perhaps in part they are caused by changes in sleeping pattern and imminent arousal, but even so such rises, being possibly secondary to hot flushes, may be of clinical significance.
Menopause is associated with an increase in arterial stiffness (Citation43), at least in part due to coinciding unfavorable changes in various cardiovascular risk factors (Citation44). We have previously shown that hot flushes in recently menopausal healthy women are not associated with structural differences in the vasculature. However, hot flushes are related to functional differences of the vasculature (Citation33) possibly due to changes in sympathetic tone. This is also in line with our current findings in BP during the hot flush episode.
In conclusion, hot flushes in normotensive, recently menopausal women do not modify ambulatory recorded BP or HR, even though severe night-time hot flushes were followed by significant rises in BP and HR. The clinical significance of these findings requests further studies.
Declaration of interest: This study was supported by unrestricted grants from the Päivikki and Sakari Sohlberg Foundation, the Emil Aaltonen Foundation, the Finnish Medical Foundation, and the Helsinki University Central Hospital Research Fund.
References
- Zanchetti A, Facchetti R, Cesana GC, Modena MG, Pirrelli A, Sega R, . Menopause-related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23:2269–76.
- Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51: 952–9.
- Barton M, Meyer MR. Post-menopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–8.
- Casiglia E, Tikhonoff V, Caffi S, Bascelli A, Schiavon L, Guidotti F, . Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. J Hypertens. 2008;26:1983–92.
- Cifkova R, Pitha J, Lejskova M, Lanska V, Zecova S. Blood pressure around the menopause: a population study. J Hypertens. 2008;26:1976–82.
- Mikkola TS, Ylikorkala O. Hormone therapy and cardiovascular disease–-still much to be learnt. Gynecol Endocrinol. 2005;20:116–20.
- van der Schouw YT, Grobbee DE. Menopausal complaints, oestrogens, and heart disease risk: an explanation for discrepant findings on the benefits of post-menopausal hormone therapy. Eur Heart J. 2005;26:1358–61.
- Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–41.
- Pinkerton JV, Stovall DW. Is there an association between vasomotor symptoms and both low bone density and cardiovascular risk? Menopause. 2009;16:219–23.
- Freedman RR, Dinsay R. Clonidine raises the sweating threshold in symptomatic but not in asymptomatic post-menopausal women. Fertil Steril. 2000;74:20–3.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–25.
- Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health. 2007;10:247–57.
- Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, . Hormone therapy to prevent disease and prolong life in post-menopausal women. Ann Intern Med. 1992;117: 1016–37.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of post-menopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133: 933–41.
- Taskinen MR. Oestrogen replacement therapy and coronary heart disease. Ann Med. 1998;30:443–51.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, . Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33.
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, . Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34.
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, . Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13.
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, . Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288:49–57.
- Nair GV, Klein KP, Herrington DM. Assessing the role of oestrogen in the prevention of cardiovascular disease. Ann Med. 2001;33:305–12.
- Hodis HN, Mack WJ. Post-menopausal hormone therapy and cardiovascular disease in perspective. Clin Obstet Gynecol. 2008;51:564–80.
- Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53:605–19.
- Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on post-menopausal hormone therapy. N Engl J Med. 2003;348:645–50.
- Mikkola TS, Clarkson TB, Notelovitz M. Post-menopausal hormone therapy before and after the women’s health initiative study: what consequences? Ann Med. 2004;36: 402–13.
- Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause. 2007;14:308–15.
- Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, . Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51:1492–8.
- Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation heart study. Circulation. 2008;118:1234–40.
- Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol. 2000;95:726–31.
- Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–90.
- Panay N, Ylikorkala O, Archer DF, Gut R, Lang E. Ultra-low-dose estradiol and norethisterone acetate: effective menopausal symptom relief. Climacteric. 2007;10: 120–31.
- Tuomikoski P, Mikkola TS, Hämäläinen E, Tikkanen MJ, Turpeinen U, Ylikorkala O. Biochemical markers for cardiovascular disease in recently post-menopausal women with and without hot flashes. Menopause. 2009;17:145–51.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–74.
- Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamäki H, Rönnback M, . Evidence for a role of hot flushes in vascular function in recently post-menopausal women. Obstet Gynecol. 2009;113:902–8.
- Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, . Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–73.
- Brown DE, Sievert LL, Aki SL, Mills PS, Etrata MB, Paopao RN, . Effects of age, ethnicity and menopause on ambulatory blood pressure: Japanese-American and Caucasian school teachers in Hawaii. Am J Hum Biol. 2001; 13:486–93.
- James GD, Sievert LL, Flanagan E. Ambulatory blood pressure and heart rate in relation to hot flash experience among women of menopausal age. Ann Hum Biol. 2004; 31:49–58.
- Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic post-menopausal women. Menopause. 2008; 15:290–5.
- Nelesen R, Krohn P, Dimsdale JE. Hot-flash hypotension. N Engl J Med. 2004;351:1577–9.
- Vongpatanasin W. Autonomic regulation of blood pressure in menopause. Semin Reprod Med. 2009;27:338–45.
- Koh KK. Effects of estrogen on the vascular wall: vasomotor function and inflammation. Cardiovasc Res. 2002;55: 714–26.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
- Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006; 119:133–41.
- Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, . Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early post-menopausal phase. Atherosclerosis. 2006;184: 137–42.
- Collins P, Rosano G, Casey C, Daly C, Gambacciani M, Hadji P, . Management of cardiovascular risk in the peri-menopausal woman: a consensus statement of European cardiologists and gynaecologists. Eur Heart J. 2007; 28:2028–40.