Abstract
This study was designed to evaluate the changes in arterial blood pressure (BP) and heart rate (HR) in moderate smokers during smoking abstinence after 7 days of treatment with bupropion alone, transdermal nicotine or bupropion combined with transdermal nicotine. Twenty-four healthy moderate smokers (12 female/12 male; 40±7 years) were evaluated randomly on five occasions and their systolic, diastolic, mean arterial blood pressure (MAP) and HR were measured by a Finapres device for 10 h, immediately after smoking interruption. All of the 24 smokers participated on five protocols during 7 days: control group (C) – no drugs; placebo group (PL); bupropion group (BUP) 150–300 mg; transdermal nicotine group (TN) – 21 mg; and BUP+TN-nicotine patch. Concomitantly, the subjects were evaluated by ABPM (ambulatory BP monitoring). All of BP parameters monitored shown significant statistical differences in the BUP, TN and BUP+TN groups compared with the controls (p<0.05), when measured by Finapres. The HR remained unaltered in all of the groups. No significant differences were seen in the BP or HR during the 24-h ABPM. These findings indicate that in moderate smokers, bupropion, transdermal nicotine or bupropion associated with transdermal nicotine caused an elevation in the BP after acute smoking interruption.
Introduction
Tobacco smoking is the most important public health problem and is the single most preventable cause of premature diseases and death in the world today. Smoking, the leading preventable cause of death globally, caused 5.4 million deaths in 2006 (one in 10 adult deaths worldwide). The annual mortality rate is projected to double by 2020 (Citation1).
A relationship between tobacco smoking and cardiovascular diseases has been demonstrated, with an increase in mortality among healthy smokers (Citation2). Approximately 92% of all smokers are well-informed about the detrimental effects of smoking on health and 70% desire to stop tobacco smoking, but only 5–10% is successful single-handedly (Citation3).
Cigarette smoke is a complex mixture that causes a variety of diseases, such as lung cancer, chronic obstructive pulmonary disease (COPD) and cardiovascular disease (Citation4). This mixture contains approximately 4000 chemical compounds, and nicotine, carbon monoxide and other toxins are suspected of being responsible for cardiovascular diseases in smokers (Citation5).
Nicotine replacement therapy (NRT) is very important for reducing the symptoms of abstinence and for increasing tobacco smoking cessation rates (Citation6). Benowitz et al. (Citation7) and Lavelle et al. (Citation8) demonstrated that transdermal nicotine is safe and has fewer side-effects in the cardiovascular system, including changes in as heart rate (HR), arterial blood pressure (BP) and catecholamine plasma levels, when compared with cigarette smoking.
Bupropion can act as a sympathomimetic amine, inhibit the reuptake of norepinephrine and dopamine. The major metabolite of bupropion is hydroxybupropion (Citation9, Citation10). Although the mechanism of action responsible for smoking cessation is unknown, there is evidence that blockade of the reuptake of catecholamines and dopamine (substances responsible for the symptoms of abstinence syndrome and drug addiction) is involved (Citation11–13).
Bupropion increases the rate of smoking cessation during a long-term use in the absence or presence of transdermal nicotine when compared with transdermal nicotine or placebo (Citation14–16). Nicotine replacement therapy and bupropion are considered safe and they are currently the first line of drug therapy for smoking cessation (Citation17).
Although transdermal nicotine (patch) has been indicated for smoking cessation in association with bupropion, there have been no studies on the acute effects of this combination on arterial BP in humans. The purpose of this study was to evaluate the changes in BP and HR in healthy smokers using bupropion alone, or in associating with transdermal nicotine during acute abstinence smoking.
Patients and methods
This observational study was approved by the institutional Ethics Committee and each subject provided informed consent prior to participating in the study. The smokers were recruited from the general public and the university hospital. Twenty-four healthy, normotensive (≤140 mmHg/ ≤90 mmHg) (Citation18,Citation19) mild/moderate smokers (12 women/12 men; ≤20 cigarettes/day) who had smoked for an average of 24±11 years (mean±SD; range: 5–38) were studied. The subjects had a body mass index of 21–27.5kg/m2 (mean: 25±2kg/m2) and ranged in age from 25 to 47 years old (mean: 41±7 years). The average numbers of cigarettes before and during the study were 16–21, respectively.
Each participant provided a complete medical history and underwent a physical examination, an electrocardiogram and laboratory analysis to exclude risk factors such as seizures, dyslipidemia, diabetes mellitus, and evidence of hepatic, renal or hematological dysfunctions. The criteria for exclusion were: pregnancy, breastfeeding, a history of hypertension, diabetes, myocardial or renal disease, the use of statins, antihypertensive drugs, acetylsalicylic acid (within the previous 7 days), oral contraceptives (within the previous 2 months) and with alterations in spirometry.
All studies were initiated at 08:00 h after overnight fasting, with the subjects lying in the supine position in a quiet air-conditioned room (22–24°C). The subjects were admitted in the outpatient clinic of the Campinas State University Hospital (HC-UNICAMP) for 10-h studies.
The study had a crossover, randomized, single-blinded design and subjects were asked to maintain the number of cigarettes usually consumed until immediately before the study period. The study had two phases; the first of which lasted for the first 6 days while the second was restricted to the 7th day. The protocol consisted of:
Control group (C): no drugs (7 days);
Placebo group (PL): placebo – bupropion and nicotine patch placebos for 7 days;
Bupropion group (BUP): bupropion 150 mg for 6 days and 300 mg on the 7th day;
Transdermal nicotine group (TN): nicotine replacement patch (21 mg) for 7 days;
Bupropion associated with nicotine group (BUP+TN): nicotine replacement patch (21 mg) for 7 days and bupropion 150 mg for 6 days and 300 mg on the 7th day.
Doses of bupropion and nicotine were chosen according its medical indications.
On the 7th day, after smoking the last cigarette, all of the subjects used the drugs (transdermal nicotine patch 21 mg – Niquitin; Alza Corporation, CA, USA – and bupropion 150 mg – Zyban; GlaxoSmithKline Inc., USA) and were monitored continuously for 10 h to obtain beat-to-beat BP and HR recordings by a non-invasive finger device (digital plethysmograph, Finapres 2300; Ohmeda, Englewood, CO, USA). The subjects remained in a supine position during the entire period and did not smoke for 10 h. Simultaneously, the subjects were underwent a 24-h ABPM, based on the oscillometric principle using a SpaceLabs device (model 90207; Redmond, WA, USA). Data analysis was done using software installed in a personal computer. After 10 h, the smokers returned home and were allowed to consume their usual amount of tobacco.
The results were expressed as the mean±standard error of the mean (SEM). The BP and HR values were compared with those of the control group using one-way analysis of variance (ANOVA) with repeated measurements followed by a contrast test for different measurements. A value of p<0.05 indicated significance.
Results
Significant increases in systolic BP (SBP), diastolic BP (DBP), and mean arterial blood pressure (MAP) were seen in the bupropion, nicotine patch and bupropion combined with nicotine patch groups when compared with the control and placebo groups (p<0.05) ().
Figure 1. Mean arterial blood pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) measured by Finapres (beat-to-beat) over a 10-h period (08:00–18:00 h) during smoking tobacco abstinence. Eight hours indicates time 0 of measurements immediately before the smoking last cigarette. □ Control (n=24); ∘ placebo (n=24); • bupropion (n=24); ◼ nicotine patch (n=24); and ▴ bupropion associated with nicotine patch (n=24). The points are the mean±SEM. *p<0.05 vs the control group; #p<0.05 vs the placebo group.
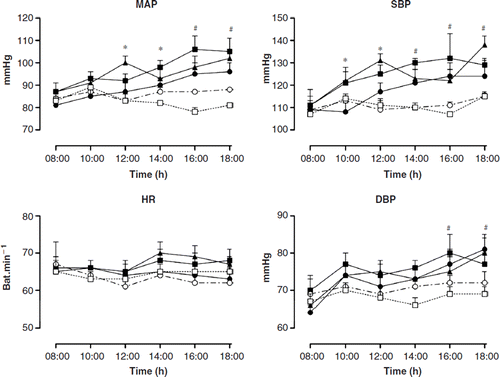
The HR did not follow in the BP increases in the treatments. The use of a placebo did not alter the BP and HR compared with the control group (p>0.05) ().
The use of a nicotine patch (21 mg), bupropion (300 mg) and both in association on the 7th day, was accompanied by a marked rise in the SBP, DBP and MAP. The highest increases in SBP using 300 mg of bupropion, the 21-mg nicotine patch and both in association, compared with the control group were, respectively, from 107±4 to 124±5 mmHg (p=0.01 at 16:00 h); from 107±4 to 132±6 mmHg (p=0.001 at 16:00 h), and 115±1 to 138±4 mmHg (p=0.01 at 18:00 h). The highest increases in MAP compared with the control group were respectively, from 78±2 to 95±4 mmHg (p=0.01 at 16:00 h), from 78±2 to 106±2 mmHg (p=0.001 at 16:00 h), and from 81±1 to 102±2 mmHg (p=0.001 at 18:00 h). Finally, the highest increases in the DBP using 300 mg of bupropion, nicotine patch and both in association, compared with the control group were, respectively, from 69±1 to 81±3 mmHg (p=0.01 at 18:00 h); from 69±3 to 80±2 mmHg (p=0.008 at 16:00 h), and from 69±15 to 80±3 mmHg (P<0.05 at 18:00 h). The pressor responses were not associated with clinical symptoms, such as tachycardia and headache. There were no significant differences in the HR among as the various groups ().
In contrast to the results obtained with the Finapres, there were no significant differences in the BP or HR of any of the groups when monitored using 24-h ABPM.
Discussion
Smoking cessation is associated with a reduction in risk of developing stroke, coronary heart disease, several types of cancer, and it is associated to an increased life expectancy (Citation20). Various combination therapies are used for smoke cessation and the selection of the pharmacologic agent should be based on the patient's comorbidities and psychosocial circumstances, as well as on adverse event profiles (Citation21).
Our data clearly show that bupropion, nicotine patch and bupropion combined with transdermal nicotine in moderate smokers elevated all of the BP parameters monitored after acute interruption of smoking.
Several studies have demonstrated that cigarette smoking increases BP from 5–10 mmHg to 30–40 min and HR by 10–20 beats/min (Citation22–24). Cigarette smoking activates the sympathetic nervous system (Citation24) in healthy smokers, and increases the HR, BP, cardiac stroke volume and output, and coronary blood flow (Citation23,Citation25). Nicotine has complex cardiovascular effects that are mediated by the stimulation of the central nervous system, the excitation of nicotinic receptors in the spinal cord and autonomic ganglia, and the discharge of adrenaline from the adrenal medulla (Citation26). These actions lead to an increase in BP and HR (Citation22,Citation27).
Cigarette smoking is associated with endothelial dysfunction (Citation28), i.e. probably mediated by the decreased availability of endothelium-derived NO in vascular beds (Citation28,Citation29). A direct, local effect of nicotine on the endothelium has been demonstrated by Chalon et al. (Citation25). In addition, the presence of acetylcholine receptors sensitive to nicotine has been reported in cultured endothelial cells from human aorta; these receptors are similar to neuronal ganglionic acetylcholine receptors. Thus, the stimulation by nicotine of acetylcholine receptors in endothelial cells may mediate the effects of nicotine in the vascular system (Citation30). Nicotine may also alter the relaxation of arterioles via an increased release of superoxide anion and subsequent inactivation of NO (Citation31). Sabha et al. (Citation32) demonstrated that transdermal nicotine mimics smoking-induced endothelial dysfunction. These authors also observed increase thromboxane B2 levels after use of transdermal nicotine in non-smokers. Finally, Moreno et al. (Citation28) observed that smoking cessation reverses smoking-induced endothelial dysfunction.
Using non-invasive monitoring BP, Benowitz et al. (Citation7) did not observe significant differences in the SBP of smokers using transdermal nicotine patches, nicotine nasal spray, placebo or cigarette smoking. A small increase in DBP was observed when smoking was compared with smoking abstinence while using transdermal patches, and there was no increase in BP when nasal spray or transdermal nicotine was compared with placebo. The authors concluded that transdermal nicotine was absorbed slowly, and had less effect on the HR and BP in healthy smokers. Intravenous nicotine, nicotine nasal spray and nicotine chewing gum acutely increase HR by 10–15 beats/min and increase the BP by 5–10 mmHg, which is similar to the effects of cigarette smoking Citation33; transdermal nicotine apparently causes fewer acute hemodynamic changes than smoking (Citation34).
Tanus-Santos et al. (Citation35) showed that acute (4-h) transdermal nicotine (21 mg) increased the BP and HR in hypertensive non-smokers and in normotensive non-smokers, but did not have a significant effect in hypertensive smokers, probably because of tolerance to the pressor effects of nicotine (Citation36). Acute tolerance can affect many of the cardiovascular actions of nicotine, including the pressor effect (Citation37). Chronic nicotine abuse can decrease baroreceptor sensitivity in smokers (Citation27, Citation37–40) and, together with raised levels of catecholamines, could increase the risk of cardiovascular diseases in smokers (Citation39).
In contrast to the foregoing study, which was short-lasting, our 10-h protocol revealed an increase in the BP levels after 2–10 h, mainly in the transdermal nicotine and bupropion combined. This finding probably reflected the fact that the cardiovascular effects were evaluated when the absorption rate of transdermal nicotine was maximal, normally 6–12 h after applying the patch (Citation41). The responses to transdermal nicotine seen in moderate smokers are probably not the same as those seen in heavy smokers.
The mechanism of action of bupropion on BP and HR, and its role in endothelial dysfunction are still unknown. Fryer and Lukas (Citation42) reported that bupropion is a potent functional inhibitor of human muscle and ganglionic nicotinic acetylcholine receptor (nAChR) subtypes. This functional blockade is non-competitive, which suggests that the use of the bupropion combined with nicotine replacement therapy may contribute to smoking cessation. Some cardiovascular side-effects of bupropion treatment include orthostatic hypotension, exacerbation of hypertension (Citation43), and increased HR (Citation44). In partial agreement with these findings, we observed a significant increase in BP, but not in the HR, after 4 h of bupropion alone or in combination with a nicotine patch (p<0.05).
This is the first study in which the effect of bupropion, alone and in combination with a nicotine patch has been examined on ambulatory BP and HR over 24 h in a randomized trial in the same individuals. Although the BP values were significantly different among the groups when evaluated with the Finapres device, the 24-h ABPM system showed no significant differences among the groups. The non-invasive Finapres equipment (beat-to-beat measurement) has a higher sensitivity than the 24-h ABPM method and is similar to methods for intra-arterial monitoring (Citation45).
Some aspects of this study deserve comments. First, the volunteers were healthy, normotensive and moderate smokers. Thus, the findings should not be extrapolated to hypertensive or heavy smokers, since the responses to transdermal nicotine and bupropion in these smokers may not be the same as those in hypertensive smokers or in heavy smokers. Second, although the number of healthy smokers (n=24) studied here can appear small, as the same individuals were evaluated under all treatment regimens (sequential treatments), we were able to reach positive significant p-values (<0.05) for BP increases. Besides, the increases in BP observed during the studies were very consistent in almost all smokers. Also, for ethical reasons, the number of healthy volunteers has to be small. Third, the design of this study did not permit us to assess whether bupropion alone or in combination with transdermal nicotine is safe or not for normotensive or hypertensive smokers. Fourth and most important is that cigarette smoke delivers not only nicotine at high doses but also a myriad of other toxins that damage the cardiovascular system (Citation46). Indeed, experimental assays cannot be compared with time of exposure of smoking (Citation47).
In conclusion, bupropion alone or in combination with transdermal nicotine increased BP in moderate smokers during acute interruption of smoking. Further studies of this combination of therapies in hypertensive and heavy smokers should be done to assess the safety patients who are at high risk of cardiovascular disease.
Acknowledgments
This work was supported by FAPESP, CAPES, CNPq and FAEPEX-UNICAMP (Brasil).
References
- Gostin LO. Global regulatory strategies for tobacco control. JAMA. 2007;298:2057–2059.
- Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. EUROASPIRE Study Group. Cardiovascular prevention guidelines in daily practice: A comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373:929–940.
- Jain A. Treating nicotine addiction. BMJ. 2003;327:1394–1395.
- Moennikes O, Vanscheeuwijck PM, Friedrichs B, Anskeit E, Patskan GJ. Reduced toxicological activity of cigarette smoke by the addition of ammonia magnesium phosphate to the paper of an electrically heated cigarette: Subchronic inhalation toxicology. Inhal Toxicol. 2008;20:647–663.
- Kuper H, Adami HO, Boffetta P. Tobacco use, cancer causation and public health impact. J Intern Med. 2002;251:455–466.
- Haustein KO. What can we do in secondary prevention of cigarette smoking?. Eur J Cardiovasc Prev Rehabil. 2003;10:476–485.
- Benowitz NL, Hansson A, Jacob P. 3rd Cardiovascular effects of nasal and transdermal nicotine and cigarette smoking. Hypertension. 2002;39:1107–1112.
- Lavelle C, Birek C, Scott DA. Are nicotine replacement strategies to facilitate smoking cessation safe?. J Can Dent Assoc. 2003;69:592–597.
- Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93.
- Shrier M, Diaz JE, Tsarouhas N. Cardiotoxicity associated with bupropion overdose. Ann Emerg Med. 2000;35:100.
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: A review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401.
- Britton J, Jarvis MJ. Bupropion: A new treatment for smokers. Nicotine replacement treatment should also be available on the NHS. BMJ. 2000;321:65–66.
- Gobbi G, Slater S, Boucher N, Debonnel G, Blier P. Neurochemical and psychotropic effects of bupropion in healthy male subjects. J Clin Psychopharmacol. 2003;23:233–239.
- Glover ED, Glover PN, Payne TJ. Treating nicotine dependence. Am J Med Sci. 2003;326:183–186.
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–202.
- Tønnesen P, Tonstad S, Hjalmarson A, Lebargy F, Van Spiegel PI, Hider A, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med. 2003;254:184–192.
- Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003;18:1053–1057.
- World Health Organization–International Society of Hypertension-Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17:151–183.
- Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3.
- Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92:990–996.
- Nides M. Update on pharmacologic options for smoking cessation treatment. Am J Med. 2008;121:(4 Suppl 1):S20–31.
- Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29:1422–1431.
- Benowitz NL, Porchet H, Sheiner L, Jacob P. 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44:23–28.
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577.
- Chalon S, Moreno H Jr, Benowitz NL, Hoffman BB, Blaschke TF. Nicotine impairs endothelium-dependent dilatation in human veins in vivo. Clin Pharmacol Ther. 2000;67:391–397.
- Marano G, Ramirez A, Mori I, Ferrari AU. Sympathectomy inhibits the vasoactive effects of nicotine in conscious rats. Cardiovasc Res. 1999;42:201–205.
- Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, et al. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. 1994;90:248–253.
- Moreno H Jr, Chalon S, Urae A, Tangphao O, Abiose AK, Hoffman BB, et al. Endothelial dysfunction in human hand veins is rapidly reversible after smoking cessation. Am J Physiol. 1998;275(3 Pt 2):H1040–H1045.
- Yugar-Toledo JC, Tanus-Santos JE, Sabha M, Sousa MG, Cittadino M, Tácito LH, et al. Uncontrolled hypertension, uncompensated type II diabetes, and smoking have different patterns of vascular dysfunction. Chest. 2004;125:823–830.
- Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439.
- Mayhan WG, Sharpe GM, Anding P. Agonist-induced release of nitric oxide during acute exposure to nicotine. Life Sci. 1999;65:1829–1837.
- Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H Jr. Transdermal nicotine mimics the smoking-induced endothelial dysfunction. Clin Pharmacol Ther. 2000;68:167–174.
- Benowitz NL, Jacob P, 3rd Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221:368–372.
- Benowitz NL, Fitzgerald GA, Wilson M, Zhang Q. Nicotine effects on eicosanoid formation and hemostatic function: Comparison of transdermal nicotine and cigarette smoking. J Am Coll Cardiol. 1993;22:1159–1167.
- Tanus-Santos JE, Toledo JC, Cittadino M, Sabha M, Rocha JC, Moreno H Jr. Cardiovascular effects of transdermal nicotine in mildly hypertensive smokers. Am J Hypertens. 2001;147 Pt 1:610–614.
- Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, et al. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–222.
- Tanus-Santos JE, Sampaio RC, Hyslop S, Franchini KG, Moreno H Jr. Endothelin ET(A) receptor antagonism attenuates the pressor effects of nicotine in rats. Eur J Pharmacol. 2000;396:33–37.
- Fattinger K, Verotta D, Benowitz NL. Pharmacodynamics of acute tolerance to multiple nicotinic effects in humans. J Pharmacol Exp Ther. 1997;281:1238–1246.
- Gerhardt U, Hans U, Hohage H. Influence of smoking on baroreceptor function: 24 h measurements. J Hypertens. 1999;17:941–946.
- Mancia G, Groppelli A, Di Rienzo M, Castiglioni P, Parati G. Smoking impairs baroreflex sensitivity in humans. Am J Physiol. 1997;273.(3 Pt 2):H1555–H1560.
- Benowitz NL, Chan K, Denaro CP, Jacob P, 3rd . Stable isotope method for studying transdermal drug absorption: The nicotine patch. Clin Pharmacol Ther. 1991;50:286–293.
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92.
- Roose SP, Dalack GW, Glassman AH, Woodring S, Walsh BT, Giardina EG. Cardiovascular effects of bupropion in depressed patients with heart disease. Am J Psychiatry. 1991;148:512–516.
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22:203–220.
- Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;136 Pt 1:647–655.
- Benowitz NL. Basic cardiovascular research and its implications for the medicinal use of nicotine. J Am Coll Cardiol. 2003;41:497–498.
- Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis. 2007;12:1927–1943.
