Abstract
Defining the vascular component(s) of the clinical diagnosis of vascular cognitive impairment (VCI) and vascular dementia (VaD) continues to be problematic. The goal of this study was to determine whether vascular stiffness, measured by pulse wave velocity (PWV), is altered in VaD, to study the utility of PWV in differentiating VaD from Alzheimer dementia (AD) and the relationship between PWV and cognitive function. A qualitative and quantitative structured analysis of the literature was conducted until September 2010, using a search strategy based on the key words: dementia, vascular dementia, dementia of vascular origin, cognitive function and arterial stiffness or pulse wave velocity. Seventeen studies assessed large vessel vascular stiff by PWV and related it to cognitive function or dementia. Six of these studies compared PWV in 154 persons with VaD, 207 with AD and 197 controls without dementia. Mean PWV was significantly (p < 0.0001) higher in VaD compared with controls. Mean PWV was significantly (p = 0.002) higher in VaD compared with AD. Fourteen studies examined the relationship between PWV and cognitive function. The majority of studies (nine of 14) reported a significant correlation between PWV and cognitive function. Four of eight studies that evaluated the relation using univariate analysis reported a significant correlation of PWV with the Mini Mental State Exam (MMSE) or Hasegawa Dementia Scale, and the correlation with MMSE between studies showed a close agreement of correlation coefficients (0.206 to 0.27). In multivariate analysis, adjusted for a wide range of possible confounding factors, the majority or 80% (eight out of 10) studies comprising a population of 6,034 individuals found a significant inverse relationship between PWV and cognitive function. In summary, vascular stiffness is inversely related to cognitive function. Vascular stiffness is greater in VaD compared with AD, suggesting PWV may be useful in identifying VaD.
Introduction
Vascular cognitive impairment (VCI) encompasses a spectrum from mild impairment in mental executive function to severe reductions in memory and executive functions or vascular dementia (VaD). VCI is unified by a conceptual framework of disorders of vascular function altering cerebral blood perfusion to areas of the brain involved in cognitive function (Citation1–4). VaD is the second most common kind of dementia after Alzheimer's disease (AD) and in some age groups maybe the most common kind of dementia (Citation3,Citation5). The clinical diagnosis of VCI has been plagued with problems in its diagnostic criteria (Citation6–8) that have lead some investigators to conclude that its definition is “a work in progress” (Citation2). VCI is a composite of impairment in cognitive and executive function with concomitant cerebrovascular disease of a severity and location that can be causally linked to the impairment in cognitive function. Multiple tests of cognitive and executive function permit the clinician to detect impairment in function in these spheres (Citation7). Tests for vascular disease, however, are more problematic. Brain imaging studies provide evidence of structural alterations in the brain but do not confirm the presence of arterial disease. The quandary with using risk factors for vascular disease as supporting evidence for VCI diagnosis is exemplified by hypertension. While hypertension is a risk factor for cerebral vascular disease, the high prevalence of hypertension in older individuals (Citation9,Citation10) limits its specificity as a diagnostic criterion for VCI, which also becomes manifested in older age groups. There is a need for a more precise measurement of the presence of vascular disease.
Recent attention has focused on the measurement of vascular stiffness, recognized by a faster speed of conduction of the pulse throughout the arterial tree, as an indicator of the status of the vasculature (Citation11,Citation12) because it is associated with premature cardiovascular disease (Citation12,Citation13). Increased pulse pressure, a manifestation of the increased arterial stiffness, is more directly applied to the brain and kidney than other organs because other tissues are protected by more ready vasoconstriction (Citation14). Increased arterial stiffness is purportedly more closely linked to pathological changes in the cerebral vasculature than other vasculatures (Citation15). Yet vascular stiffness was not considered amongst the potential adjunctive diagnostic factors in an intensive review of the diagnostic criteria for VCI and VaD (Citation2). The principal goal of this study is to conduct a qualitative and quantitative structured analysis of the data to determine whether vascular stiffness is altered in VaD, to determine its utility in differentiating VaD from AD and to examine the relationship between vascular stiffness and cognitive function.
Methods
Study identification
A MEDLINE/Pubmed/Embase search was conducted covering the period until August 2010, using a search strategy based on the key words: dementia, vascular dementia, dementia of vascular origin, cognitive function and arterial stiffness, or pulse wave velocity, as either text words or Subject heading terms. Only English language studies were evaluated. All articles were reviewed and the reference lists were searched for additional papers on the subject.
The strategy was to be inclusive of all possible studies and no papers were excluded based on the nature of the scientific journal in which they were published or the nature of the study. For comparison of arterial stiffness in patients with VaD or AD, the only preset exclusion criteria were studies that did not identify individuals with dementia as having AD or VaD. Six studies were identified that compared vascular stiffness in persons with VaD, AD or controls – without dementia. Two studies were excluded from comparative analysis – one study in which the entry criteria specifically excluded individuals with VaD (Citation16) and another which did not evaluate vascular stiffness separately according to the type of dementia (i.e. VaD vs AD) (Citation17). All studies were included regardless of the criteria that were used for the diagnosis of dementia, VaD or Alzheimer dementia. All studies were included regardless of the methodology used to measure cognitive function. Pulse wave velocity (PWV) was the index of vascular stiffness reported in all studies, so that PWV was considered the measurement of vascular stiffness, regardless of the instrumentation used to measure it. All vascular segments were considered, which include carotid to femoral (cfPWV), heart to brachial (hbPWV) or brachial to ankle/tibial artery (baPWV). If studies reported more than one vascular segment, then only cfPWV data were used.
Studies were reviewed and data extracted on the details of the diagnostic criteria for VaD and AD, specifically which clinical, brain imaging (computerized tomography, CT, or magnetic resonance imaging, MRI) or tests of cognitive function were used. The subject characteristics were also extracted, specifically individual's age, sex the presence of risk factors for cardiovascular disease (hypertension, diabetes mellitus, dyslipidemia and cigarette consumption), the use of antihypertensive drugs and their type were also collected.
Data analysis
The data are presented as the mean ± SD. Data analysis used STATA version 10 with online updates, using Metan. Tests of significance compared the mean difference between groups (Z statistic).
Results
Six studies compared vascular stiffness in persons with VaD and performed comparisons with controls without dementia or persons with AD (Citation18–23) (). Diagnostic criteria for VaD varied widely between studies using different diagnostic criteria and in some studies MRI. Three studies used international standards such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) for VaD and AD (Citation20,Citation21,Citation22), one study used primarily the Hashinski Score plus MRI (stroke or multiple lacuna infarcts) (Citation18), one study relied mainly on MRI (deep white matter lesions or periventricular hyperintensities) (Citation23) and one study did not report its diagnostic criteria (Citation19). A total of 561 persons were in the analysis, which included 154 with VaD, 209 with AD and 198 controls (). The populations were mainly in the 70–80-year age group. The proportion of women predominated in most but not all studies. The proportion of persons with hypertension varied from 36% to 76%. While antihypertensive drug usage was high, there was not enough information reported to analyze the data according to the type of antihypertensive drug. Most studies had the expected larger proportion of AD than VaD subjects, with one notable exception that had almost twice as many VaD than AD persons (Citation20). In that study, the investigators allocated persons with criteria for both conditions into the VaD group (Citation20).
Table Ia. The diagnostic criteria for vascular dementia (VaD) in studies comparing pulse wave velocity in VaD, Alzheimer's disease and controls.
Table Ib. The diagnostic criteria for Alzheimer's disease (AD) in studies comparing pulse wave velocity in vascular dementia, AD and controls.
Table II. Characteristics of the subjects in studies comparing pulse wave velocity in persons with vascular dementia (VaD), Alzheimer's disease (AD) and controls.
PWV was reported in all studies and was the index of arterial stiffness used for meta-analysis. PWV, however, was either brachial–ankle or carotid–femoral segments (). The data was consistently presented as the mean and SD for each group, which provided the basis for the meta-analysis (Citation24). Five studies directly compared VaD and controls, and four out of five studies reported a higher PWV in VaD than controls, of which three studies showed a statistically significantly greater PWV (). The mean PWV was higher in all persons with VaD compared with all those in the control group (1809 ± 414 vs 1487 ± 418 cm/s) (, inset). Comparison of the difference in PWV showed a significant (p < 0.0001) difference between VaD and controls ().
Figure 1. Pulse wave velocity (PWV) in persons with vascular dementia (VaD) compared with individuals without dementia (Control). (a) The PWV, mean ± SD for persons with vascular dementia and those without dementia is shown for all studies that compared the two. The significance level in each study is also provided. The inset shows the pooled mean + SD and total number of persons with vascular dementia and those without dementia. (b) Comparison of the differences in PWV for all studies that compared vascular dementia and those without dementia. The data is shown as the mean difference and the 95% confidence interval (CI). The weighting that each study contributed to the overall mean is indicated as well as the overall mean (CI) and statistical testing.
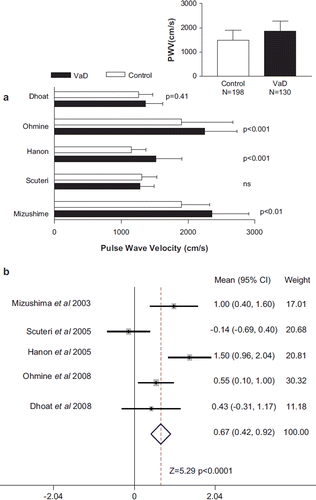
Table III. Univariate analysis of the correlation of pulse wave velocity (PWV) and cognitive function.
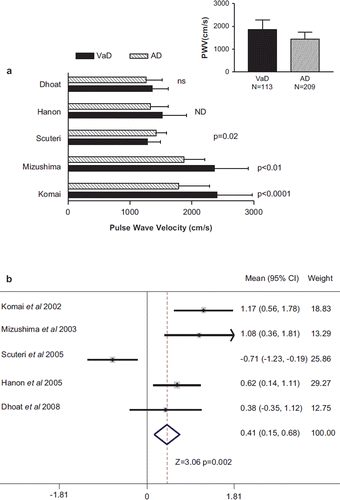
PWV was higher in VaD compared with AD in four studies, of which two reported a statistically significant difference (). In contrast, one study reported a significantly lower PWV in VaD than AD (Citation20). The mean PWV was higher in all persons with VaD compared with all those with AD (1862 ± 414 vs 1430 ± 310 cm/s) (, inset). Meta-analysis showed that there was a significant (p = 0.002) difference in PWV in VaD compared with AD.
Figure 2. Pulse wave velocity (PWV) in persons with vascular dementia compared with individuals with Alzheimer dementia (AD). (a) The PWV, mean ± SD, for persons with vascular dementia (VaD) and those with Alzheimer dementia (AD) is shown for all studies that compared the two. The significance level in each study is also provided. ns, not significant. ND indicated that the p-value was not reported. The inset shows the pooled mean + SD and total number of persons with VaD and AD. (b) Comparison of the differences in PWV for all studies that compared VaD and those with AD. The data is shown as the mean difference and the 95% confidence interval (CI). The weighting that each study contributed to the overall mean is indicated as well as the overall mean (CI) and statistical testing.
No study examined data on a potential gradient of risk, i.e. a correlation between PWV and cognitive impairment, in VaD. We next sought to analyze the relationship between PWV and cognitive function in all studies reporting such an association, regardless of the aetiology of any impairment in cognitive function, recognizing that a proportion of individuals will have VCI. Eight studies were identified that reported such data in univariate or almost univariate analysis (). Three of them were considered above (Citation18,Citation20,Citation21) and five additional studies were identified (Citation16,Citation25–28). The correlation between PWV and cognitive function assessed by the Mini Mental State Exam (MMSE) showed remarkably similar correlation coefficients in three studies with r values between 0.213 and 0.27, despite differences in sample size (Citation20,Citation21,Citation26). The correlation coefficient was larger in one study if persons with white matter hyperintensity lesions (WML) on brain imaging were included in the analysis (Citation16), suggesting that VCI is more closely linked to PWV than with other types of cognitive impairment. One study did not find a significant association between PWV and MMSE, perhaps because all the participants already had dementia (Citation18). Two studies did not examine the continuous relationship between PWV and cognitive function, but compared PWV in persons with impaired MMSE (<24) to persons with higher MMSE (24 or higher) and found no difference in mean PWV (Citation27,Citation28). In one study, a subset of persons over 60 years of age with the metabolic syndrome showed a significant inverse correlation between PWV and MMSE (Citation27). In another study, although there was no significant difference in MMSE in different tertiles of PWV, in one of these studies, its noteworthy that the highest tertile of PWV had an odds ratio of 1.6 times the lowest tertile for impaired cognitive function (Citation28).
Five additional studies were identified that used multivariate analysis to adjust for the role of age and other factors in that influence cognition (Citation28–32). Two of the studies that used univariate analysis did not use multivariate analysis (Citation18,Citation26). The majority of studies showed a statistically significant association between PWV and cognitive function, even after adjusting for age, sex, mean blood pressure, educational level, body mass index, lipids and cardiovascular medications (). The testing for cognitive function was more extensive in some of these studies than the MMSE and included other tests of cognitive and executive function – Stroop, Weschler Adult Intelligence Scale, Blessed Memory IMC, Verbal fluency, Grober–Buske and Benton Visual Retention tests (Citation25,Citation29–32). One study that found no relationship in univariate analysis, as expected, also found no relationship in multivariate analysis perhaps because PWV was analyzed according to tertiles of PWV (Citation28). One of the studies presented the data only for men, as they did not find an association between cognitive function and vascular factors in women (Citation32). Taken together, in multivariate analysis, adjusted for a wide range of possible confounding factors, the majority or 80% (eight out of 10) studies comprising a population of 6,034 individuals found a significant inverse relationship between PWV and cognitive function. There is additional supportive data. In a cross-sectional association study in 203 subjects (87 men, 116 women), all of whom were 85 years old, PWV was significantly increased in the impaired MMSE group compared with the normal MMSE group and the association remained evident after multivariate analysis considering hypertension, diabetes mellitus and hypercholesterolemia (Citation33).
Table IV. Multivariate analysis of the correlation of pulse wave velocity (PWV) and cognitive function after adjusting for other factors.
Discussion
This meta-analysis showed a statistically significantly greater PWV in persons with VaD compared with persons without dementia as well as a statistically greater PWV in persons with VaD compared with persons with AD. The present analysis is the first meta-analysis on this subject and encompasses 561 persons of whom 154 had VaD, 209 had AD and 198 were controls without dementia. A salient feature of the literature was the existence of only a few studies that have examined the relationship between PWV and VaD, and the small sample size of most of these studies. The advantage of meta-analysis is the strength in combining studies with small sample sizes to reach conclusions that might not be otherwise evident (Citation24). Our meta-analysis demonstrated an overall effect that was not uniformly evident in each study but was evident in several studies within each diagnostic category. The higher PWV in VaD validates the “vascular component” of VaD.
Qualitative analysis of the studies showed that the definitions of VaD varied widely. This finding reflects between study lack of uniformity in diagnostic criteria (for VaD) as well as the difficulties in arriving at universally accepted diagnostic criteria for this condition (Citation2,Citation34,Citation35). The studies also did not further subgroup the diagnostic criteria to identify the distribution of the different types of diseases (e.g. large vessel or small vessel disease) that was responsible for VaD (Citation36). The disparate nature of the diagnostic criteria between studies should not be grounds for discounting their reliability but rather is reflective of the state of the art in defining VaD (Citation2).
VaD is one extreme of the spectrum of cognitive impairment on a vascular basis (VCI) (Citation1,Citation2,Citation37). It was not possible to examine PWV across the spectrum of VCI because there were no studies that examined the relationship between PWV and cognitive function only in VCI. It is noteworthy, however, that the correlation between PWV and cognitive impairment was higher when persons with brain imaging evidence for VCI, namely WML, were included in the analysis compared with when they were excluded from analysis (Citation16), suggesting that VCI is more closely linked to PWV than other types of cognitive impairment. Additional support for a link between PWV and VaD is the neuroimaging data, showing a correlation between greater arterial stiffness and more extensive the evidence of WML on MRI evaluated by a grading system or the Fazekas score (Citation32).
The majority of studies report a statistically significant correlation between PWV and MMSE with similar correlation coefficients from 0.206 to 0.256 (Citation20,Citation21,Citation26,Citation33). There were several large studies that did not report an association between cognitive function and PWV but these studies dichotomized MMSE to values below 24 and 24 or higher, and compared the mean PWV (Citation27,Citation28). This kind of analysis stands in contrast to studies that examined the relationship across the range of MMSE, which showed significant correlations (Citation38). The more compelling case for a relationship between PWV and cognitive function comes from studies using multivariate analysis. A significant relationship between PWV and cognitive function was evident in multivariate analysis, in the majority of studies that adjusted for a range of other factors including age, sex, education and risk factors for atherosclerotic vascular disease such as diabetes mellitus, hypertension, lipids and cigarette smoking. Because PWV is higher in VaD than AD, we speculate that a meaningful part of the relationship between PWV and cognitive function is related to the presence of VCI.
We found a statistically significantly higher PWV in persons with VaD compared with persons with AD. A greater PWV in VaD was noted in all but one study, which reported the reverse (Citation20). There are two factors that were unique in that study. First the number of persons with VaD was unusually high – almost twice the number with AD (Citation20) compared with all the other studies. The investigators classified persons with features of AD plus VaD into the VaD group likely producing a group that primary had AD rather than VaD (Citation20). Second there was a high proportion of their study population on nitrate therapy (Citation20). These factors may account for the differences between that study and the others, and may account for the significant heterogeneity between studies. Other kinds of analysis strengthen the contention that PWV is higher in VaD than AD. Categorizing patients into those with higher or lower PWV showed a preponderance of VaD in those with high PWV and a higher proportion of persons with AD in the group with lower PWV (Citation18). After adjusting for age, gender, systolic blood pressure, education level, use of antihypertensive medications and the presence of cardiovascular disease, each 2-m/s increment in PWV (cfPWV) was associated with an odds ratio of VaD of 3.5 compared with only 1.7 for AD (Citation21). Thus the present meta-analysis in conjunction with other analysis (Citation18,Citation21) suggests that PWV may be useful in differentiating VaD from AD and perhaps useful in the diagnosis of VCI and VaD compared with AD.
Several underlying phenomena may explain the relationship between PWV and reduced cognitive function leading to VaD. PWV as an index of arterial stiffness is also an indicator of the status of the vasculature and thereby is a reflection of the status of the cerebral vasculature. Atherosclerosis in the cerebral arteries will eventually compromise cerebral perfusion leading to VaD (Citation39). The link between high PWV and cognitive impairment may explain the link between left ventricular hypertrophy (LVH) and cognitive impairment because high PWV may induce greater LVH (Citation40). Cardiovascular risk factors may be responsible for arterial stiffness and a decline in cognitive function through different mechanisms. However, the more intriguing possibility is that stiff arteries are the cause of cerebral vascular small vessel disease leading to cognitive decline. Because the brain is not shielded from the pulsatile nature of blood flow, increases in pulsation associated with arterial stiffness may directly damage intracerebral large and small vessels as well as adversely affecting the integrity of the blood-brain barrier (Citation14,Citation15,Citation41).
Distinguishing VaD from AD on the basis of vascular factors alone has limitations. Some patients with VaD and AD have overlapping features such that they cannot be easily separated into only one of the two categories. In addition, vascular factors have been implicated in the production of AD as well as VaD (Citation42), to blur the understanding of pathophysiological mechanisms that should produce two discrete diseases. To the extent that vascular factors are operative in both AD and VaD, this meta-analysis suggests that VaD is more likely associated with large vessel disease that can be detected by PWV.
The criteria for “proof” that a cardiovascular risk factor is causally related to a cardiovascular disease are stringent (Citation43). PWV satisfies some but not all of these criteria. First, PWV is increased in patients with the most severe form of cognitive impairment – VaD. Second, there is a gradient of risk with increasing PWV being associated with lesser cognitive function (Citation20,Citation21,Citation26). Third, multivariate analysis demonstrated that the association of PWV and cognitive impairment is independent of factors that affect cognitive function such as age and education (Citation20,Citation25,Citation29,Citation31). Furthermore, the association of PWV and cognitive impairment is independent of other factors that may affect PWV such as blood pressure, cigarette smoking and lipids (Citation16,Citation20,Citation21,Citation29,Citation31). Fourth, PWV predicts the development of impaired cognitive function or future cognitive decline (Citation31,Citation44). Fifth, experimental evidence provides a biological mechanism for a causal role for PWV in the production of cerebral arterial disease (Citation14,Citation15,Citation41). Crucial pieces of evidence are still missing specifically relating PWV to cognitive function only in persons with VCI and data that reduction of vascular stiffness can improve cognitive function or prevent its decline.
Limitations of this meta-analysis largely revolve around the underlying nature of the studies in the field specifically differences in diagnostic criteria for VaD and AD, subject characteristics and sample size. It might have been preferable to relate the data to cognitive decline from a vascular or non-vascular cause but such data are not consistently available. The certainty to which subjects were correctly classified as VaD or AD is unknown and one can only speculate on the extent to which it might have influenced the results. Studies of VaD are inherently difficult. Some investigators believe that the link between the vasculature and dementia is “an uncertain presumption in some patients” (Citation4). Whether a lacunar infarct (stroke) is or is not responsible for symptoms or cognitive impairment in a given person can be debated. Despite these caveats, the higher PWV in VaD validates the concept of the “vascular component” of VaD. The number of studies available to examine the question concerning the value of PWV as a technique to asses VCI is small and the sample size of most of these was also small. However, the meta-analysis was able to include almost 560 persons. Another issue is that we included all studies, which meant combining studies using PWV in different arterial segments, which may affect the results. The alternative of excluding studies would have lead to bias in the selection studies for inclusion. The studies, in this meta-analysis, were done during a period when there was no standardized or recommended arterial segment to assess vascular stiffness. Subsequently, European guidelines suggest that carotid–femoral PWV should be considered the standard measurement of arterial stiffness (Citation12). The majority of studies evaluated herein used carotid–femoral PWV. While we assessed only PWV because it was the only index of arterial stiffness measured in all studies that investigated VaD, augmentation index also correlates strongly with VaD even after multivariate analysis considering blood pressure, cigarette consumption, heart rate and body mass index (Citation23).
Our conclusion that PWV is higher in VaD compared with AD suggests the potential value for PWV as an additional diagnostic criterion for VaD. This subject requires further research in the construction of a diagnostic algorithm for VCI that is inclusive of PWV. The significant correlation between PWV and detailed assessment of cognitive impairment suggests that PWV should be evaluated as a target for intervention to prevent cognitive decline.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hachinski V. Shifts in thinking about dementia. JAMA. 2008; 300:2172–2173.
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis D, Black SE, . National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241.
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, . Vascular cognitive impairment. Lancet Neurology. 2003;2:89–98.
- Meyer JS, Shirai T, Akiyama H. Vascular dementia. Welch KMA, Caplan LR, Reis DJ, Siesjo BK, Weir B. Primer on cardiovascular diseases. San Diego: Academic Press; 1997. 364–366.
- Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. New Engl J Med 1993;328:153–158.
- Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD–10, NINDS-AIREN). Stroke 1996;27:30–36.
- Wiederkehr S, Simard M, Fortin C, van Reekum R. Validity of the clinical diagnostic criteria for vascular dementia: A critical review. Part II. J Neuropsychiatry Clin Neurosci. 2008;20:162–177.
- Wiederkehr S, Simard M, Fortin C, van Reekum R. Comparability of the clinical diagnostic criteria for vascular dementia: A critical review. Part I. J Neuropsychiatry Clin Neurosci. 2008;20:150–161.
- Rabkin SW, Mathewson AL, Tate RB. Predicting risk of ischemic heart disease and cerebrovascular disease from systolic and diastolic blood pressures. Ann Intern Med 1978; 88:342–345.
- Rabkin SW, Mathewson FA, Tate RB. Long term changes in blood pressure and risk of cerebrovascular disease. Stroke 1978;9:319–327.
- Blacher J, Safar ME. Large-artery stiffness, hypertension and cardiovascular risk in older patients. Nature Clin Pract Cardiovasc Med. 2005;2:450–455.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, . Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension. 2005;46:200–204.
- Byrom FB. The evolution of acute hypertensive arterial disease. Progr Cardiovasc Dis 1974;17:31–37.
- Nagai K, Akishita M, Machida A, Sonohara K, Ohni M, Toba K. Correlation between pulse wave velocity and cognitive function in nonvascular dementia. J Am Geriat Soc. 2004; 52:1037–1038.
- Meaume S, Rudnichi A, Lynch A, Bussy C, Sebban C, Benetos A, . Aortic pulse wave velocity as a marker of cardiovascular disease in subjects over 70 years old. J Hypertens. 2001;19:871–877.
- Komai N, Ohishi M, Kaibe M, Jinno T, Katsuya T, Higaki J, . Arterial pulse wave velocity in elderly patients with dementia. Geriat Gerontol Int. 2002;2:193–198.
- Mizushima Y, Oobasawa H, Yoshida S, Irie H, Urata T, Shimoda H. Pulse wave velocity in persons with vascular dementia. J Am Geriat Soc. 2003;51:1329–1330.
- Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: A pilot study. J Hypertens. 2005;23: 1211–1216.
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, . Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197.
- Dhoat S, Ali K, Bulpitt CJ, Rajkumar C. Vascular compliance is reduced in vascular dementia and not in Alzheimer's disease. Age Ageing. 2008;37:653–659.
- Ohmine T, Miwa Y, Yao H, Yuzuriha T, Takashima Y, Uchino A, . Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res Clin Exp. 2008;31:75–81.
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley; 2000. 2–35.
- Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190: 143–149.
- Abbatecola AM, Barbieri M, Rizzo MR, Grella R, Laieta MT, Quaranta E, . Arterial stiffness and cognition in elderly persons with impaired glucose tolerance and microalbuminuria. J Gerontol SerA Biol Sci Med Sci. 2008;63:991–996.
- Kim Y-S, Kim D-H, Choi BH, Sohn E-H, Lee AY. Relationship between brachial-ankle pulse wave velocity and cognitive function in an elderly community-dwelling population with metabolic syndrome. Arch Gerontol Geriat. 2009;49: 176–179.
- Sugawara N, Yasui-Furukori N, Umeda T, Kaneda A, Sato Y, Takahashi I, . Comparison of ankle-brachial pressure index and pulse wave velocity as markers of cognitive function in a community-dwelling population. BMC Psychiatry. 2010; 10:46.
- Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, . Arterial stiffness, cognitive decline, and risk of dementia: The Rotterdam study. Stroke. 2007;38:888–892.
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–673.
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104.
- Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, . Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229–1236.
- Fukuhara M, Matsumura K, Ansai T, Takata Y, Sonoki K, Akifusa S, . Prediction of cognitive function by arterial stiffness in the very elderly. Circulation J. 2006;70:756–761.
- Roman GC. Facts, myths, and controversies in vascular dementia. J Neurol Sci. 2004;226:49–52.
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, . Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260.
- Portera-Sanchez A, Porta-Etessam J. Microvascular pathology in vascuar and related dementia. Meyer JS, Rauch GM, Lechner H, Loeb C. Vascular dementia. New York: Futura Publishing Co.; 2001. 77–99.
- Roman GC, Sachdev P, Royall DR, Bullock R, Orgogozo J, López-Pousa S, . Vascular cognitive disorder: A new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87.
- Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, . Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. AmJ Hypertens. 2009;22:525–530.
- Roman GC. Brain hypoperfusion: A critical factor in vascular dementia. Neurol Res. 2004;26:454–458.
- Scuteri A, Coluccia R, Castello L, Nevola E, Brancati AM, Volpe M. Left ventricular mass increase is associated with cognitive decline and dementia in the elderly independently of blood pressure. Eur Heart J. 2009;30:1525–1529.
- Seo W-K, Lee J-M, Park MH, Park KW, Lee DH. Cerebral microbleeds are independently associated with arterial stiffness in stroke patients. Cerebrovasc Dis. 2008;26:618–623.
- Weller RO, Boche D, Nicoll JAR. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol. 2009; 118:87–102.
- Rabkin SW, Sackett DL. Epidemiology of arterial thromboembolism. Colman RW, Hirsh J, Marder VJ, Salzman EW.Hemostasis and thrombosis. Basic principles and clinical practice. Philadelphia, PA: J.B. Lippincott Co.; 1982. 873–888.
- Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–40.
