Abstract
Purpose: Masked hypertension (MH) independently predicts mortality but cannot be diagnosed from clinic blood pressure (BP) taken under resting conditions. We sought to determine if MH could be identified from BP taken during a single bout of low-intensity exercise. Methods. BP was recorded at rest and during brief low-level cycling exercise (60–70% of age-predicted maximal heart rate) in 75 untreated subjects with a hypertensive response to exercise (aged 54 ± 9 years). All subjects underwent 24-h ambulatory BP monitoring (ABPM) and MH was defined as clinic BP < 140/90 mmHg and ABPM BP ≥ 130/80 mmHg. Results. There were 42 (56%) patients with MH, and at rest systolic (SBP) was higher in subjects with MH compared with those without MH (127 ± 9 vs 120 ± 9 mmHg; p < 0.05). During exercise, MH subjects had significantly higher SBP (188 ± 22 vs 168 ± 15 mmHg; p < 0.05), with a greater change from baseline (61 ± 21 vs 48 ± 15 mmHg; p < 0.05). Low-level exercise SBP was independently associated with MH, and if ≥175 mmHg, identified MH with 74% sensitivity and 67% specificity (p < 0.001). Conclusion. MH can be identified in untreated individuals from low-intensity exercise SBP. Further research on the diagnostic value of BP during early phases of exercise stress testing is needed.
Introduction
Clinic blood pressure (BP) predicts cardiovascular morbidity and mortality (Citation1). A limitation of this method is the inability to detect certain BP abnormalities such as masked hypertension (MH). This condition is associated with increased mortality (Citation2), and is defined by normal clinic BP but elevated BP outside the office environment (Citation3). Currently, the methods to identify MH involve 24-h ambulatory BP monitoring (ABPM) or home BP monitoring in conjunction with clinic BP. An alternative and rapid screening test to identify suspected MH may be clinically useful. Exercise stress testing is widely used to reveal cardiovascular abnormalities that are not identifiable at rest. Interestingly, a hypertensive response to exercise (HRE) has been shown to predict the future onset of hypertension, irrespective of apparently normal office BP (Citation4–6). Additionally, since patients with MH exhibit higher BP on ABPM, we hypothesized that an exaggerated BP elevation may be detected during a single bout of low-stress physical activity, and that this may differentiate between people with and without MH. We sought to test this hypothesis in a study of subjects with exaggerated exercise BP (Citation7), as these patients may have increased propensity for MH.
Methods
Subjects and protocol
This study was performed in 75 untreated subjects with exaggerated exercise BP. Clinical characteristics of the study population are outlined in . All participants had previously undergone an exercise stress test (Bruce protocol to volitional fatigue), as part of usual clinical care, and were identified from hospital records as having an exaggerated exercise BP, defined from Framingham data (Citation8) as peak exercise systolic BP (SBP) ≥ 210 mmHg for males and ≥190 mmHg for females. Patients were only included if clinic BP was <140/90 mmHg (on two separate clinic visits) and they had not previously been diagnosed with hypertension and were not taking antihypertensive medication. Patients with a clinical history of coronary or renal disease were excluded. MH was defined according to current guidelines (Citation3,Citation9) as clinic BP <140/90 mmHg and ABPM BP ≥ 130/80 mmHg. Non-MH (normotensive with exaggerated BP response to exercise) was defined as a clinic BP < 140/90 mmHg and ABPM BP < 130/80 mmHg. Each patient underwent assessment of exercise BP during a single laboratory visit. Additional fasting blood samples were obtained via venipuncture, and ABPM began that day. The research was approved via local human research ethics committee and all procedures were carried out in accordance with the Declaration of Helsinki (2000) with all participants providing informed consent.
Table I. Clinical characteristics of study population (n = 75).
BP
All clinic BP measures were taken by a trained technician using a mercury sphygmomanometer and appropriately sized cuff (Baumanometer, W.A. Baum Co., New York, NY, USA) after 5 min of rest in the supine position as per JNC 7 guidelines (Citation3). The average of two measures was used as the baseline clinic BP value. Exercise BP was also measured using a mercury sphygmomanometer and recorded as the average of duplicate measures. ABPM was recorded using a validated device (TM2430, A&D Mercury, A&D Medical, Thebarton, South Australia) on a typical mid-week day. Measures were recorded each 30 min during the day (06:00 to 22:00 h) and each hour over night (22:00 to 06:00 h).
Exercise protocol
Exercise BP measurements were performed during upright cycling using a cycle ergometer (model 818E; Monark, Varberg, Sweden). Load was variably set for each individual in order to achieve a steady state heart rate at 60–70% of age-predicted maximal heart rate (defined as 220 − age × 0.60 or 0.70) as previously described (Citation10). All patients achieved this intensity, which typically equated to “very light” to “moderate” exercise, or a rating of 1–3 out of the Borg 10-point scale. Once the desired intensity was reached, exercise was performed at steady state for approximately 3–5 min during which time all exercise BP measures were recorded.
Arterial stiffness
We were interested in assessing arterial stiffness between subjects with and without MH, because this has been associated with exaggerated exercise BP (Citation11). As a measure of regional artery stiffness, pulse wave velocity (PWV) was calculated during supine rest using electrocardiogram-gated hand-held applanation tonometry (SphygmoCor 7.1; AtCor Medical., Sydney, Australia). Aortic PWV was determined from sequential carotid and femoral waveforms, whereas brachial PWV was determined from carotid and radial waveforms.
Blood biochemistry
Blood biochemistry analysis of fasting plasma glucose, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride levels was performed through hospital pathology services via standard procedures.
Statistics
Data was analysed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). The clinical characteristics and hemodynamic differences between those with MH and those without MH were assessed by independent t-tests and chi-square analysis. Analysis of covariance (ANCOVA) was additionally undertaken to assess between-group differences, correcting for any baseline differences. Pearson product moment correlations were performed to assess relationships between variables. Binary logistic regression using a forced entry method was utilised to determine independent predictors of MH. Multi-collinearity was defined as an r-value for the association between independent variables of ≥0.70 or if tolerance was <0.10. Receiver operator curve analysis was undertaken to determine light exercise brachial BP cut-off values for predicting the presence of MH. p < 0.05 was considered significant.
Results
Among the entire study population (n = 75), there were 42 (56%) participants with MH (). There was a predominance of male subjects in the MH group (p < 0.05). MH patients were taller, with increased daytime SBP and DBP, increased nocturnal SBP and DBP, and increased ABPM SBP and DBP compared with those without MH (p < 0.05 for all). Furthermore, those with MH had increased plasma triglycerides, plasma glucose, plasma HbA1c, aortic and brachial PWV compared with those without MH (p < 0.05 for all). Smoking status did not differ between groups. Resting SBP was significantly higher in MH subjects compared with those without MH (p < 0.001; ).
Table II. Hemodynamic differences between normotensives with exaggerated blood pressure (BP) response to exercise and masked hypertensive individuals.
Exercise BP for delineation of MH
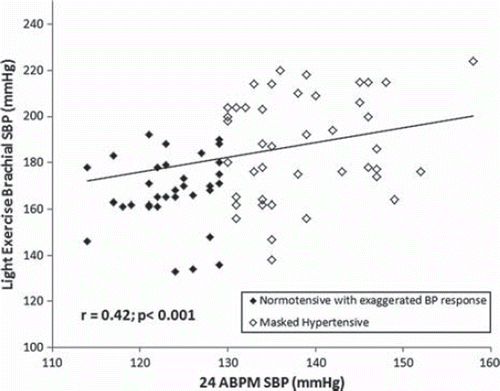
After correction for resting brachial SBP, MH subjects had significantly higher brachial SBP during light exercise, with greater changes from resting conditions ( and ). These differences remained significant after additional correction for sex, height, triglyceride and blood glucose levels (p < 0.05). Light exercise brachial SBP was significantly correlated with brachial SBP acquired by ABPM (r = 0.42, p < 0.001; ).
Figure 1. Change in systolic blood pressure (SBP) from rest to light exercise (60–70% maximum heart rate, HRmax) for the study population (n = 75). Values are significantly different between normotensives with exaggerated BP response to exercise and masked hypertensives and remain significant after analysis of covariance (ANCOVA) correction for height, sex, fasting triglycerides and plasma glucose levels. *p < 0.05. Error bars are standard error of the mean.
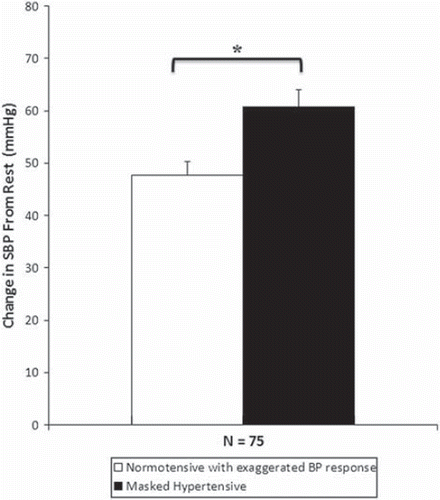
Figure 2. Pearson correlation graph showing the relationship between 24-h ambulatory systolic blood pressure (SBP) and light exercise brachial SBP in the study population (n = 75).
A logistic regression model for predictors of MH was constructed with the following independent variables entered in the model; light exercise brachial SBP, resting brachial SBP, sex (0 = female, 1 = male), body mass index, age, aortic PWV, fasting triglyceride, glucose and total cholesterol (). Significant predictors of MH were light exercise brachial SBP, sex and aortic PWV (p < 0.05). The overall model accounted for 48–64% of the variability in MH. The model also predicted MH with 82% specificity and 80% sensitivity (p < 0.001). However, when aortic PWV was removed from the model, light exercise SBP was no longer predictive of MH (p = 0.053). A light exercise brachial SBP value of 175 mmHg was identified as the optimal cut-off point to reveal MH, with 74% sensitivity and 67% specificity (p < 0.001). However, if light exercise brachial SBP was ≥190 mmHg, specificity increased to 97%, but with low sensitivity (48%; p < 0.001).
Table III. Logistic regression model for predictors of masked hypertension.
Discussion
The novel finding of this study was that MH could be “unmasked” (with high positive predictive value) via the BP response to light exercise in untreated subjects known to be at risk of MH. The adverse clinical consequences of MH are recognized in guidelines on the evaluation of high BP, but no recommendations are provided with respect to further BP investigations in people presenting with apparently normal office BP but in who MH may be suspected (e.g. HRE, increased left ventricular mass or other end-organ abnormalities) (Citation9). Thus, a significant proportion of people with heightened cardiovascular risk from MH may remain undetected according to current diagnostic recommendations. To our knowledge, these data provide the first preliminary evidence for the potential diagnostic value of light exercise BP.
Exercise BP and MH
The clinical significance of MH is demonstrated by an association with left ventricular hypertrophy (Citation12), increased risk for future development of sustained hypertension (Citation4–6) and increased mortality (Citation2). Although MH has not been extensively studied, there is data to suggest that it is common, with reported prevalence ranging from 15% in apparently healthy subjects (Citation2) to 30% in higher risk individuals such as those with diabetes (Citation13). The presence of exaggerated exercise BP may be a critical marker for the presence of MH because the prevalence of the condition seems to be markedly higher in those with exercise hypertension. This was identified in a recent study, which indicated 41% prevalence of MH in patients with an exaggerated exercise BP (Citation14). Indeed, subjects with a HRE in this current study had a 56% prevalence of MH, and a recent study in diabetic individuals with an exaggerated exercise BP response found the prevalence of MH to be 71% (Citation7).
Many studies have reported cardiovascular sequelae associated with an exaggerated exercise BP response attained at maximal (Citation15) or modest exercise workloads (Citation16–20). Considering that maximal exercise intensity is seldom reached in everyday life by the general population, measurement of BP during light to moderate exercise may be a more suitable tool to assess the true risk related to BP, since this medium would be analogous to the chronic BP load during daily life activities. In the present study, we found that light exercise brachial SBP was an independent predictor of MH, and significantly correlated with ABPM SBP. While cycle ergometry may not be incorporated into routine clinical practice, our results highlight the need for further research into the diagnostic value of BP during early stages of a standard exercise stress test, such as the commonly used Bruce treadmill protocol. This may provide another avenue by which poor BP control could be identified.
Mechanisms of abnormal exercise BP in MH
The mechanisms of an abnormal elevation of exercise BP remain unclear, but are likely to be multifactorial. Impaired endothelial function, which may act to hinder an appropriate vasodilatory response to exercise (Citation21) may contribute to an unusually sharp rise in BP during exercise (Citation22). Furthermore, the tone of the large elastic arteries during exercise should also contribute to the magnitude of the exercise BP rise. Naka et al. (Citation23) found that exercise results in an elevation in brachial PWV (indicating increased stiffness) and an overall vasoconstrictive response in a cohort of healthy subjects (Citation23). Our study showed that MH patients have increased aortic and brachial PWV under resting conditions. Whether this would translate to excessive large artery stiffness during exercise is unknown, but if this was the case, it may contribute to exaggerated exercise BP. Certainly, it is interesting that aortic PWV independently predicted the presence of MH.
Under resting conditions, central artery stiffness (aortic PWV) is also known to be associated with elevated BP (Citation24) and global endothelial dysfunction (Citation25), possibly related to decreased nitric oxide bioavailablity (Citation26). Although, whilst under the physical stressor of exercise, the effects of nitric oxide on vascular resistance appear to be less marked than at rest (Citation27) and nitric oxide may only play a minor role in the modulation of exercise BP changes (Citation28). Thus, exercise BP and vasodilatation of the muscular arteries supplying the skeletal muscle during exercise (Citation29) could be arbitrated by other unknown factors. Potential candidates may include blood rheology such as increased blood plasma viscosity (Citation30). Additionally, metabolic factors influencing vascular reactivity, including cholesterol and insulin resistance, and sensitivity may abnormally alter exercise BP changes from basal conditions (Citation31,Citation32). Indeed, patients with MH in this study had elevated triglyceride and glucose levels. Further studies are required to ascertain the pathways responsible for the abnormal exercise BP response in MH individuals.
Limitations
This was a study confined to patients with exaggerated exercise BP on the grounds that exercise BP may be a useful medium to differentiate MH in these individuals (Citation7). This hypothesis proved correct, but in order to confirm this, future studies should include a control group with normal exercise BP. Furthermore, we chose to increase the time intervals between night-time BP measures in order to decrease patient discomfort. This may have resulted in some participants being incorrectly assigned as MH or non-MH.
Conclusion
Although MH is a clinically significant condition known to be associated with cardiovascular morbidity and mortality (Citation2,Citation12,Citation33), current guidelines do not provide recommendations regarding diagnosis and management of the phenomenon (Citation3,Citation9). This study shows that brachial BP measurements taken during a low-intensity bout of exercise are significantly raised in subjects with MH. Moreover, light exercise brachial SBP predicts the presence of MH with high specificity. These findings suggest that an exaggerated BP response to light exercise may be a marker to delineate those at an increased risk related to BP. Thus, further studies should be directed towards determining the diagnostic value of BP acquired during the early stages of an exercise stress test in varying patient and healthy populations.
Acknowledgments
In part by a National Health and Medical Research Council project grant (reference 569669), Canberra, Australia. Dr Sharman was supported by a National Health and Medical Research Council Career Development Award (reference 569519). Dr Hare was supported by a scholarship co-funded by the National Heart Foundation of Australia (PC 07B 3407) and the National Health and Medical Research Council of Australia (reference 511266).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913.
- Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, . Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252.
- Tsumura K, Hayashi T, Hamada C, Endo G, Fujii S, Okada K. Blood pressure response after two-step exercise as a powerful predictor of hypertension: The Osaka Health Survey. J Hypertens. 2002;20:1507–1512.
- Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC, . Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–1836.
- Manolio TA, Burke GL, Savage PJ, Sidney S, Gardin JM, Oberman A. Exercise blood pressure response and 5-year risk of elevated blood pressure in a cohort of young adults: The Cardia Study. Am J Hypertens. 1994;7:234–241.
- Kramer CK, Leitao CB, Canani LH, Ricardo ED, Pinto LC, Gross JL. Blood pressure responses to exercise in type II diabetes mellitus patients with masked hypertension. J Hum Hypertens. 2009;23:620–622.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol. 1995;26:1630–1636.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the management of arterial hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536.
- Holland DJ, Sacre JW, McFarlane SJ, Coombes JS, Sharman JE. Pulse wave analysis is a reproducible technique for measuring central blood pressure during hemodynamic perturbations induced by exercise. Am J Hypertens. 2008;21: 1100–1106.
- Tsioufis C, Dimitriadis K, Thomopoulos C, Tsiachris D, Selima M, Stefanadi E, . Exercise blood pressure response, albuminuria, and arterial stiffness in hypertension. Am J Med. 2008;121:894–902.
- Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, . Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione arteriose monitorate e loro associazioni [PAMELA] study). Circulation. 2001;104: 1385–1392.
- Leitao CB, Canani LH, Kramer CK, Boza JC, Pinotti AF, Gross JL. Masked hypertension, urinary albumin excretion rate, and echocardiographic parameters in putatively normotensive type 2 diabetic patients. Diabetes Care. 2007;30:1255–1260.
- Kayrak M, Bacaksiz A, Vatankulu MA, Ayhan SS, Kaya Z, Ari H, . Exaggerated blood pressure response to exercise – A new portent of masked hypertension. Clin Exp Hypertens. 2010;32:560–568.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol. 1999;83: 371–375.
- Laukkanen JA, Kurl S, Salonen R, Lakka TA, Rauramaa R, Salonen JT. Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle-aged men. Hypertension. 2004;44:820–825.
- Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O'Donnell CJ, . Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2008;101:1614–1620.
- Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts mortality from myocardial infarction. Hypertension. 1996;27:324–329.
- Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension. 1994;24:56–62.
- Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens. 2001;19:1343–1348.
- Tzemos N, Lim PO, MacDonald TM. Exercise blood pressure and endothelial dysfunction in hypertension. Int J Clin Pract. 2009;63:202–206.
- Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, . Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320.
- Naka KK, Tweddel AC, Parthimos D, Henderson A, Goodfellow J, Frenneaux MP. Arterial distensibility: Acute changes following dynamic exercise in normal subjects. Am J Physiol Heart Circ Physiol. 2003;284:H970–978.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, . Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, . Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608.
- Sugawara J, Komine H, Hayashi K, Yoshizawa M, Yokoi T, Otsuki T, . Effect of systemic nitric oxide synthase inhibition on arterial stiffness in humans. Hypertens Res. 2007;30: 411–415.
- Brett SE, Cockcroft JR, Mant TG, Ritter JM, Chowienczyk PJ. Haemodynamic effects of inhibition of nitric oxide synthase and of l-arginine at rest and during exercise. J Hypertens. 1998;16:429–435.
- Sharman JE, McEniery CM, Campbell R, Pusalkar P, Wilkinson IB, Coombes JS, . Nitric oxide does not significantly contribute to changes in pulse pressure amplification during light aerobic exercise. Hypertension. 2008;51:856–861.
- Munir S, Jiang B, Guilcher A, Brett S, Redwood S, Marber M, . Exercise reduces arterial pressure augmentation through vasodilation of muscular arteries in humans. Am J Physiol Heart Circ Physiol. 2008;294:H1645–1650.
- Sharman JE, Brown J, Holland DJ, Macdonald G, Kostner K, Marwick TH. Influence of altered blood rheology on ventricular-vascular response to exercise. Hypertension. 2009;54:1092–1098.
- Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation. 2000; 101:611–615.
- Fossum E, Hoieggen A, Moan A, Rostrup M, Kjeldsen SE. Insulin sensitivity is related to physical fitness and exercise blood pressure to structural vascular properties in young men. Hypertension. 1999;33:781–786.
- Stergiou GS, Rarra VC, Yiannes NG. Prevalence and predictors of masked hypertension detected by home blood pressure monitoring in children and adolescents: The Arsakeion School Study. Am J Hypertens. 2009;22:520–524.
