Abstract
Objective. Hypertensive response at peak exercise and blunted blood pressure (BP) recovery, altered BP responses obtained from exercise stress testing, have been suggested as risk factors for future onset of hypertension in previous studies. Epicardial fat, a new cardiometabolic risk factor, has been linked to hypertension in some recent studies. In this study, we tested the primary hypothesis suggesting that the epicardial fat thickness (EFT) is related to altered BP responses to treadmill exercise testing. We also evaluated the sensitivity and specificity of the EFT as a predictor of hypertensive response to peak exercise. Methods. Normotensive subjects underwent to treadmill stress testing and transthoracic echocardiography. Hypertensive response to peak treadmill exercise testing was defined as ≥ 210/105 mmHg and ≥190/105 mmHg at peak exercise in males and females, respectively. BP recovery index (BPRI) was defined as the ratio of the BP at the 3rd minute of the recovery phase to BP at peak exercise. EFT was measured by echocardiography. Thirty-two subjects with hypertensive response to peak exercise constituted Group 1 and 48 subjects with normal response constituted Group 2. Results. The mean EFT of subjects in Group 1 was significantly higher (8.2 ± 1.1 mm vs 5.1 ± 1.5 mm; p = 0.0001) than subjects in Group 2. In correlation analysis performed in Group 1, EFT was found to be significantly correlated with BPRI (r = 0.51, p < 0.003). An EFT of ≥6.5 mm predicted the hypertensive response to peak exercise test with 68.8% sensitivity and 87.5% specificity (receiving operator characteristic area under curve: 0.879, 95% CI 0.793–0.965, p < 0.001). Patients with EFT ≥6.5 mm showed a significantly increased BPRI (0.89 ± 0.07 vs 0.74 ± 0.09, p < 0.0001) and peak systolic BP (198.4 ± 15.3 mmHg vs 169.4 ± 19.8 mmHg, p < 0.0001). There were significant differences in metabolic equivalents, maximum heart rate, homeostatic model assessment of insulin resistance, high-density lipoprotein-cholesterol, waist circumference and age values between two patients groups dichotomized according to the cut-off value of EFT. BPRI was the only independent variable related to EFT in the multivariate analysis (odds ratio = 1.4, 95% CI 2.75–7.16, p = 0.001). Conclusions. EFT was found to be related to altered BP responses to exercise stress testing. The echocardiographic measurement of EFT may serve as a useful non-invasive indicator of heightened risk of future hypertension.
Introduction
Hypertension remains the leading cause of death worldwide and one of the world's great public health problems (Citation1). Exercise stress testing is generally used as a tool to predict coronary artery disease. Apart from the ST-segment changes, exercise stress testing can also give some valuable information like blood pressure (BP) responses. Hypertensive response at peak exercise and blunted BP recovery obtained from exercise stress testing have been suggested as a risk factors for future onset of hypertension in previous studies (Citation2–4).
The relationship between obesity and hypertension is well established in adults (Citation5,Citation6). Epicardial fat tissue, another form of visceral adiposity, has been proposed as a new cardiometabolic risk factor and the possible association of epicardial fat with hypertension has been shown in some recent studies (Citation7–9). Although it has been demonstrated that healthy normotensive obese subjects showed an enhanced response of systolic BP (SBP) to a fixed, moderate level of exercise compared with non-obese subjects (Citation10), the role of excess and ectopic visceral fat in altered BP responses is unexplored.
In this study, we aimed to test the primary hypothesis suggesting that EFT predicts altered BP responses to exercise stress testing in normotensive individuals. We also assessed the sensitivity and specificity of the EFT as a predictor of hypertensive response to peak exercise stress testing.
Methods
Study subjects
Subjects were the volunteers for a routine check-up in a cardiology outpatient clinic. The subjects’ medical histories were obtained through a questionnaire. Those who had history of established heart disease, hypertension, known diabetes, overt liver disease, obesity, cancer, chronic renal disease, alcohol or drug abuse, thyroid problems were not included in the study. Participants were classified as hypertensive if resting SBP was ≥140 mmHg and/or diastolic BP (DBP) was ≥90 mmHg or if they were taking antihypertensive medications. Participants were classified as diabetic if fasting blood glucose level was ≥126 mg/dl or if they were taking any antidiabetic medication. Participants were classified as obese if body mass index (BMI) was ≥30. All subjects underwent transthoracic echocardiography and exercise stress testing to measure EFT and BP responses. Subjects who showed signs suggestive of ischemia, with ejection fraction below 50%, moderate to severe valvular stenosis or regurgitation, left ventricular (LV) hypertrophy more than mild level (interventricular septum and/or posterior wall thickness ≥1.2–1.3 cm) were excluded from the study (Citation11). Thirty-two subjects with hypertensive BP response at peak exercise constituted Group 1 and 48 subjects with normal BP response at peak exercise constituted Group 2. Written consents were obtained from all subjects and the institutional review board approved the study.
Anthromorphometry and laboratory tests
BMI was defined as weight (in kilograms) divided by the square of height (in meters). Waist circumference was recorded as the average of two measurements while the subject was standing at midpoint between the lowest rib and the iliac crest. Blood sampling was obtained at least 14 h of fasting. Levels of total cholesterol, triglycerides (TG), low-density lipoprotein cholesterol (LDL) and high-density lipoprotein cholesterol (HDL), glucose, creatinine, hemoglobin were assayed by routine laboratory techniques. Plasma concentration of insulin was measured in duplicate using commercial available kit (Biosource, Camarillo, CA). Homeostasis model assessment index (HOMA) was defined as fasting plasma insulin (mu/l) × fasting glucose (FG; mg/dl)/405 by using Matthews's equation (Citation12). The coefficients of variation were less than 5% for every measurement.
Echocardiograms
Complete transthoracic two-dimensional echocardiograms were obtained. Standard parasternal and apical views were obtained in the left lateral decubitus position using available equipment (Vivid 3 pro, GE Vingmed, Milwaukee, USA). Images were digitally stored and reviewed by a single cardiologist blinded to the subject's information in order to avoid inter-reader variability. Echocardiograms of 10 subjects were randomly selected and a second measurement of epicardial fat thickness (EFT) was performed 1 week later to assess the intraobserver variability. Measurement of the left ventricle (LV) and left atrial (LA) diameters was performed on M-mode traces recorded from the parasternal long axis view according to established standards (Citation13). Left ventricular mass (LVM) was calculated by using the Devereux Formula as described (Citation14). EFT was measured according to the method previously described and validated (Citation15). The epicardial fat was identified as the echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium. EFT was measured perpendicularly on the free wall of the right ventricle at end-diastole in three cardiac cycles. The maximum EFT was measured at the point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus. For the mid-ventricular parasternal short-axis assessment, maximum EFT was measured on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the interventricular septum at mid-chordal and the tip of the papillary muscle level, as the anatomic landmark. Intraobserver variability of the EFT was 2.7%.
Exercise stress testing
All the subjects instructed not to eat, drink any beverages or smoke for 4 h before the test underwent a symptom-limited exercise stress testing according to Bruce protocol (Quinton Treadmill system, Quinton, Inc., Bothell, WA, USA). The Tango exercise BP monitor (SunTech Medical, Morrisville, North Carolina) was used to automatically measure and display a subject's SBP, DBP and heart rate (HR). BP and HR were measured before, at 2 min of each stage of exercise and during the recovery. Exercise was stopped when the subjects demanded cessation because of exhaustion, or if the HR achieved was more than 95% of estimated maximal HR (220 – age). HR and BP at peak exercise were recorded. During the recovery phase, the subjects continued to walk for 60 s at a speed of 1.5 mph and they sat down for 3 min with continued BP, HR and rhythm monitoring. Myocardial ischemia was defined as J-point and ST80 (the point 80 ms from the J-point) depression of 0.1 mV.
Hypertensive response to treadmill exercise testing was defined as a BP of ≥ 210/105 mmHg and ≥ 190/105 mmHg at peak exercise in males and females, respectively (Citation3). BP recovery index (BPRI) was defined as the ratio of the SBP at 3rd minute of recovery phase to SBP at peak exercise (Citation16). Metabolic equivalents (METS) were calculated from the treadmill speed and the grade at peak exercise according to the previously described formula (Citation17).
Statistical analysis
Statistical analysis was carried out with a commercially available statistical package (SPSS for Windows version 10.0; SPSS Inc Chicago, IL, USA). Data are presented as mean ± SD for continuous variables and as relative frequencies for discrete variables. Mann–Whitney U test was used for continuous variables and the chi-square test was used for categorical changes. Relationships between variables were examined with Pearson correlation coefficients. The cut-off value of EFT for predicting the hypertensive response to exercise test with corresponding sensitivity and specificity was estimated by receiving operator characteristic (ROC) curve analysis. Multivariate logistic regression analysis was performed to assess which factors independently influence EFT. Variables selected for inclusion in the model were those significant at univariate analysis. A p-value <0.05 was considered to indicate statistical significance.
Results
Among 80 subjects, 52 (65%) were men and 28 (35%) were women. The mean (SD) for age was 50 ± 8 years. There was no significant difference between the two groups with regard to age, gender, smoking, waist circumference, BMI, serum glucose level, homeostatic model assessment of insulin resistance (HOMA-IR) index and serum lipid parameters. shows baseline demographic properties of the study subjects.
Table I. Demographic and biochemical variables.
Comparison of exercise stress testing and echocardiographic parameters is shown in . The mean EFT and BPRI of Group 1 were significantly higher than Group 2 (EFT; 8.2 ± 1.1 mm vs 5.1 ± 1.5 mm; p = 0.0001 and BPRI; 0.92 ± 0.06 vs 0.77 ± 0.09; p = 0.0001). LV end-diastolic and end-systolic diameters, left atrium (LA) dimension, and LVM were no different between the two groups. Basal HR, maximal HR, basal BP values, peak DBP and METS also were not different between two groups. Peak SBP of Group 1 was significantly higher (205.8 ± 12 mmHg vs 175 ± 16.8 mmHg; p = 0.0001) than that of Group 2.
Table II. Comparison of exercise stress testing and echocardiographic parameters.
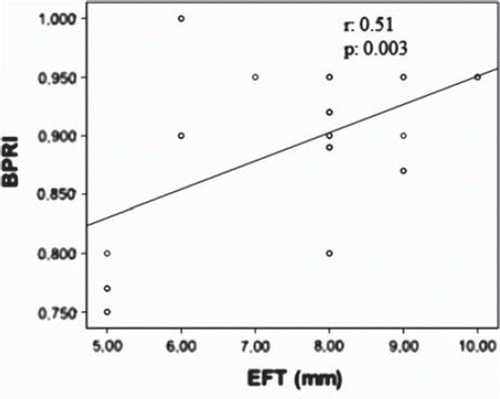
In correlation analysis performed in Group 1, EFT showed a strong correlation with BPRI (r = 0.51, p < 0.003) () and with peak SBP (r = 0.60, p = 0.001). Both EFT and BPRI were found to correlate positively with waist circumference (EFT: r = 0.44, p = 0.01 and BPRI: r = 0.30, p = 0.05) and negatively with METS (EFT: r = −0.40, p = 0.008 and BPRI: r = −0.32, p = 0.05).
Figure 1. Scatter plot of the relation between blood pressure recovery index (BPRI) and epicardial fat thickness (EFT).
An EFT of ≥6.5 mm predicted the hypertensive response to peak exercise test with 68.8% sensitivity and 87.5% specificity (ROC area under curve: 0.879, 95% CI 0.793–0.965, p < 0.001). Patients with EFT ≥ 6.5 mm showed a significantly increased BPRI (0.89 ± 0.07 vs 0.74 ± 0.09, p < 0.0001) and peak SBP (198.4 ± 15.3 mmHg vs 169.4 ± 19.8 mmHg, p < 0.0001). Moreover, there were significant differences in METS, maximum HR, HOMA-IR, HDL-cholesterol, waist circumference and age values between two patients groups dichotomized according to the cut-off value of EFT (). Multivariate logistic regression analysis was performed with EFT as a dependent variable and with BPRI, age, waist circumference, HOMA-IR, HDL, METS and maximum HR as independent variables. Peak SBP was not included in the model because BPRI was derivative of it. BPRI was the only independent variable related to EFT in the multivariate analysis (odds ratio = 1.4, 95% CI 2.75–7.16, p = 0.001).
Table III. Clinical and laboratory parameters according to epicardial fat thickness (EFT).
Discussion
The primary findings of this study were that epicardial fat was thicker in subjects with hypertensive response to peak exercise stress testing and EFT was significantly associated with BPRI. The secondary findings of this study were that an EFT of ≥6.5 mm predicted the hypertensive response to peak exercise test with 68.8% sensitivity and 87.5% specificity, and BPRI was the only independent variable significantly related to EFT in the multivariate analysis. To our knowledge, this is the first report showing the relationship between EFT and altered BP responses to exercise stress testing in normotensive subjects.
Ectopic fat is an important predictor of metabolic and cardiovascular disease, carrying more risk than general fat accumulation. Within this concept, epicardial fat has been emerged as a new cardiometabolic risk factor recently (Citation7). It has been reported that EFT reflects intra-abdominal visceral fat (Citation18). Supporting this, EFT was found to be correlated with waist circumference in our study.
There is some data about possible association between epicardial fat and hypertension in literature. Several studies have shown a link between epicardial fat accumulation, and development of coronary heart disease and hypertension (Citation19–21). Sironi et al. (Citation21) demonstrated that fat was preferentially accumulated intra-abdominally and intrathoracically in newly found, untreated men with essential hypertension. It was recently shown that early hypertension was associated with epicardial ectopic fat along with visceral fat (Citation9). Altered BP responses such as hypertensive response at peak exercise and blunted BPRI have been suggested as risk factors for future onset of hypertension in previous studies (Citation2–4). Although the mechanisms responsible for this altered BP responses have not been fully revealed, endothelial dysfunction and disturbed cardiovascular autonomic system have been suggested as a possible candidates (Citation4,Citation16,Citation22,Citation23). Burger et al. (Citation10) have recently demonstrated that healthy obese subjects showed an enhanced response of SBP to a fixed, moderate level of exercise. A recent study showed that post-exercise BP recovery was lower in type 2 diabetic patients when compared with non-diabetic persons (Citation22). Although there was no difference between the BMI and HOMA in our two groups, the significantly higher EFT in subjects with hypertensive response to peak exercise and significant correlation between the EFT and BPRI could suggest the importance of the EFT as an early cardiometabolic risk factor even before the general fat accumulation in non-obese individuals.
Supporting the previous studies suggesting an association between epicardial fat tissue and hypertension, the results of the present study raise the question of possible function of epicardial adipose tissue as an endocrine or paracrine organ. Although epicardial fat tissue is the true visceral fat depot of the heart, it is also an extremely active organ that produces cytokines and adipokines. Epicardial fat tissue-derived adiponectin production has been shown to be decreasing in patients with hypertension (Citation8) and decreased adiponectin has been shown to be related to increased collagen deposition and arterial stiffness (Citation24).
Lastly, our study showed that EFT and BPRI both correlated negatively with METS, an indirect measure of cardiorespiratory fitness. This result can be partly related to interactions between physical fitness level and visceral fat accumulation. Poor physical fitness has been shown to be associated with higher levels of total and abdominal fat, independent of BMI in the Canada Fitness Survey (Citation25). As a result, it could be argued that poor physical fitness might cause excess body fat and these two disorders could constitute a vicious circle.
We acknowledge that there are limitations to our findings. Firstly, EFT was not found to correlate with HDL, TG, FG, HOMA and age in our study, as opposed to previous studies (Citation15), but we have to admit that our subjects were middle-aged, healthy and without metabolic syndrome. Secondly, the lack of data about cytokines specific epicardial fat tissue does not permit any causal inference. Future research is certainly needed in large number of patients with more direct measurements of EFT and epicardial fat volume, including computed tomography. It is important to measure adipokines to find a causal link between adiposity and hypertension.
In summary, we have shown that epicardial fat tissue, a new cardiometabolic risk factor, was related to altered BP responses to exercise stress testing in non-obese, normotensive individuals. The echocardiographic measurement of EFT may serve as a useful non-invasive indicator of heightened risk of future hypertension.
Acknowledgments
The authors wish to express their deepest gratitude to all investigators who actively participated in this study. The authors have no relevant conflict of interest to disclose.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Campanini B. The World Health Report: Reducing risks. Promoting healthy life. Geneva: World Health Organization; 2002.
- Lim PO, Donan PT, MacDonald TM. How well do office and exercise blood pressure predict sustained hypertension? A Dundee Step Test Study. J Hum Hypertens. 2000;14: 429–433.
- Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC, . Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–1836.
- Wilson MF, Sung BH, Pincomb GA, Lovallo WR. Exaggerated pressure response to exercise in men at risk for systemic hypertension. Am J Cardiol. 1990;66:731–736.
- MacMahon S, Cutler J, Brittain E, Higgins M. Obesity and hypertension: Epidemiological and clinical issues. Eur Heart J. 1987;8:57–70.
- Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24-h ambulatory blood pressure and hypertension. Hypertension. 2005;45:602–607.
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationship with the heart. Nat Clin Pract Cardivasc Med. 2005;2:536–543.
- Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens. 2008;22:856–863.
- Sironi AM, Pingitore A, Ghione S, De Marchi D, Scattini B, Positano V, . Early hypertension is associated with reduced regional cardiac function, insulin resistance, epicardial, and visceral fat. Hypertension. 2008;51:282–288.
- Burger JP, Serne EH, Nolte F, Smulders YM. Blood pressure response to moderate physical activity is increased in obesity. Neth J Med. 2009; 67:342–346.
- Julius S, Li Y, Brant D, Krause L, Buda AJ. Neurogenic pressor episodes fail to cause hypertension, but do induce cardiac hypertrophy. Hypertension. 1989;13:422–429.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28: 412–419.
- Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, . Report of the American Society of Echocardiography Committee on nomenclature and standards in two dimensional echocardiography. Circulation. 1980;62: 212–217.
- Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography: Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9:19–26.
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, . Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168.
- Miyahara T, Yokota M, Iwase M, Watanabe M, Matsunami T, Koide M, . Mechanism of abnormal postexercise systolic blood pressure response and its diagnostic value in patients with coronary artery disease. Am Heart J. 1990;120:40–49.
- Franklin BA, Whaley MH, Howley ET. American College of Sports Medicine. Guideliness for exercise testing and prescription. 6th Baltimore, MD: Lippincott; 2000.
- Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005:6300–6302.
- Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: What is the link? Nutr Metab Cardiovasc Dis. 2010;20: 481–490.
- Djaberi R, Schuijf JD, Van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102: 1602–1607.
- Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, . Visceral fat in hypertension: Influence on insulin resistance and beta-cell function. Hypertension. 2004; 44:127–133.
- J Simões GC, Moreira SR, Kushnick MR, Simões HG, Campbell CS. Postresistance exercise blood pressure reduction is influenced by exercise intensity in type-2 diabetic and nondiabetic individuals. Strength Cond Res. 2010;24:1277–1284.
- Hashimoto M, Okamoto M, Yamagata T, Yamane T, Watanabe M, Tsuchioka Y, . Abnormal systolic blood pressure response during exercise recovery in patients with angina pectoris. J Am Coll Cardiol. 1993 22:659–664.
- Tsai WC, Lin CC, Chen JY, Huang YY, Lee CH, Li WT, . Association of adiponectin with procollagen type I carboxyterminal propeptide in non-diabetic essential hypertension. Blood Press. 2008;17:233–238.
- Ross R, Katzmarzyk PT. Cardiorespiratory fitness is associated with diminished total and abdominal obesity independent of body mass index. Int J Obes Relat Metab Disord. 2003;27:204–210.
