Abstract
Objectives. The aim of the study was to assess the relationship between non-invasively (NIA) and invasively assessed (IA) aortic pulsatile indices and the presence and severity of coronary artery disease (CAD). Subjects and Methods. The study group consisted of 58 patients who were admitted to our institute for elective coronary angiography (CAG). We measured the aortic systolic, diastolic and mean blood pressure (BP) using non-invasive and invasive techniques. We assessed the pulsatile indices of the aortic pressure waveform (APW) including pulse pressure (PP), fractional PP (FPP, the ratio of PP to mean BP) and pulsatility index (PI, the ratio of PP to diastolic BP). The severity of CAD was assessed by Gensini score. Results. IA aortic PP, FPP and PI were significantly higher in patients with CAD than without CAD, but NIA indices did not show significant differences between two groups. After multivariate stepwise adjustment, the odds ratio (OR) and confidence interval (CI) of having significant CAD was: PP per 10 mmHg, OR = 2.51 (95% CI 1.12–5.63); FPP per 0.1, OR = 3.30 (95% CI 1.25–8.72); and PI per 0.1, OR = 1.88 (95% CI 1.09–3.23). In linear regression analysis, IA aortic systolic BP (SBP), PP, FPP and PI were significantly correlated with Gensini score, but NIA indices were not correlated. The NIA aortic PP was lower than IA aortic PP (mean difference: 6.1 ± 15.8 mmHg). Conclusion. IA aortic PP, FPP and PI were related to the presence and severity of CAD, but NIA assessed indices of APW were not related. NIA aortic PP underestimated IA aortic PP.
Introduction
The importance of central blood pressure (BP) is increasing for decades. It has been demonstrated that central BP predicts cardiovascular (CV) events better than peripheral BP in several studies (Citation1,Citation2). Central BP may better represent the load imposed on the coronary and cerebral arteries, and thereby bear a stronger relationship to vascular damage and prognosis. Also, arterial stiffness of the large, elastic conduit arteries is considered a risk marker of vascular aging, as well as a new biomarker of CV disease (Citation3–6). The pulsatile index of central BP, especially pulse pressure (PP), is significantly related to arterial stiffness and atherosclerosis. Increased PP may be both a cause and an effect of atherosclerosis. This may result in a vicious cycle wherein elevated PP promotes vascular endothelial damage, an antecedent to atherosclerosis, which results in large-vessel stiffening and increased wave reflection, thus, further amplifying PP (Citation7). Furthermore, new parameters (fractional PP and pulsatility index) of the pulsatile indices of BP have been developed (Citation8,Citation9). Fractional PP (FPP, pulsatility) is calculated as PP divided by mean BP (MBP) and pulsatility index (PI) is calculated as PP divided by diastolic BP (DBP). These two indices are not correlated with MBP and may be seen as indicator of the relative changes of BP in opposition to PP, which is an index of absolute BP changes (Citation10,Citation11). This observation may be very useful in research on atherosclerosis pathogenesis and its complications development (Citation12). In recent years, a number of studies were carried out to assess the relationship of pulsatile indices of central BP and coronary artery disease (CAD) (Citation8–11,Citation13–19). However, in most of studies, central BP-derived indices were measured by invasive techniques. Invasive techniques have limited value for screening and risk stratification in larger patient groups. Central BP-derived indices can now be assessed non-invasively with a number of devices. Radial applanation tonometry has been commonly used. The pressure waveform from the radial artery is recorded non-invasively with applanation tonometry, and then the aortic pressure waveform (APW) is derived by a generalized transfer function (Citation20), but the validity of non-invasive techniques using a generalized transfer function is still controversial (Citation21–23). Therefore, we assessed indices of APW non-invasively and invasively, and investigated the relationship between pulsatile indices and the presence and severity of CAD. Then, we evaluated that non-invasively assessed (NIA) indices of APW that could be applied in daily practice.
Methods
Study population
Patients from March 2010 to June 2010 who underwent elective coronary angiography (CAG) for the evaluation of CAD and follow-up of prior coronary intervention were studied. We excluded patients from the analysis who had acute myocardial infarction (MI) within a month period before angiography, impaired left ventricular function (defined as ejection fraction below 50%), hemodynamically significant valvular heart disease, renal insufficiency and cancer.
Fasting blood samples were taken before CAG for the analysis of glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high sensitivity C-reactive protein (Hs CRP), erythrocyte sedimentation rate (ESR), fibrinogen, mean platelet volume (MPV), blood urea nitrogen and creatinine. The height and weight for each patient were recorded. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Hypertension was present with repeated measurements ≥ 140 mmHg systolic BP (SBP) and/or ≥ 90 mmHg DBP or permanent antihypertensive drug treatment. Diabetes mellitus was defined as a fasting blood glucose concentration ≥ 126 mg/dl or antihyperglycemic drug treatment. Current smoking was defined as having smoked the last cigarette less than 1 week before CAG. Creatinine clearance was estimated using the modification of diet in renal disease (MDRD) formula.
Measurement of hemodynamic indices
All antihypertensive medicines were withdrawn for at least 24 h before the study and patients had not eaten for at least 12 h. Hemodynamic measurements were obtained from the patient in the supine position after 10 min rest. Measurements were made under standardized condition between 08:00 to 10:00 AM. Non-invasive measurements of brachial BP and APW were done prior to the invasive measurement of APW. The peripheral BP was recorded non-invasively with the use of an oscillometric method (Microlife BP 3BM1–3®, Switzerland) at the brachial artery. Non-invasive measurement of APW was performed using radial applanation tonometry (SphygmoCor®, AtCor Medical, Australia). The radial pressure waveform was recorded with a micro-manometer and calibrated with a brachial BP. APW was derived from on-line reconstruction of the pressure waveform characteristic for an ascending aorta with the use of the generalized transfer function that is incorporated in the SphygmoCor device. Finally, aortic SBP, DBP and PP were obtained and MBP was calculated (2 × DBP + SBP)/3. Invasive measurement of APW was performed before CAG. Aortic SBP and DBP were measured using low-compliance fluid-filled system (5F pigtail catheter) at the ascending aorta. MBP was obtained by direct integration of the BP curve and PP was calculated as (SBP – DBP). Aortic FPP was calculated as (PP/MBP) and PI was calculated as (PP/DBP) from both non-invasively and invasively measured indices.
Measurement of angiographic variables
CAG was performed mainly through the percutaneous radial approach using the standard technique. Invasive assessments of APW and CAG were performed after intra-arterial infusion of nitroglycerin to prevent radial artery spasm. The three major coronary vessels (the left anterior descending artery, the circumflex artery and the right coronary artery) were considered for evaluation of the extent of coronary atherosclerosis. Optimal views of the arteries from all technically suitable angiograms were analyzed. The guiding catheter was used as the reference dimension. A significant diseased artery was defined as having ≥ 50% stenosis of at least one of its segments or prior coronary intervention. Significant left main artery stenosis was coded as a two-vessel disease. The severity of CAD was assessed by the Gensini scoring system. This scoring system assigns a different severity score depending on geometrically increasing severity of the lesion, the cumulative effects of multiple obstructions and the significance of their locations (Citation24). The Gensini score was used widely in previous years to assess the severity and extent of coronary atherosclerosis. Assessment of CAD extent and Gensini score in patient undergoing follow-up angiography used the angiographic findings of prior coronary intervention.
Statistical analysis
All data were analyzed using the SPSS 15.0 software. Categorical variables were reported as percentages and continuous variables as means ± standard deviation (SD). The Pearson's chi-squared test or Fisher's exact test was applied to all categorical variables. Normally distributed continuous variables were compared using Student's t-test. A stepwise logistic regression analysis was performed to evaluate the independent effects of hemodynamic indices on the risk of having significant CAD starting with a model including a number of potential confounders (age, gender, smoking, hypertension, diabetes, BMI, the history of MI, cholesterol and creatinine levels were included). Correlations between hemodynamic indices and Gensini score were calculated using univariate linear regression analysis. Multivariate linear regression analysis using a general linear model was performed to evaluate the independent effects of hemodynamic indices on the Gensini score with a same model of logistic regression analysis. A p-value < 0.05 was considered statistically significant.
Results
Baseline clinical characteristics
The study group consisted of 58 patients (38 male, aged 63.4 ± 10.8 years). CAG revealed that 13 patients (22.4%) had no significant CAD and 45 patients (77.6%) had significant CAD. Patients with CAD were more likely to be men when compared with subjects without CAD. The mean ages of both groups were similar, and the distributions of hypertension, diabetes, current smoking status and previous MI were no different between both groups. The laboratory findings associated with coronary atherosclerosis (total cholesterol, LDL-cholesterol, glucose, Hs CRP, ESR, fibrinogen, MPV and creatinine) were higher in subjects with CAD than without CAD, but were statistically insignificant. Baseline clinical characteristics of the study population were summarized in .
Table I. Baseline clinical characteristics of the study population.
Hemodynamic indices and CAD
Mean values of brachial BP-derived indices, and NIA and invasively assessed (IA) indices of APW were shown in . IA aortic PP, FPP and PI were significantly higher in patients with CAD than without CAD (PP, 57.4 ± 14.7 vs 47.2 ± 14.9 mmHg, p = 0.043; FPP, 0.61 ± 0.12 vs 0.52 ± 0.14, p = 0.046; PI, 0.83 ± 0.21 vs 0.68 ± 0.23, p = 0.049), but brachial BP-derived indices, NIA indices of APW and IA aortic MBP did not show significant differences between two groups ().
Table II. Mean values of hemodynamic indices according to the presence of coronary artery disease.
IA aortic PP, FPP and PI were related to the presence of CAD in univariate logistic regression analysis as well as after adjustments for a number of potential confounders including age, gender, smoking, hypertension, diabetes, BMI, the history of MI, cholesterol and creatinine (). After multivariate stepwise adjustment, the odds ratio (OR) and confidence interval (CI) of having significant CAD were: PP per 10 mmHg OR = 2.51 (95% CI 1.12–5.63, p = 0.025); FPP per 0.1 OR = 3.30 (95% CI 1.25–8.72, p = 0.016); and PI per 0.1 OR = 1.88 (95% CI 1.09–3.23, p = 0.023). In each model of IA aortic PP, FPP and PI, OR and CI of having significant CAD in male were: OR = 0.03 (95% CI 0.001–0.545, p = 0.018); OR = 0.03 (95% CI 0.002–0.496, p = 0.013); and OR = 0.05 (95% CI 0.004–0.621, p = 0.020). However, NIA aortic MBP and pulsatile indices and IA aortic MBP were not related to the presence of CAD in logistic regression analysis.
Table III. Odds ratios for the association between NIA and IA aortic MBP and pulsatile indices and risk of presence of significant coronary artery disease.
In univariate linear regression analysis, IA aortic SBP, PP, FPP and PI were significantly correlated with Gensini score (), but NIA indices and IA aortic MBP were not correlated. Furthermore, in multivariate linear regression analysis, IA aortic SBP, PP, FPP and PI were significantly correlated with Gensini score, but NIA indices and IA aortic MBP were not correlated ().
Figure 1. The correlation of invasively assessed aortic SBP and pulsatile indices and the severity of coronary artery disease using the Gensini score in univariate linear regression analysis. Invasively assessed aortic (A) SBP, (B) PP, (C) FPP and (D) PI had a positive correlation with Gensini score. IA, invasively assessed; SBP, systolic blood pressure; PP, pulse pressure; FPP, fractional pulse pressure; PI, pulsatility index.
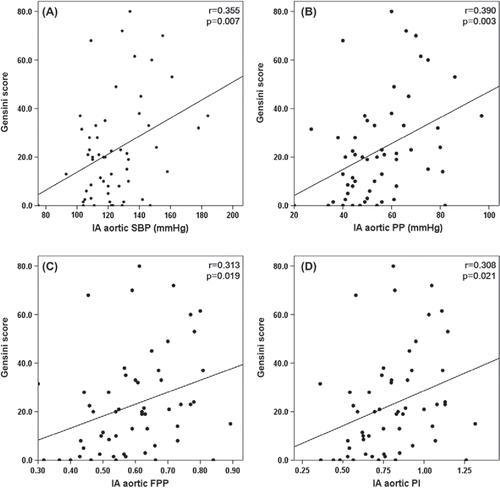
Table IV. The linear regression analysis of non-invasively and invasively assessed aortic pulsatile indices and the severity of coronary artery disease using Gensini score.
The comparison of NIA and IA indices of APW
Mean values of hemodynamic indices of study group and mean differences between NIA and IA indices of APW were shown in . For the group as a whole, NIA aortic SBP and DBP overestimated IA aortic SBP and DBP by an average of 1.5 ± 18.76 mmHg and 7.5 ± 9.2 mmHg, respectively. Consequently, NIA aortic PP underestimated IA aortic PP by an average of 6.1 ± 15.8 mmHg.
Table V. Mean values of hemodynamic indices of study population and mean differences between non-invasively and invasively assessed indices of aortic pressure waveform
Discussion
To the best of our knowledge, this is the first study not only assessing the indices of APW non-invasively and invasively, but also investigating the relationship between both assessed indices and CAD.
In our study, IA aortic pulsatile indices including PP, FPP and PI were related with presence and severity of CAD, but brachial BP-derived indices and steady index including MBP were not related. These findings were similar with previous studies (Citation8–11, Citation13–19). Jankowski et al. (Citation18) showed that IA aortic FPP was related to the extent of coronary atherosclerosis irrespective of the presence of hypertension. Importantly, IA aortic FPP determined to be a better predictor of both coronary atherosclerosis and CV events when compared with IA aortic PP (Citation19). Similarly, the OR of having significant CAD of IA aortic FPP was higher than IA aortic PP in our study.
However, there are no published studies showing the relation between CAD and central BP using NIA and IA methods. Wykretowicz et al. (Citation25) studied only the non-invasive method to measure the central BP showing that NIA aortic FPP was related to the presence of CAD in contrast with our result. The mean age of participants of that study (53 ± 0.9 years) was younger than in our study (63 ± 10.8 years). The discrimination power of FPP might be marked in subjects with younger age, as we refer to the suggestion that an increase of aortic augmentation index was non-linear and more prominent in subjects under 50 years and a decrease in PP amplification (ratio of peripheral to central PP) with age that was non-linear and more marked in those under 50 years (Citation26). Also, we assessed the indices of APW non-invasively and invasively. The reason for our results was that NIA aortic PP underestimated IA aortic PP. This difference may be related to a calibration error of the radial pulse wave. The generalized transfer function in radial applanation tonometry yields accurate results when invasive peripheral BP is used (Citation27) for the calibration of the radial pulse wave. However, the controversy of this technique is that non-invasive BP recordings in the brachial artery are used to calibrate the radial pulse wave (Citation21,Citation23). The output error at the aorta is associated with the input errors at the radial artery, attributable to the under- or overestimation of aortic SBP and DBP, depending on which non-invasive technique is applied (Citation28), and the presence of brachial-to-radial pressure amplification (Citation29). Moreover, this output error may be magnified by the generalized transfer function in the setting of slower heart rates and higher BPs (Citation30). Consequently, the NIA aortic PP is usually underestimated in previous studies (Citation21–23,Citation29) and similar findings were shown in our study.
Although NIA indices of APW were not correlated with CAD in our study, a non-invasive technique using radial applanation tonometry has been used widely in several large studies. In particular, NIA aortic PP is known to be a strong predictor of CV events and mortality. In subjects with hypertension, but without chronic renal failure, findings from the SHS (Strong Heart Study) have shown that brachial PP, a simple indirect index of arterial stiffness, was associated with a higher CV mortality level, independent of traditional risk factors, left ventricular hypertrophy and reduced ejection fraction in adults without overt CAD (Citation31). Results from the same study showed that during the 5-year follow-up the NIA aortic PP predicted incident CV disease better than the corresponding brachial PP did, possibly because of a more accurate representation of the vascular load on the left ventricle (Citation1). The large CAFE (Conduit Artery Function Evaluation) study reported that NIA aortic PP independently predicted CV outcomes in treated patients with hypertension (Citation32). Therefore, NIA indices of APW could be useful tool in daily practice for risk stratification and follow-up of CV patients. In the future, a more accurate calibration method and convenient measurement of central BP are necessary.
The reference values for pulsatile indices are not yet determined. Recently, central PP ≥ 50 mmHg predicts adverse CV disease outcome in SHS (Citation33). In our study, the proportion of ≥ 50 mmHg of IA aortic PP in the CAD group was higher than the no-CAD group (66.7%, 30/45 vs 38.5%, 5/13, p = 0.11). In the European study, the proposed value for the arterial measurement according to age was approximately 40 mmHg for the central PP (Citation34). In the Korean study, the proposed diagnostic value (upper limit of the 95 percentile) was 50 mmHg for the central PP (Citation35). However, there is no study for the reference values of FPP and PI. Further large clinical trials of the general population may provide us with reference values of central pulsatile indices, and increase the prediction power for coronary atherosclerosis and clinical outcome according to age and sex.
There are several limitations in our study. First, the study population was small in number, at a single center. The study populations of previous studies that assessed central BP non-invasively and invasively were smaller than in our study, but the findings were similar (Citation21–23). Therefore, our study population may be responsible for our findings. Second, the study population was not evenly distributed. The risk of having significant CAD was higher in the male group in our study. These results were related to selection bias, in that males were dominant in the CAD group. Third, we used a fluid-filled system to record the ascending aortic pressure. The use of a high-fidelity pressure transducer could have increased the accuracy of the recorded pressure waveform. However, this equipment is not cost effective, which explains why most of previous studies used a fluid-filled system to record the ascending aortic pressure. Finally, most participants of the study were prescribed CV drugs including antihypertensive agents. Although these drugs affected hemodynamic indices, our results agree with the findings of previous studies (Citation18,Citation19). In particular, FPP and PI are indicators of the relative changes of BP, therefore these indices are less affected by drugs.
Conclusion
IA aortic pulsatile indices including PP, FFP and PI were correlated with the presence and severity of CAD, but NIA indices of APW were not correlated with CAD. The reason for these results was that NIA aortic PP underestimated IA aortic PP. This difference may be related to calibration error of the radial pulse wave in radial applanation tonometry.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, . Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The Strong Heart Study. Hypertension. 2007;50:197–203.
- Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, . Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: The ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, . Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, . Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, . Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation. 2006;113:657–663.
- Dart AM, Kingwell BA. Pulse pressure – A review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001; 37: 975–984.
- Nakayama Y, Tsumura K, Yamashita N, Yoshimaru K, Hayashi T. Pulsatility of ascending aortic pressure waveform is a powerful predictor of restenosis after percutaneous transluminal coronary angioplasty. Circulation. 2000;101:470–472.
- Lu TM, Hsu NW, Chen YH, Lee WS, Wu CC, Ding YA, . Pulsatility of ascending aorta and restenosis after coronary angioplasty in patients >60 years of age with stable angina pectoris. Am J Cardiol. 2001;88:964–968.
- Jankowski P, Kawecka-Jaszcz K, Bryniarski L, Czarnecka D, Brzozowska-Kiszka M, Posnik-Urbanska A, . Fractional diastolic and systolic pressure in the ascending aorta are related to the extent of coronary artery disease. Am J Hypertens. 2004;17:641–646.
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Styczkiewicz M, . Ascending aortic, but not brachial blood pressure-derived indices are related to coronary atherosclerosis. Atherosclerosis. 2004;176: 151–155.
- Jankowski P, Bilo G, Kawecka-Jaszcz K. The pulsatile component of blood pressure: Its role in the pathogenesis of atherosclerosis. Blood Press. 2007;16:238–245.
- Nishijima T, Nakayama Y, Tsumura K, Yamashita N, Yoshimaru K, Ueda H, . Pulsatility of ascending aortic blood pressure waveform is associated with an increased risk of coronary heart disease. Am J Hypertens. 2001;14:469–473.
- Danchin N, Benetos A, Lopez-Sublet M, Demicheli T, Safar M, Mourad JJ. Aortic pulse pressure is related to the presence and extent of coronary artery disease in men undergoing diagnostic coronary angiography: A multicenter study. Am J Hypertens. 2004;17:129–133.
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Bryniarski L. Ascending aortic blood pressure waveform may be related to the risk of coronary artery disease in women, but not in men. J Hum Hypertens. 2004;18:643–648.
- Ozaki M, Masuoka H, Kawasaki A, Ito M, Nakano T. Intraortic pulse pressure is correlated with coronary artery stenosis. Int Heart J. 2005;46:69–78.
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Posnik-Urbanska A, Styczkiewicz K. Ascending aortic blood pressure-derived indices are not correlated with the extent of coronary artery disease in patients with impaired left ventricular function. Atherosclerosis. 2006;184:370–376.
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D. Ascending aortic blood pressure waveform is related to coronary atherosclerosis in hypertensive as well as in normotensive subjects. Blood Press. 2007;16:246–253.
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, . Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51: 848–855.
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, . Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084.
- Cloud GC, Rajkumar C, Kooner J, Cooke J, Bulpitt CJ. Estimation of central aortic pressure by SphygmoCor requires intra-arterial peripheral pressures. Clin Sci (Lond). 2003;105: 219–225.
- Davies JI, Band MM, Pringle S, Ogston S, Struthers AD. Peripheral blood pressure measurement is as good as applanation tonometry at predicting ascending aortic blood pressure. J Hypertens. 2003;21:571–576.
- Smulyan H, Siddiqui DS, Carlson RJ, London GM, Safar ME. Clinical utility of aortic pulses and pressures calculated from applanated radial-artery pulses. Hypertension. 2003;42: 150–155.
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983; 51:606.
- Wykretowicz A, Metzler L, Milewska A, Balinski M, Rutkowska A, Adamska K, . Noninvasively assessed pulsatility of ascending aortic pressure waveform is associated with the presence of coronary artery narrowing. Heart Vessels. 2008;23:16–19.
- McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity. The Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005; 46:1753–1760.
- Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937.
- Smulyan H, Sheehe PR, Safar ME. A preliminary evaluation of the mean arterial pressure as measured by cuff oscillometry. Am J Hypertens. 2008;21:166–171.
- Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: Importance of brachial-to-radial pressure amplification. Hypertension. 2005;46:244–248.
- Papaioannou TG, Lekakis JP, Karatzis EN, Papamichael CM, Stamatelopoulos KS, Protogerou AD, . Transmission of calibration errors (input) by generalized transfer functions to the aortic pressures (output) at different hemodynamic states. Int J Cardiol. 2006;110:46–52.
- Palmieri V, Devereux RB, Hollywood J, Bella JN, Liu JE, Lee ET, . Association of pulse pressure with cardiovascular outcome is independent of left ventricular hypertrophy and systolic dysfunction: The Strong Heart Study. Am J Hypertens. 2006;19:601–607.
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, . Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225.
- Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, . High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009;54:1730–1734.
- Wojciechowska W, Staessen JA, Nawrot T, Cwynar M, Seidlerova J, Stolarz K, . Reference values in white Europeans for the arterial pulse wave recorded by means of the SphygmoCor device. Hypertens Res. 2006;29:475–483.
- Chung JW, Lee YS, Kim JH, Seong MJ, Kim SY, Lee JB, . Reference values for the augmentation index and pulse pressure in apparently healthy Korean subjects. Korean Circ J. 2010;40:165–171.
