Abstract
Hypertension management guidelines recommend titrating antihypertensive drugs stepwise every 4–6 weeks. We compared efficacy and safety of early versus late titration after 10 weeks’ treatment with irbesartan/hydrochlorothiazide. Hypertensive patients uncontrolled on monotherapy were randomized into two groups. In the early titration group (E), patients received irbesartan/hydrochlorothiazide 150/12.5 mg for 2 weeks; uncontrolled patients were up-titrated to 300/25 mg at weeks 2 and 6. In the late titration group (L), patients received 150/12.5 mg for 6 weeks; uncontrolled patients were up-titrated to 300/25 mg at week 6 (W6). The change of mean systolic (SBP) and diastolic blood pressure (DBP) from baseline to week 10 (W10) were studied using a covariance analysis model. The percentage of controlled patients at W10 was compared between groups using Fisher's exact test. Of 833 patients enrolled from 14 countries, the intent-to-treat (ITT) population included 795 (mean age 58 ± 12 years, female 60%, obesity 38%, diabetes 22%). At W6, mean SBP decrease was: E − 28.8 mmHg vs L − 26.3 mmHg (p = 0.02). At W10, there was similar mean SBP decrease: E − 29.5 mmHg vs L − 31.0 mmHg (p = 0.14). The control rate at W10 was 58% (E) and 64% (L), p = 0.06. Serious adverse events were more frequent in E (2.5% vs 0.7%, p = 0.044). Both early and late titration regimens provide similar BP decrease and control rate.
Introduction
Hypertension is a major health concern, affecting approximately one billion people worldwide –20% of the adult population (Citation1). Despite increased awareness and availability of many antihypertensive compounds, the blood pressure (BP) goals set by hypertension management guidelines, i.e. <140/90 mmHg in uncomplicated hypertension or <130/80 mmHg in type 2 diabetes or kidney disease, are not achieved in a high proportion of patients. Recent surveys indicate that in Europe only 50% of those regularly taking antihypertensive treatments are at the BP goal, while in the USA this figure is as high as 70% (Citation2). This raises concerns and allows asking the question whether the recommended strategy in antihypertensive management should be modified.
Major hypertension management guidelines recommend the initiation of antihypertensive drugs in all patients with systolic BP (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg, and to adjust the treatment to ensure that patients remain below these values. The European Society of Hypertension (ESH)/European Society of Cardiology (ESC) guidelines indicated that initial treatment can make use of monotherapy with a subsequent increase in drug doses or number, if needed (Citation3). The initiation monotherapy strategy was challenged in the 2007 ESH/ESC guidelines in subjects with initial BP in the grade 2 or 3 range or in those with high/very high cardiovascular risk because of the presence of organ damage, diabetes, renal disease or a history of cardiovascular disease (Citation3). In these patients, a combination of two antihypertensive drugs was recommended for treatment initiation, although no evidence from morbidity/mortality trials was provided.
In the majority of subjects, antihypertensive treatment is initiated as monotherapy, and if monotherapy is insufficient to achieve BP goal, a two-drug combination is prescribed. In several cases, a fixed-dose combination (FDC) is decided, but a vast choice of dosages is available, in particular in the case of addition of a diuretic to an angiotensin receptor blocker (ARB). The use of an FDC containing the lowest dosage of hydrochlorothiazide (HCTZ) is recommended at this step and adjustment of doses for one or both components is eventually realized after 4–6 weeks of follow-up. However, to achieve BP goal more promptly, a quicker adjustment of doses of the FDC after only 2 weeks of follow-up should be realized.
The ACTUAL study was designed to test this hypothesis. The study was conducted in hypertensive subjects unsuccessfully controlled with monotherapy and switched to irbesartan/HCTZ 150/12.5 mg as first step, and then to irbesartan/HCTZ 300/25 mg in fixed doses combined.
The aim of this study was to evaluate the efficacy and safety of two hypertension management strategies: a conservative strategy with a late stepwise titration after 6 weeks of follow-up; and an early strategy with a rapid stepwise titration after 2 weeks of follow-up. The primary objective was to compare the antihypertensive efficacy of the two strategies. Secondary objectives were to determine the incidence and severity of adverse events (AEs) and to compare the antihypertensive efficacy of the two strategies in the sub-group of obese patients and in the population of patients with type 2 diabetes.
Methods
Study design
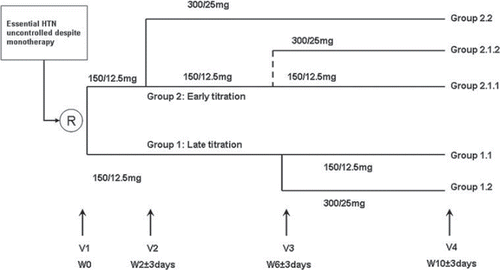
This international study was carried out at 89 centers in 14 countries (Algeria, Argentina, Brazil, Colombia, Ecuador, Egypt, Lebanon, Mexico, Morocco, Peru, Saudi Arabia, Tunisia, United Arab Emirates and Venezuela). Two hypertension management strategies were compared in a randomized, open-label, parallel-group, prospective study. The first strategy used was a late titration regimen based on a decision to titrate the antihypertensive regimen (by doubling initial dosage) after 6 weeks of follow-up. The second was an early titration regimen based on a decision to double the initial dosage after only 2 weeks of follow-up (). Patients who fulfilled inclusion/exclusion criteria and signed informed consent were randomized using an interactive voice response system. Ethics committee approval was sought and granted for all centers in the participating countries.
Study population
Outpatients ≥18 years of age were enrolled if they had established essential hypertension treated for at least 4 weeks by antihypertensive monotherapy, had uncontrolled BP (defined as SBP ≥ 160 mmHg and/or DBP ≥ 100 mmHg in patients without diabetes or SBP ≥ 150 mmHg and/or DBP ≥ 90 mmHg in patients with diabetes) and if they signed the written informed consent.
Patients were excluded if they had any of the following: SBP ≥ 180 mmHg and/or DBP ≥ 110 mmHg at baseline visit (V1); known or suspected causes of secondary hypertension; associated cardiovascular conditions that do not allow stopping the current antihypertensive drug; known hypersensitivity or contraindications to the study drugs; administration of any other investigational drug within 30 days prior to study entry.
Study treatments and concomitant medications
The investigational products: irbesartan/HCTZ (CoAprovel®) 150 mg/12.5 mg and 300 mg/25 mg were supplied by the sponsor. Irbesartan/HCTZ 150/12.5 mg was administered orally, once daily in the morning, from the day following V1 (W0). Thereafter, treatment continuation/titration at each visit was as per the study design (). Concomitant antihypertensive drugs were not permitted during the study.
Study assessments
Office BP measurement was taken manually using OMRON® 7 series (OMRON Healthcare, Kyoto, Japan) – a printer-equipped, semiautomatic, digitized, validated electronic device – provided to the investigator at the beginning of the study. BP measurements were made between 07:00 and 11:00 with no treatment taken on the morning of the visit. All BP measurements were obtained in a seated position after 5 min rest. Mean SBP and DBP were calculated based on three measurements made at 1-min intervals.
Laboratory assessments of serum sodium, potassium and creatinine values were done by a local laboratory at least 3 days prior to V2 (W2), V3 (W6) and V4 (W10).
Statistical analysis
Sample size was calculated using nQuery Advisor® 6.01 (Statistical Solutions Ltd, Cork, Ireland). Using a two-group t-test with a two-sided significance level of 0.05, a sample size of 296 in each group would have 80% power to detect a clinically relevant difference of 3 mmHg between the means of SBP between the groups, assuming that the common standard deviation (SD) is 13 mmHg. To take into account an attrition rate of 10%, 658 patients (329 per study arm) were to be randomized.
Efficacy analyses were carried out in intent-to-treat (ITT) population. AEs were described by treatment groups in the full analysis set. Continuous variables were summarized using number of documented patients, number of missing data, mean, SD, minimum, maximum and median. Categorical variables were summarized using numbers and percentages. The change of mean SBP and mean DBP between V1 (W0) and V4 (W10) was studied in a covariance analysis model (ANCOVA), adjusted by SBP or DBP at randomization, respectively. The treatment effect test was a two-sided one with a Type-I error equal to 5%. The percentage of controlled patients at W10 was compared between the groups using the Fisher's exact test. Same analyses were conducted for the BP changes between V1 (W0) and V3 (W6) and for the percentage of controlled patients at V3 (W6).
Results
Baseline and demographic characteristics
Between June 16, 2008 and April 14, 2009, 832 patients were randomized. Demographics and baseline characteristics of ITT population (n = 795) are presented in . About 54.6% patients were less than 60 years old. The most common comorbidities were dyslipidemia and type 2 diabetes. The most commonly prescribed previous antihypertensives were angiotensin-converting enzyme (ACE) inhibitors, ARBs and calcium-channel blockers.
Table I. Baseline and demographic characteristics of the intent-to-treat population.
Change in BP from baseline to week 6 and week 10
On average, SBP decreased by 31.0 mmHg in the late and 29.5 mmHg in the early titration group, between W0 and W10. Results of ANCOVA showed an adjusted mean difference of 1.48 mmHg (95% CI − 0.50 to 3.45), which was not significant (p = 0.14).
On average, DBP decreased by 14.5 mmHg in the late and 14.1 mmHg in the early titration group, between W0 and W10. Results of ANCOVA showed an adjusted means difference of −0.12 mmHg (95% CI − 1.27 to 1.02), which was not significant (p = 0.83).
Whereas changes in SBP and DBP at W6 were statistically significant, no significant difference was noted in the change of SBP or DBP at W10 ().
Table II. Change in BP (ANCOVA model) from baseline in the intent-to-treat population.
Similar results were seen in per-protocol (PP) population.
BP control at week 6 and week 10
The proportion of controlled patients (BP < 140/90 mmHg) at W6 was 56.5% in the late and 65.4% in the early titration group (p < 0.05). The proportion of controlled patients at W10 was 70.9% in the late and 66.2% in the early titration group. shows the proportion of controlled patients, taking into account the recommended goal for subjects with diabetes (SBP < 130 mmHg and DBP < 80 mmHg). Similar results were seen in the PP population.
Table III. BP control at week 6 and week 10 in the intent-to-treat population.
Antihypertensive efficacy of irbesartan/HCTZ combination in obese patients and patients with diabetes
Between W0 and W10, mean SBP decreased by 29.6 mmHg in obese (BMI > 30 kg/m2) and 30.6 mmHg in non-obese (BMI ≤ 30 kg/m2) patients (p = 0.48, ANCOVA), whereas mean DBP decreased by 14.6 mmHg in obese and 14.1 mmHg in non-obese patients (p = 0.06, ANCOVA). The proportion of controlled patients (SBP < 140 mmHg and DBP < 90 mmHg) at W10 was 69.8% in obese and 67.7% in non-obese patients. The proportion of controlled patients at W10, taking into account the recommended goal for subjects with diabetes (SBP < 130 mmHg and DBP < 80 mmHg), was 59.7% in obese and 61.5% in non-obese patients.
Mean SBP decreased by 26.0 mmHg in patients with diabetes and 31.5 mmHg in patients without diabetes, between W0 and W10 (p < 0.001, ANCOVA). Mean DBP decreased by 11.8 mmHg in patients with diabetes and 15.0 mmHg in patients without diabetes, between W0 and W10 (p = 0.30, ANCOVA). The proportion of controlled patients at W10, was 31.7% in patients with diabetes, taking into account the recommended goal for subjects with diabetes (SBP < 130 mmHg and DBP < 80 mmHg) and 69.4% in patients without diabetes (p < 0.0001). Similar results were seen in the PP population.
Safety evaluation
The safety population included 804 patients (408 in the late and 395 in the early titration group). The mean laboratory values of serum sodium, potassium and creatinine remained stable between W2 and W10 in both groups.
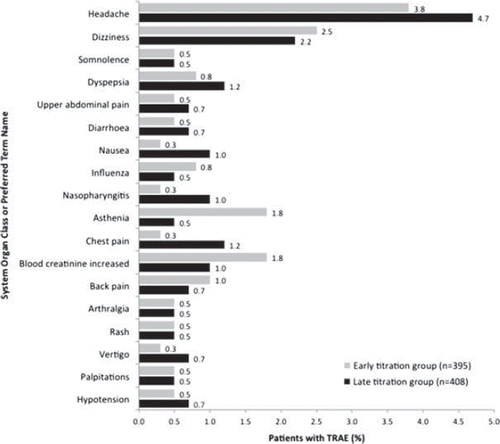
At least one treatment-related AE (TRAE) was reported in 177 (22.0%) patients. Of these, 30 (3.7%) had at least one TRAE leading to permanent discontinuation of the study drug (27 AEs, two lack of efficacy, one lost to follow-up), and 57 (7.1%) had at least one TRAE possibly related to the study drug (31 in the late vs 26 in the early titration group). There were no significant differences between the groups for TRAEs. TRAEs reported by at least 0.5% of the safety population are presented in .
Figure 2. Treatment-related adverse events by system organ class and preferred term according to randomization group – safety population. *One patient with missing randomization group. TRAE, treatment-related adverse events.
Sixteen serious AEs (SAEs) were reported in 13 (1.6%) patients, with a significantly higher proportion in the early (10, 2.5%) than in the late titration group (3, 0.7%) (p < 0.05). The most frequent SAEs belonged to the following system organ classes: cardiac disorders and nervous system disorders. No deaths were reported.
Discussion
The ACTUAL study showed that in hypertensive subjects uncontrolled with monotherapy and switched to irbesartan/HCTZ combination, after 10 weeks of follow-up, an early regimen with titration after 2 weeks provides similar BP decrease and control rate to a late regimen with titration after 6 weeks. Both strategies achieved target BP in about 60% patients; however, because serious adverse effects were observed more frequently with early titration, to double the dosage of a FDC after 2 weeks of follow-up seems unreasonable.
Our primary objective was to evaluate the antihypertensive efficacy of a stepwise titration strategy using irbesartan/HCTZ FDC in hypertensive subjects uncontrolled by monotherapy. Our results highlight that the mean decrease in SBP/DBP at W10 was −31.0/−14.5 mmHg in the late and −29.5/−14.1 mmHg in the early titration group. This antihypertensive effect is higher than the mean decrease in SBP/DBP described in studies of irbesartan/HCTZ FDC in patients failing on monotherapy (Citation4–11). In an Asian study, patients received FDC irbesartan/HCTZ 150/12.5 mg for 2 weeks, with rapid titration to 300/12.5 mg (after 2 weeks) and 300/25 mg (after 4 weeks), if required. After 8 weeks, the mean reduction was 22.0/16.1 mmHg (Citation12). Thus, our results confirm the efficacy of irbesartan/HCTZ combination in patients failing on monotherapy, and favor the implementation of current hypertension management guidelines indicating monotherapy as the initial treatment for mild BP elevation followed by combination therapy if the BP goal is not achieved (Citation3).
The ACTUAL study was designed to evaluate the efficacy and safety of an early titration regimen based on a decision to titrate the antihypertensive regimen (by doubling initial dosage) after 2 weeks of follow-up. Our results indicate that in the ITT population, both groups had similar mean decrease of BP and proportion of controlled patients at W10. However, the mean BP decrease was significantly higher at W6 in the early than in the late titration group and the proportion of controlled patients at W6 was significantly higher in the early than in the late titration group (65.4% vs 56.5%). Thus, our results suggest that early titration enables earlier BP decrease and BP control, although the final BP decrease and BP control are comparable with late titration. Our results are not consistent with another study in which participants were randomized either to a fast (every 2 weeks) or slow (every 6 weeks) drug titration. Results indicated that slower dose escalation of an ACE inhibitor provided higher BP control rates than more rapid drug dose escalation (Citation13).
The safety records of the effects of an early titration strategy based on the doubling, after 2 weeks of follow-up, of the initial dosage of irbesartan/HCTZ 150/12.5 mg combination is an important result of our study. In this population, at least one TRAE was reported in 22.0% patients, though there were no significant differences between early and late groups for TRAEs. Sixteen SAEs were reported in 13 (1.6%) patients, with a significantly higher proportion in the early (2.5%) than in the late titration group (0.7%) (p < 0.05). The global rate of SAEs in the ACTUAL study (1.6%) was similar to that described in the INCLUSIVE study (1.0%) (Citation7). Our results are consistent with the study in which participants were randomized either to a fast (every 2 weeks) or slow (every 6 weeks) drug titration (Citation13). A similar proportion of patients in the two groups experienced AEs (10.8% in slow vs 10.7% in fast group). Nevertheless, the frequency distribution of AEs differed by severity between the two treatments groups and the slow group experienced fewer severe (21% vs 12%) and more mild (52% vs 39%) AEs than the fast titration group.
Safety and tolerability are of paramount importance, as aggressive antihypertensive drug therapy can be associated with hypotension, syncope, headache and hypokalemia. Higher doses (25 mg/day) of HCTZ may provide additional BP control but may be associated with increased incidence of AEs, especially metabolic effects (Citation14). However, irbesartan tends to ameliorate the dose-related biochemical abnormalities associated with HCTZ alone (Citation15). In the current study, no clinical side effects or significant metabolic changes were observed after 10 weeks. Studies of FDCs of other ARBs with high-dose (25 mg/day) HCTZ have also shown better efficacy of the combinations without compromising the safety (Citation16,Citation17).
The early strategy evaluated in the ACTUAL study of assessing BP responses after 2 weeks and, if the BP reduction is inadequate, to double the drug dose, is not supported by our findings. Irrespective of our findings, however, it is imperative to deliberate on the following question: what is the benefit of more rapid BP lowering? Most patients with hypertension, especially those having stage 1 or 2 BP elevations, are at risk of BP-related complications over the years. Additionally, the benefits of rapid BP lowering/normalization have not been well defined. The 2007 ESH/ESC guidelines recommend achieving the BP goal more promptly in hypertensive patients who have high initial BP or are classified as being at high/very high cardiovascular risk (Citation3). This recommendation is justified by a post hoc analysis of the VALUE trial (Citation18), but these data obviously are not compelling enough to establish the advantage of early BP control, because there is a possibility that the immediate responders were at a lower cardiovascular risk, and hence, had a more prompt reduction in BP with treatment. To validate the benefit of rapid BP lowering, an appropriate morbidity–mortality trial should be conducted comparing earlier BP control to later control. Although, the ACTUAL study is not a morbidity–mortality trial, our results are suggestive of a slower drug escalation strategy, as recommended in the guidelines, until valid benefits of rapid (over a few weeks) reduction of BP are established.
The ACTUAL study has some limitations. Despite randomization, patients of the early titration arm presented slightly more severity criteria than the late titration arm. These small differences may have an impact on the mean decrease of SBP/DBP and on the proportion of controlled patients. The active treatment period was relatively short (10 weeks), allowing only short-term evaluation of efficacy and tolerability.
In conclusion, our findings suggest that in hypertensive subjects uncontrolled with monotherapy and switched to irbesartan/HCTZ combination, a rapid drug dose titration strategy not only does not yield tangible improvements in BP control after 10 weeks of treatment, but rather is associated with less desirable outcomes on potentially important safety profile parameters. While the ACTUAL study does not permit any definitive conclusions to be drawn, our results suggest, in the absence of any proven benefit from rapid BP lowering accomplished over a few weeks, a slower drug dose titration strategy as recommended in the guidelines seems reasonable.
Acknowledgements
Bristol-Myers Squibb Company was responsible for the worldwide safety database for irbesartan/HCTZ.
Editorial support for preparing this manuscript was provided by Dr Sujata Shah, Ms Anahita Gouri, and Mr Satyendra Shenoy of sanofi-aventis (India).
The study protocol was prepared by Dr Nathalie Genes, who was employed by sanofi-aventis.
We would like the following investigators, who participated in the ACTUAL Study: Algeria: Nora Henine, Brahim Kichou, Belkacem Benbouabdallah, Mohamed Foughali, Abdelmounène Mekarnia, Zahoua Belkadi, Boudfer and Benkouar; Argentina: Claudio Rodolfo Majul, Hugo Daniel Sanabria, Daniel Leonardo Piskorz, Ricardo Gabriel López Santi, Marcelo Orías, Carlos Alberto Mario Borrego, Olga Beatriz Liliana Paéz, Pablo Alejandro Puleio, Miguel Visser, Cesar Javier Zaidman, Miguel Aron Cartía, Luis Esteban Keller, Carlos Enrique Lamas, María Rosa Siegel, Hugo Gabriel Tonín, Graciela Rosana Reyes, Pablo Gabriel Romia, Julio Oscar Bono, Patricia M. Flammia, Pablo Antonio Novoa, Eduardo Moreyra, Viviana Elizabeth Arias, María Victoria Borrego and Hector Ballestero; Brazil: Décio Mion Jr., Celso Amodeo, Fernando Almeida, Hilton Chaves, José Saraiva, Osvaldo Kohlmann, Paulo Jardim, Wille Oigman, Katia Ortega, Murilo Coelho, Carolina Gonzaga, Márcio Sousa, Maria Valéria Pavan, José Roberto Silva, Maria Helena Vidotti, Frida Plavnik, Marcelo Uehara, Thiago Jardim, Weimar Souza and Mário Fritsch; Colombia: Julio Cesar Duran, Miguel Urina, Alonso Merchan, Carlos Silva, Andres Almanzar, Jose Balaguera and Stase Slotkus; Ecuador: Mayra Sánchez, Carlos Chacón, Alfredo Luna and Luis Falconí; Egypt: Ramez Raouf Guindy, Sameh Mohamed Shaheen, Hossam El-Din Ghanem El-Hossary, Hossam Ibrahim Kandil, Ahmed Shawky El-Serafy, Ahmed Ashraf Eissa, Mohamed Abd El-Lateef Khaleel and Yasser Abd El-Hameed Sharaf; Lebanon: Adel Berbari, Roland Kassab, Georges Ghanem, Chaouki Abdallah, Samer Kabbani, Georges Badawi, Antoine Sarkis and Rabih Azar; Mexico: Miguel Esteban Estrella Garza, Joel Rodríguez Saldaña, Jorge Carrillo Calvillo, Ricardo Alvarado Ruiz, Fernando Antonio Reyes Cisneros, Raúl Gerardo Velasco Sánchez, Edmundo Alfredo Bayram Llamas, René Narvaez David, Eduardo Santiago Uruchurtú Chavarín, Osvaldo Corona, Gregorio Magno Fernández, Hugo López, Marco Antonio Morales de Teresa, José Luis Leiva Pons, Natalia Nikitina, Paulina Sida Pérez, Francisco Javier Robles, Roberto Orozco and Joel Dorantes García; Morocco: Saadia Abir, Said Chraibi, Hafid Akoudad, Laila Azzouzi and Ikram Lahlou; Peru: José Tordoya, Carlos Chavez, Walter Yañez, Mario Carrión, Juan Urquiaga, Victor Herrera, Javier Chumbe and Yudi Roldan; Saudi Arabia: Abdulrahman Ziada, Ibrahim Wasfy, Mohamed Said El Nazer, Yousef Maleeh, Hesham Yehia and Samy Abdel Rahman; Tunisia: Lotfi Slimen, Habib Haouala, Ali Ben Khalfalla, Faouzi Maatouk, Samir Kammoun, Salem Kachboura, Lahidheb Dhaker, Saidi Imen, Othman Salah, Gommidh Mehdi, Samia Chaouachi, Hamrouni Mounir, Leila Abid, Faten Triki and Chine Samira; United Arab Emirates: Al Mahmeed, Binbrek, Agrawal, Sabry, Misra and Munther; and Venezuela: Gloria Vergara, Lempira Guevara, Luis López Gómez, Andrés Carmona, Aaron Cohen, Carlos Mogollón and José Félix Oletta.
Information about previous presentations of the work presented in the article
None.
Sources of support
The ACTUAL study was sponsored by sanofi-aventis.
Conflict of interest statement
Professor Xavier Girerd received honorarium from sanofi-aventis, Daiichi-Sankyo, IPSEN, Novartis Pharma, and Boehringer Ingelheim France during the past 3 years. Dr Joseph Aoun is employed by sanofi-aventis. Dr David Rosenbaum declared no financial disclosure.
Clinical Trials Registry (clinicaltrials.gov) Number
NCT00708344
References
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Bobrie G, Delonca J, Moulin C, Giacomino A, Postel-Vinay N, Asmar R. A home blood pressure monitoring study comparing the antihypertensive efficacy of two angiotensin II receptor antagonist fixed combinations. Am J Hypertens. 2005;18:1482–1488.
- Coca A, Calvo C, Sobrino J, Gomez E, Lopez-Paz JE, Sierra C, . Once-daily fixed-combination irbesartan 300 mg/ hydrochlorothiazide 25 mg and circadian blood pressure profile in patients with essential hypertension. Clin Ther. 2003; 25:2849–2864.
- Cushman WC, Neutel JM, Saunders E, Bakris GL, Ferdinand KC, Ofili EO, . Efficacy and safety of fixed combinations of irbesartan/hydrochlorothiazide in older vs younger patients with hypertension uncontrolled with monotherapy. Am J Geriatr Cardiol. 2008;17:27–36.
- Neutel JM, Saunders E, Bakris GL, Cushman WC, Ferdinand KC, Ofili EO, . The efficacy and safety of low- and high-dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: The INCLUSIVE trial. J Clin Hypertens (Greenwich). 2005;7:578–586.
- Neutel JM, Smith D. Ambulatory blood pressure comparison of the anti-hypertensive efficacy of fixed combinations of irbesartan/hydrochlorothiazide and losartan/hydrochlorothiazide in patients with mild-to-moderate hypertension. J Int Med Res. 2005;33:620–631.
- Ofili EO, Ferdinand KC, Saunders E, Neutel JM, Bakris GL, Cushman WC, . Irbesartan/HCTZ fixed combinations in patients of different racial/ethnic groups with uncontrolled systolic blood pressure on monotherapy. J Natl Med Assoc. 2006;98:618–626.
- Saunders E, Cable G, Neutel J. Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: A secondary analysis of the irbesartan/ hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) study. J Clin Hypertens (Greenwich). 2008;10:27–33.
- Sowers JR, Neutel JM, Saunders E, Bakris GL, Cushman WC, Ferdinand KC, . Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes. J Clin Hypertens (Greenwich). 2006;8:470–480.
- Sun NL, Jing S, Chen J. [The control rate of irbesartan/ hydrochlorothiazide combination regimen in the treatment of Chinese patients with mild to moderate hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2005;33:618–621.
- Flack JM, Yunis C, Preisser J, Holmes CB, Mensah G, McLean B, Saunders E. The rapidity of drug dose escalation influences blood pressure response and adverse effects burden in patients with hypertension: The Quinapril Titration Interval Management Evaluation (ATIME) Study. ATIME Research Group. Arch Intern Med. 2000;160:1842–1847.
- Neldam S, Edwards C, Telmisartan/Hydrochlorothiazide I. Results of increasing doses of hydrochlorothiazide in combination with an angiotensin receptor blocker in patients with uncontrolled hypertension. J Clin Hypertens (Greenwich). 2008;10:612–618.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens. 1999;12: 797–805.
- Gradman AH, Brady WE, Gazdick LP, Lyle P, Zeldin RK. A multicenter, randomized, double-blind, placebo-controlled, 8-week trial of the efficacy and tolerability of once-daily losartan 100 mg/hydrochlorothiazide 25 mg and losartan 50 mg/hydrochlorothiazide 12.5 mg in the treatment of moderate-to-severe essential hypertension. Clin Ther. 2002;24: 1049–1061.
- Ruilope LM, Malacco E, Khder Y, Kandra A, Bonner G, Heintz D. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: The VAST study. Clin Ther. 2005; 27:578–587.
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet. 2004; 363:2022–2031.