Abstract
Aims. To assess safety and efficacy of valsartan/amlodipine combination in hypertensive Taiwanese patients. Methods. This 12-week, multi-center, prospective, observational, post-marketing study enrolled 1029 patients to receive valsartan/amlodipine combination alone or as add-on to other antihypertensives. Efficacy was evaluated by blood pressure (BP) control rate (in mmHg; non-diabetics, < 140/90; diabetics, < 130/80) at Week 12 and BP-lowering ability at Weeks 4 and 12. Additionally, responder rate (sitting-SBP < 140 for baseline SBP ≥ 140 or sitting-DBP < 90 for baseline DBP ≥ 90, or SBP reduction > 20 or DBP reduction > 10 from baseline) was determined. Major findings. Adverse events (AEs) were reported in 12.15% patients; dizziness, cough, and peripheral edema were the most commonly reported AEs. Overall BP control rate was 48.27%. Greater BP reduction was noted at Week 12 than at Week 4 between all groups and subgroups. Greater SBP/DBP reduction was observed in patients with stage 2 hypertension than stage 1 hypertension at baseline. The overall responder rate was 78.52%. Subgroup analysis showed greater BP reduction in non-diabetics than diabetics; only SBP reduction reached statistical significance (− 13.7 [18.3] vs. − 10.7 [17.4] mmHg; p < 0.0093). Principal conclusion. Valsartan/amlodipine combination was well tolerated, with no safety concerns identified and an effective treatment option for hypertensive Taiwanese patients.
Introduction
Although the life-time risk for developing hypertension is about 90% (Citation1), the appropriate first-line therapy for hypertensive patients still remains an unmet challenge. Elevated blood pressure (BP) is also associated with a greater risk of cardiovascular (CV) and related diseases (Citation2), leading to higher mortality and morbidity worldwide (Citation3,Citation4). The Taiwanese Society of Cardiology has reported an increased impact of hypertension on CV diseases in Asians as compared to Caucasians (Citation5). In a 2002 Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia (TwSHHH), the nationwide prevalence rates of hypertension were reported to be 25% in men and 18% in women, with higher rates up to 47% noted among individuals ≥ 60 years of age. Additionally, as compared to the Nutrition and Health Survey in Taiwan (NAHSIT, 1993–1996) study, an increase in hypertension control rates from 2.4% to 21% in men, and 5% to 29% in women were observed (Citation6). However, no single country has reported an overall control rate > 40%, which is defined by office BP < 140/90 mmHg in non-high-risk patients and < 130/80 mmHg in high-risk patients (Citation2).
Interesting insights regarding the treatment patterns for hypertension were obtained from a study conducted in Taiwan that used the 200,000-person representative random sample from the computerized reimbursement database of the National Health Insurance (NHI), between January 1997 and December 2004 (Citation7). Due to lack of specific guidelines for the treatment of hypertensive Taiwanese patients, most physicians seemed to adopt the recommendations of the Joint National Committee in the United States (US JNC) or the World Health Organization-International Society Hypertension for the Management of Hypertension (WHO/ISH) guidelines which recommended low dosages of thiazide diuretics as the first-line drug for essential hypertension in patients with no compelling indications. Additionally, a summary of the total number of prescriptions for the different categories of antihypertensive drugs showed that calcium channel blockers (CCBs) (n = 92,574; 51.8%) were the most frequently prescribed antihypertensive agents, followed by beta-blockers, and then by angiotensin-converting enzyme (ACE) inhibitors, diuretics, others, and angiotensin receptor blocker (ARBs). However, with the passage of time from the date of initial therapy, a significant increase in the prescription rate for ARBs was noted, from 3.8% in the first year to 10.3% in the seventh year (p < 0.0001). Furthermore, the percentage of monotherapy treatments declined over time from the initial diagnosis, whereas a gradual increase in the percentage of combination therapies was observed (Citation7). Other studies have also shown that in a majority of hypertensive adults, two or more antihypertensive drugs from different classes may be required for BP control (Citation8–10).
Both diuretics and beta-blockers have been reported to accelerate new-onset diabetes (NOD) in patients with hypertension (Citation11–14). Many studies have reported that CCBs have a neutral effect on lipid and glucose metabolism and are not independently associated with an increased risk of NOD (Citation11,Citation15,Citation16). Recent studies have indicated that ARBs that interrupt the renin-angiotensin axis are the preferred drugs in diabetic patients (Citation14,Citation17). Many hypertension treatment guidelines including the 2010 Taiwan Society of Cardiology guidelines have acknowledged the use of combination therapy ([ACE or ARB] + [CCB] or [thiazide diuretic]) in high-risk individuals and patients with stage 2 or 3 hypertension to achieve specific BP goals (< 140/90 mmHg or < 130/80 mmHg in diabetics) (Citation5,Citation18,Citation19).
Exforge® (Novartis Pharma AG, Switzerland), a single-pill fixed dose combination of 160/320 mg valsartan and 5/10 mg amlodipine has been approved by the United States Food and Drug Administration (US FDA) as initial and first-line therapy in treatment of hypertension (Citation20), and is also approved for treatment of hypertensive patients in Taiwan in 2008. In ARB/CCB combination therapy, the ARB blocks the angiotensin II type I (AT1) receptor-mediated vasoconstriction (by blocking Ca ++ release from intracellular storage pool) and the CCB blocks Ca ++ entry through dihydropyridine inhibited channels; this co-operative antagonism causes vasodilation and vascular protection, thereby decreasing blood pressure and vascular damage (Citation21). Studies have shown the valsartan/amlodipine combination to be highly effective, providing adequate BP control in patients unresponsive to previous monotherapy (Citation22–24), in the elderly population (Citation25), or in patients with mild, moderate, and severe hypertension (Citation26).
The safety and efficacy profile of valsartan/ amlodipine combination as compared to monotherapy and other combination therapies has been well documented in different ethnicities worldwide (Citation26–28). However, no studies so far have been reported with this combination drug in Taiwan. The objective of this post-marketing surveillance study was to evaluate the efficacy and safety of valsartan/amlodipine combination in the Taiwanese population.
Patients and methods
Study design
This was a 12-week, multi-center, prospective, open-label, non-comparative, observational, post-marketing surveillance study designed and implemented in accordance with the ICH GCP guidelines and the ethical principles laid down in the Declaration of Helsinki. The study was approved by the respective Institutional Review Boards. The data was collected across seven centers in Taiwan from October 29, 2009 to October 12, 2010. The primary objective of the study was to evaluate the safety of Exforge (a single-pill combination of 80 mg valsartan and 5 mg amlodipine) as antihypertensive therapy under daily life conditions during a planned treatment and observation period for at least 12 weeks (± 4 weeks). The secondary objective was to evaluate the efficacy of the valsartan/amlodipine combination for the same time period.
Patient selection
Male and female hypertensive patients aged ≥ 20 years, medically recommended valsartan/amlodipine combination, were included in the study. Patients with known hypersensitivity to the active substances valsartan or amlodipine besilate, dihydropyridine derivatives, or to any of the excipients; or women who were pregnant, intended to become pregnant, or breastfeeding were excluded. All patients provided written informed consent before inclusion in the study.
Treatment and assessments
At Visit 1, in addition to physical examination, demographic details, baseline characteristics, relevant medical history including CV risk factors, details about current antihypertensive therapy, sitting BP values, and heart rate were recorded. The patients were prescribed a single-pill combination of 5 mg amlodipine and 80 mg valsartan either alone or as add-on therapy with diuretics, beta blockers, angiotensin converting enzyme (ACE) inhibitors, or any other antihypertensive medication based on investigator's judgment. Assignment of patients to therapy was based on current practice standards and medical indication and was distinct from the decision to include the patient in the study. At Visit 2 (4 ± 2 weeks from baseline), sitting BP values were recorded, and any change in the clinical signs or in the current antihypertensive medication and AEs were noted. Similar assessments were made at Visit 3 (12 ± 4 weeks from baseline).
Outcome measures
The primary objective was assessment of safety based on the incidence of any AEs and serious adverse events (SAEs) along with their severity and relationship to the study drug. The secondary objective was to evaluate the efficacy of the valsartan/amlodipine combination in terms of the percentage of patients who achieved therapeutic treatment goal according to the WHO criteria (BP control rate; diastolic blood pressure (DBP) < 90 mmHg and systolic blood pressure (SBP) < 140 mmHg for non-diabetics or DBP < 80 mmHg and SBP < 130 mmHg for diabetics at Week 12); BP-lowering ability (reduction in mean sitting SBP [MSSBP] and mean sitting DBP [MSDBP] at Week 12; and proportion of responder at the end of the study (MSDBP < 90 mmHg for non-diabetics or < 80 mmHg for diabetics; MSSBP < 140 mmHg for non-diabetics or < 130 mmHg for diabetics] or a reduction of ≥ 10 mmHg DBP or ≥ 20 mmHg SBP from baseline at Week 12). Subgroup analysis for the BP-lowering effect was performed for patients with stage 1, stage 2, or isolated systolic hypertension (ISH) (stage 1 hypertension was defined as 140 mmHg ≤ SBP < 160 mmHg or 90 mmHg ≤ DBP < 100 mmHg; stage 2 hypertension was defined as SBP ≥ 160 mmHg or DBP ≥ 100 mmHg; ISH was defined as SBP ≥ 140 mmHg and DBP < 90 mmHg); similar analysis was performed for the BP control rate and BP-lowering effect of the valsartan/amlodipine combination between diabetics and non-diabetics.
Assessment of efficacy and tolerability (very good, good, average, and below average) was performed by the investigators at the end of the study (Week 12).
Statistical analysis
The incidence rate of AEs in the overall population was estimated at 5.4% from a global study. Assuming that incidence rate of AEs in Taiwan was 3.5%, under the superiority hypothesis, an estimated sample size of 957 was required for the test to have 80% power and a significance level of 0.05, considering a drop-out rate of 10%. Thus, 1029 patients were enrolled in the study. The safety and efficacy analyses were performed on the enrolled set (ENR) which consisted of all patients who were eligible and provided written informed consent. Continuous variables were reported as descriptive statistics including mean, median, standard deviation (SD), minimum, maximum, and 95% confidence intervals (CIs); categorical variables were presented as frequencies and percentages. The t-test or the Wilcoxon rank-sum test was used for analysis of all continuous variables, and the chi-square test was used for analysis of the categorical variables. The paired t-test was used for comparisons of change in blood pressure within a cohort and between subgroups. Paired comparisons of continuous variables other than blood pressure were analyzed by the Wilcoxon rank-sum test. The BP control rate and the responder rate between subgroups were assessed by the chi-square test. The statistical significance was set as p < 0.05. All analyses were performed using the SAS version 9.2.
Results
Patient demographics and baseline characteristics
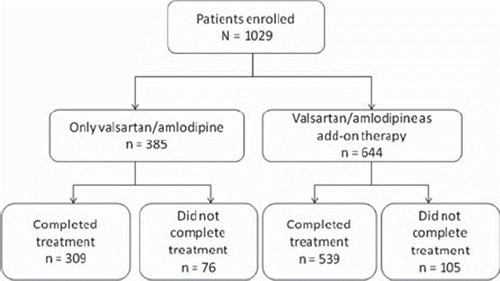
A total of 1029 patients were screened and enrolled in the study (). Of these, 385 patients received the valsartan/amlodipine combination pill alone and 644 patients received it as add-on therapy with other antihypertensive drugs. shows patient demographics and baseline characteristics. The mean (SD) age of the patients was 63.3 (12.9) years and about 47% patients were aged ≥ 65 years. All patients but one were Taiwanese. About 62% patients with CV risk factors were prescribed the valsartan/amlodipine combination as add-on therapy with diuretics, beta blockers, ACE inhibitors, or other antihypertensive medication. The mean (SD) number of concomitant medications at baseline was 3.6 (2.5). Over 60% patients with stage 1, stage 2, and ISH received the valsartan/amlodipine combination as add-on therapy (60.15%, 65.59%, and 67.16%, respectively).
Table I. Demographic and baseline characteristics.
Primary endpoint
Safety. A total of 164 AEs were reported in 125 patients (12.15%). Dizziness (2.62%) and cough (0.97%) were the most frequently reported AEs. A majority of the AEs were of mild (76.83%) or moderate (13.41%) intensity. A total of 28 AEs (17.07%) were considered related to the study drug; dizziness, cough, palpitations, and peripheral edema were the most common AEs considered related to study treatment. Only 14 patients (1.36%) discontinued the drug due to an AE. Twenty-seven SAEs were reported in 17 patients (1.65%); none of which were considered related to the study drug. Overall, the valsartan/amlodipine was well tolerated, with no deaths reported during the treatment period.
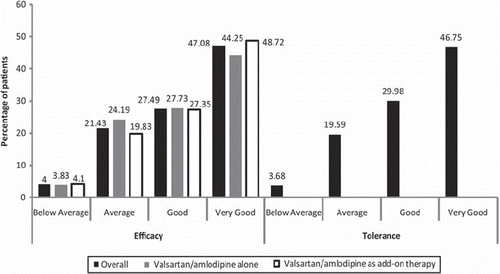
In the investigators assessment of tolerance (), the tolerability was judged as “good” or “very good” in more than 75% patients.
Secondary endpoint
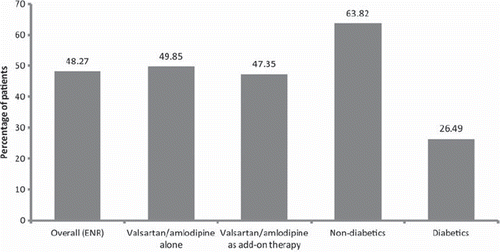
Efficacy. After 12 weeks of treatment with the valsartan/amlodipine combination, 446 out of 924 patients (848 patients completed the study and 76 patients discontinued prior to Week 12) achieved the desired therapeutic BP goal (DBP < 90 mmHg and SBP < 140 mmHg for non-diabetics or DBP < 80 mmHg and SBP < 130 mmHg for diabetics), with an overall BP control rate of 48.27%. Similar control rates were observed in patients prescribed the combination alone and as add-on therapy. Subgroup analysis showed that twice the percentage of non-diabetic patients achieved the desired BP goal as compared to the diabetic patients (63.82% vs. 26.49%) (p < 0.0001). The control rates for all the groups and between subgroups have been shown in .
shows the mean reduction in SBP and DBP from baseline to Weeks 4 and 12 in the ENR set as well as with the study drug alone and as add- on therapy subgroups. The BP-lowering effect from baseline was statistically significant (p < 0.0001) at all time points. Subgroup analysis showed that the BP reduction were greater in the non-diabetic patients than in the diabetic patients, although the statistical significance was reached only for SBP reduction. Subgroup analysis based on the type of hypertension at baseline showed greater reduction in SBP and DBP in patients with stage 2 hypertension at Week 4 and Week 12 ().
Table II. Summary of adverse events (AEs).
Table IIIa. Overall change in blood pressure by treatment groups.
Table IIIb. Change in blood pressure in non-diabetic and diabetic patients (Subgroup analysis).
Table IIIc. Change in blood pressure based on the baseline BP condition.
The overall responder rate of patients with MSDBP < 90 mmHg or MSSBP < 140 mmHg for non-diabetics or MSDBP < 80 mmHg or MSSBP < 130 mmHg for diabetics at Week 12 was 78.52%. Significant difference was observed between responder rates of non-diabetic and diabetic subgroups (86.59% vs. 66.34%; p < 0.0001). In the investigators evaluation of efficacy, the effectiveness of the valsartan/amlodipine combination was judged as “good” or “very good” in about 75% patients ().
Discussion
In patients with coexisting hypertension and diabetes, there is an increased risk of CV disease and diabetes-specific complications, including retinopathy and nephropathy. Tighter BP control in hypertensive patients with type 2 diabetes has been documented to reduce the risk of both microvascular and macrovascular diseases (Citation29,Citation30). A lower risk for stroke was observed with intensive therapy in diabetic patients with SBP < 120 mmHg (Citation31,Citation32). Furthermore, as compared to subjects with BP levels < 120/80 mmHg, the relative risk for developing end-stage renal disease increased with increase in BP (Citation33,Citation34). Efficacy in our study was judged based on the BP control rate after 12 weeks of treatment. The overall BP control rate was 48.27%. The EX-STANDstudy reported similar BP control rate with the valsartan/amlodipine combination (49.8%) at Week 8; this value increased to 57.2% at Week 12 (Citation35). However, it may not be suitable to draw a direct comparison with the EX-STAND study due to differences in baseline characteristics between blacks and an Asian population. Better control rates than ours have been observed in hypertensive patients who switched to the valsartan/amlodipine combination from amlodipine monotherapy (Citation8,Citation23,Citation36). This could be because our study had patients with very few exclusion criteria, to replicate the real-life hypertension treatment scenario in Taiwan. The 2002 (TwSHHH) reported a control rate of 24.3% (Citation6). Although the TwSHHH study and the present study are not directly comparable, our better results may indicate improved BP control with the use of ARB/CCB combination therapy. This can be attributed to single-pill fixed dose combination of the valsartan/amlodipine, which is convenient and known to increase patient compliance (Citation37). Our results showed mean SBP/DBP reductions of –12.5 (18.0) mmHg/–6.5 (11.8) mmHg at Week 12, which were an improvement from Week 4.
Twice the percentage of non-diabetic patients achieved the desired BP goal as compared to the diabetic patients (63.82% vs. 26.49%). Hypertensive patients with coexistent diabetes are generally known to be most resistant to treatment (Citation38) and are also known to benefit from the ARB/CCB combination (Citation19). The addition of valsartan to CCB is effective as it causes improvement in resistance artery remodeling in diabetic hypertensive patients (Citation39). Over 30% patients in our study were diabetic; mean SBP/DBP reductions of –10/–5.8 mmHg were observed in these patients.
Numerous studies have demonstrated the excellent BP-lowering ability of valsartan/amlodipine combination as compared to either monotherapy or other combination therapies (Citation40–42). Data from other studies have revealed that mean (SD) reductions in BP were significantly greater with the valsartan/amlodipine combination than with their individual components or losartan/amlodipine combination or lisinopril/hydrochlorothiazide (Citation40,Citation43,Citation44). The valsartan/amlodipine combination is also known to be effective in hypertensive patients uncontrolled with previous monotherapy (Citation23,Citation45) and other combination therapies such as olmesartan/amlodipine (Citation46). Hypertensive patients non-responsive to previous therapies such as ACE inhibitors and CCB combination showed reduction in MSSBP and MSDBP of –15.4 mmHg and –7.0 mmHg, respectively, with the valsartan/amlodipine combination (Citation47).
The BP reduction in our study was lower than that in other studies; a possible explanation could be a large number of patients with stage 2 and ISH included in our study. Previous studies have shown the valsartan/amlodipine combination to be efficacious in patients with various grades of hypertension (Citation26), including ISH (EX-STAND study) (Citation35). In our study too, we observed statistically significant reductions in both SBP and DBP in patients with stage 1, 2, and ISH at Week 12 compared to baseline. The overall response rate in our study was 78.52%. Similar response rate (79%) was reported with the valsartan/amlodipine combination as compared to monotherapy with amlodipine (70.1%) (Citation48). However, two, multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension reported slightly higher response rates (over 88%) with the combination (Citation40). The lower response rate in our study may be a result of low compliance with antihypertensive medication in a real-life scenario. However, we believe that further research may help to validate this assumption.
The valsartan/amlodipine combination treatment was well tolerated in our study, with > 85% patients not experiencing any AEs. Dizziness, a common AE associated with valsartan therapy, was reported by 2.62% patients, while < 1% patients experienced cough. The incidence of dizziness was similar to that observed with valsartan 20–160 mg monotherapy (2%–3.5%), but much lower than the 5.4% with placebo (Citation24) and with losartan (Citation49). The incidence of cough in our study was much lower than that previously reported in patients on ACE inhibitors (7.9%), valsartan therapy (2.6%), and on ACE/diuretics combination therapy (11.3%) (Citation37,Citation50). Only four patients (0.49%) in our study reported peripheral edema, supporting a low incidence of peripheral edema with ARB/CCB combination therapy as compared to CCB monotherapy in other studies (Citation27,Citation43,Citation51). The valsartan/amlodipine combination also resolved peripheral edema in > 50% patients who switched from amlodipine to this combination (Citation36). This effect has been attributed to valsartan which counteracts the microcirculatory changes responsible for amlodipine-induced edema (Citation52). The low incidence of peripheral edema (0.49%) in our study as compared to previous research (5.4%–7.6%) may be attributed to ethnic differences in various populations (Citation40,Citation48). In the investigators’ evaluation of tolerability, more than 75% patients on the valsartan/amlodipine combination were judged as having “good” and “very good” tolerance. Our results indicate that the valsartan/amlodipine combination was well tolerated in the Taiwanese population, with no new safety concerns identified.
The results of the present study must be considered against the background of several potential limitations. First, the study was not controlled, therefore, the observed effects cannot be entirely attributed to study medication and the effectiveness results may be overestimated due to the presence of other antihypertensive agents. Second, in the absence of a randomization procedure, the influence of patient selection biases cannot be assessed. Furthermore, various types of patients were assessed jointly for the effectiveness analysis and may have biased the overall effectiveness results. However, the large number of patients selected from the general population with few exclusion criteria make this study results relevant and provide real-life evidence of the efficacy and safety of the valsartan/amlodipine combination in Taiwanese patients with hypertension.
In conclusion, the valsartan/amlodipine combination can be an effective treatment option for Taiwanese hypertensive patients.
Acknowledgements
We would like to thank all the investigators participating in this project, and Diana Liu and Carol Yang (Novartis Pharmaceuticals Ltd., Taipei, Taiwan) for their valuable contributions. We would like to acknowledge the services of Dawn Chien (Formosa Biomedical Technology Corp.) for statistical assistance. We also thank the study participants and their families for their personal time and commitment to this project. Supportive medical writing services were offered by Cactus Communications Pvt. Ltd. and paid for by Novartis Taiwan.
Declaration of interest: The current study was sponsored by Novartis for post-marketing surveillance of valsartan/amlodipine combination pill with regard to its tolerability and efficacy in hypertensive Taiwanese patients. None of the authors have any conflicts of interest to declare.
References
- Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010.
- Chobanian AV. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42: 1206–1252.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360: 1903–1913.
- Lawes CMM, Hoorn SV, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371: 1513–1518.
- Chiang CE, Wang TD, Li YH, Lin TH, Chien KL, Yeh HI, . 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2010;109:740–773.
- Su TC, Bai CH, Chang HY, You SL, Chien KL, Chen MF, . Evidence for improved control of hypertension in Taiwan: 1993–2002. J Hypertens. 2008;26:600–606.
- Liu PH, Wang JD. Antihypertensive medication prescription patterns and time trends for newly-diagnosed uncomplicated hypertension patients in Taiwan. BMC Health Serv Res. 2008;8:133.
- Allemann Y, Fraile B, Lambert M, Barbier M, Ferber P, Izzo JL, Jr. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX-FAST) study. J Clin Hypertens (Greenwich). 2008;10:185–194.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther. 2007;29:61–73.
- Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, . Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404.
- Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29:1065–1070.
- Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, . A comparison of outcomes with angiotensin-converting—enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592.
- Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, . Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356:359–365.
- Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, . Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. JHypertens. 2009;27:2121–2158.
- Liou YS, Ma T, Tien L, Chien C, Chou P, Jong GP. Long-term effects of antihypertensive drugs on the risk of new-onset diabetes in elderly Taiwanese hypertensives. Int Heart J. 2008;49:205–211.
- Stump CS, Hamilton MT, Sowers JR. Effect of antihypertensive agents on the development of type 2 diabetes mellitus. Mayo Clin Proc. 2006;81:796–806.
- Andraws R, Brown DL. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials). Am J Cardiol. 2007;99:1006–1012.
- Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, . Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary: The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2006;28:88–136.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536.
- Krzesinski JM, Cohen EP. Exforge (amlodipine/valsartan combination) in hypertension: the evidence of its therapeutic impact. Core Evid. 2010;4:1–11.
- Ferri C, Croce G, Desideri G. Role of combination therapy in the treatment of hypertension: Focus on valsartan plus amlodipine. Adv Ther. 2008;25:300–320.
- Brachmann J, Ansari A, Mahla G, Handrock R, Klebs S. Effective and safe reduction of blood pressure with the combination of amlodipine 5 mg and valsartan 160 mg in hypertensive patients not controlled by calcium channel blocker monotherapy. Adv Ther. 2008;25:399–411.
- Ke Y, Zhu D, Hong H, Zhu J, Wang R, Cardenas P, Zhang Y. Efficacy and safety of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr Med Res Opin. 2010;26:1705–1713.
- Oparil S, Giles T, Ofili EO, Pitt B, Seifu Y, Hilkert R, . Moderate versus intensive treatment of hypertension with amlodipine/valsartan for patients uncontrolled on angiotensin receptor blocker monotherapy. J Hypertens. 2011;29: 161–170.
- Fogari R, Zoppi A, Mugellini A, Corradi L, Lazzari P, Preti P, Derosa G. Efficacy and safety of two treatment combinations of hypertension in very elderly patients. Arch Gerontol Geriatr. 2009;48:401–405.
- Chazova IE, Dongre N, Vigdorchik AV. Real-life safety and effectiveness of amlodipine/valsartan combination in the treatment of hypertension. Adv Ther. 2011;28: 134–149.
- Flack JM, Hilkert R. Single-pill combination of amlodipine and valsartan in the management of hypertension. Expert Opin Pharmacother. 2009;10:1979–1994.
- Ofili EOOparil S, Giles T, Pitt B, Purkayastha D, Hilkert R, . Moderate versus intensive treatment of hypertension using amlodipine/valsartan and with the addition of hydrochlorothiazide for patients uncontrolled on angiotensin receptor blocker monotherapy: results in racial/ethnic subgroups. J Am Soc Hypertens. 2011.
- Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316:823–828.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317: 703–713.
- Nilsson PM. ACCORD and Risk-Factor Control in Type 2 Diabetes. N Engl J Med. 2010;362:1628–1630.
- Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, . Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, . Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18.
- Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005;165:923–928.
- Flack JM, Calhoun DA, Satlin L, Barbier M, Hilkert R, Brunel P. Efficacy and safety of initial combination therapy with amlodipine/valsartan compared with amlodipine monotherapy in black patients with stage 2 hypertension: the EX-STAND study. J Hum Hypertens. 2009;23:479–489.
- Schrader J, Salvetti A, Calvo C, Akpinar E, Keeling L, Weisskopf M, Brunel P. The combination of amlodipine/ valsartan 5/160 mg produces less peripheral oedema than amlodipine 10 mg in hypertensive patients not adequately controlled with amlodipine 5 mg. Int J Clin Pract. 2009;63:217–225.
- Black HR, Bailey J, Zappe D, Samuel R. Valsartan: more than a decade of experience. Drugs. 2009;69:2393–2414.
- Brown MJ, Castaigne A, de Leeuw PW, Mancia G, Palmer CR, Rosenthal T, Ruilope LM. Influence of diabetes and type of hypertension on response to antihypertensive treatment. Hypertension. 2000;35:1038–1042.
- Savoia C, Touyz RM, Endemann DH, Pu Q, Ko EA, De Ciuceis C, Schiffrin EL. Angiotensin Receptor Blocker Added to Previous Antihypertensive Agents on Arteries of Diabetic Hypertensive Patients. Hypertension. 2006;48: 271–277.
- Philipp T, Smith TR, Glazer R, Wernsing M, Yen J, Jin J, . Two multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther. 2007;29:563–580.
- Smith TR, Philipp T, Vaisse B, Bakris GL, Wernsing M, Yen J, Glazer R. Amlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: subgroup analyses of 2 randomized, placebo-controlled studies. J Clin Hypertens (Greenwich). 2007;9: 355–364.
- Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55: 1314–1322.
- Fogari R, Mugellini A, Preti P, Zoppi A, Derosa G. Valsartan addition to amlodipine is more effective than losartan addition in hypertensive patients inadequately controlled by amlodipine. Vasc Health Risk Manag. 2010;6:87–93.
- Poldermans D, Glazes R, Kargiannis S, Wernsing M, Kaczor J, Chiang YT, . Tolerability and blood pressure-lowering efficacy of the combination of amlodipine plus valsartan compared with lisinopril plus hydrochlorothiazide in adult patients with stage 2 hypertension. Clin Ther. 2007;29:279–289.
- Sinkiewicz W, Glazer RD, Kavoliuniene A, Miglinas M, Prak H, Wernsing M, Yen J. Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on valsartan monotherapy. Curr Med Res Opin. 2009;25:315–324.
- Braun N, Ulmer H-J, Ansari A, Handrock R, Klebs S. Efficacy and safety of the single pill combination of amlodipine 10mg plus valsartan 160mg in hypertensive patients not controlled by amlodipine 10mg plus olmesartan 20mg in free combination. Current Medical Research and Opinion. 2009;25:421–430.
- Trenkwalder P, Schaetzl R, Borbas E, Handrock R, Klebs S. Combination of amlodipine 10 mg and valsartan 160 mg lowers blood pressure in patients with hypertension not controlled by an ACE inhibitor/CCB combination. Blood Press. 2008;17 Suppl 2:13–21.
- Schunkert H, Glazer RD, Wernsing M, Yen J, Macarie CE, Vintila MM, Romanova J. Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on amlodipine monotherapy. Curr Med Res Opin. 2009;25:2655–2662.
- Weber M. Clinical safety and tolerability of losartan. Clin Ther. 1997;19:604–616; discussion 603.
- Ishimitsu T, Yagi S, Ebihara A, Doi Y, Domae A, Shibata A, . Long-term evaluation of combined antihypertensive therapy with lisinopril and a thiazide diuretic in patients with essential hypertension. Jpn Heart J. 1997;38: 831–840.
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med. 2011;124:128–135.
- Fogari R, Zoppi A, Derosa G, Mugellini A, Lazzari P, Rinaldi A, . Effect of valsartan addition to amlodipine on ankle oedema and subcutaneous tissue pressure in hypertensive patients. J Hum Hypertens. 2007;21:220–224.