Abstract
Background and aim. The impact of aging on the relationship between left ventricular (LV) mass/geometry and diastolic function as assessed by updated echocardiographic methods, such as tissue Doppler, is poorly defined. We investigated this issue in a cohort of hypertensive patients. Methods. A total of 660 hypertensives (mean age 65 ± 13 years, 48% men) with preserved LV systolic function underwent a comprehensive echo-Doppler examination for routine clinical indications. For the present analysis, the subjects have been divided in two age groups (<65 or ≥65 years). Results. Overall, 61% of subjects fulfilled the criteria for LVH, 18% for left atrial (LA) enlargement and 11% for altered LV filling index. Concentric LV geometry was 1.4-fold higher in older hypertensives than in younger counterparts; also the prevalence of LA enlargement and altered LV filling was 2.0- and 1.9-fold higher in the former group, respectively. In older hypertensives, at variance from younger ones, neither LV mass nor relative wall thickness (RWT), a continuous index of LV geometry, were independently correlated to conventional as well as tissue Doppler LV diastolic indexes. Conclusions. Our findings suggest the relationship between cardiac hypertrophy and diastolic function in hypertensive subjects is affected by aging-associated factors unrelated to the amount of LV mass as assessed by standard echocardiography.
Introduction
Arterial hypertension and aging are major determinants of left ventricular hypertrophy (LVH) and LV diastolic dysfunction in the general population (Citation1–4). Progression from normal LV morphology to LVH encompasses a variety of structural alterations. Long-term exposure to pressure overload occurring in sustained arterial hypertension, in particular in the older fraction of the hypertensive population, may induce complex changes in myocardial texture (Citation4,Citation5). In hypertensive LVH, cardiac structure is affected by two major pathological processes, namely hypertrophy of cardiac myocytes and accumulation of interstitial fibrosis; thus, in hypertensive LVH, tissue homogeneity is disrupted and abnormalities in cardiac texture are associated with an impaired function as a result of accumulation of cells other than cardiac myocytes (Citation6–8).
Variable combinations of pressure and volume overload associated with hypertension and frequent comorbidities (i.e. obesity, metabolic syndrome, diabetes) induce different LV geometric adaptations. Echocardiographically assessed LV geometry has been traditionally categorized in four patterns based on LV mass index and relative wall thickness (RWT, the ratio of wall thickness to chamber diameter) and defined as normal geometry, concentric remodeling (normal LV mass index with increased RWT), eccentric hypertrophy (increased LV mass index with normal RWT) and concentric hypertrophy (increased LV mass index and increased RWT) (Citation9). Alterations of LV geometry are associated to biological variables such as age, hemodynamic profile, LV systolic/ diastolic myocardial performance, clinic/ambulatory BP, body mass index (BMI) and extra-cardiac organ damage (Citation10–14). More importantly, pathological geometric patterns are associated with increased incidence of cardiovascular events – in particular concentric hypertrophy is associated with the highest risk (Citation15,Citation16).
Findings linking LV geometries and diastolic function are mostly based on LV diastolic properties as assessed by conventional mitral flow velocity measurements; limited evidence is available about the relationship between LV geometries and diastolic function as assessed by an updated echocardiographic method, such as tissue Doppler, and even fewer results are available about the impact of age on this relationship. Therefore, we examined the distribution of LV geometric patterns across a large cohort of hypertensive subjects according to an age-based analysis and their association with diastolic function assessed either by conventional trans-mitral flow or by tissue Doppler parameters.
Methods
A total of 660 hypertensive subjects, referred to the outpatient echocardiographic service of the Istituto Auxologico Italiano (Meda, Italy) by their general practitioners during a 15-month period from February 2009 to April 2010, were enrolled in the study. Main exclusion criteria were: (i) impaired LV ejection fraction (< 40%); (ii) previous myocardial infarction or coronary bypass history; (iii) previous hospitalization for heart failure; (iv) significant cardiac valve disease (regurgitation > 1 + at Doppler examination, stenosis of any degree, presence of prosthesis and mitral annulus calcification); (v) atrial fibrillation; (vi) left bundle branch block. The study protocol was approved by the Ethics Committee of one of the Institutions involved.
After an informed consent had been obtained, patients’ demographic data, medical history and medications were collected at the echo-lab in a questionnaire administered by the attending physician. Obesity was identified as BMI ≥ 30 kg/m2. High blood pressure (BP) was defined as systolic BP (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg in untreated subjects or current antihypertensive therapy regardless BP values (Citation17). Diabetes mellitus was defined by patient's self-report.
Measurements
Body weight was recorded to the nearest 0.1 kg using a calibrated electronic scale in subjects wearing indoor clothing without shoes. Height was recorded to the nearest 0.5 cm using a standardized wall-mounted height board. BMI was computed as weight in kg divided by the squared height in meters.
Clinic BP was measured by mercury sphygmomanometer with appropriate sized cuffs; measurements were performed after the subjects had been resting for 3–5 min in the sitting position. Three measurements were taken from the non-dominant arm at 1-min intervals and the average was used to define patient's representative values.
Echocardiography
Echo and Doppler examinations were carried out according to a standardized protocol with a commercially available instrument (Vivid 7, GE, Horten, Norway).
Briefly, end-diastolic (d) and end-systolic (s) LV internal diameters (LVID), interventricular septum thickness (IVS) and posterior wall thickness (PW) were measured from two-dimensionally guided M- mode tracings recorded at a speed of 50–100 cm/s, during at least three consecutive cycles according to the Penn convention. RWT was defined by the ratio of PW plus IVS thickness to LVIDd; LV mass was estimated by Devereux's formula {1.04[(IVSd + PWd + LVIDd)3–LVIDd3] − 13.6} (Citation18) and indexed to height to the allometric power of 2.7; LVH was defined as LV mass index > 50 g/h2.7 in both genders (Citation19). Patterns of abnormal LV geometry were defined as follows: (i) LV concentric remodeling (normal LV mass index combined with RWT ≥ 0.43); (ii) eccentric LVH (increased LV mass index combined with RWT < 0.43); (iii) concentric LVH (increased LV mass index combined with RWT ≥ 0.43) (Citation9).
LV ejection fraction was measured from the four-chamber apical projection in the standard fashion from LV end-diastolic and LV end-systolic volume.
Left atrium (LA) diameter was determined at end-systole in the parasternal long-axis view; LA volume (LAV) was measured at end-systole in the apical four-chamber view by planimetry. LAV was calculated by the biplane method of discs according to Simpson rule and indexed to body surface area (LAVI); LA enlargement was defined as LAVI > 29 ml/m2 in both genders (Citation9).
Conventional Doppler measurements
LV filling was assessed by recording mitral flow by standard pulsed Doppler technique in apical four-chamber view; the following parameters were considered: early diastolic peak flow velocity (E), late diastolic peak flow velocity (A), their ratio (E/A) and E wave deceleration time (measured from peak E-wave to baseline).
Tissue Doppler Measurements
Tissue Doppler imaging of the mitral annulus movement was obtained from the apical four-chamber view; a sample volume of 2.0 mm was sequentially placed at the lateral and septal annular sites. Filters and gains were adjusted to minimize background noise and maximize tissue signal. For the present study, analysis was performed for lateral and septal early (Ei) and late (Ai) diastolic peak velocities and their ratio (Ei/Ai); average lateral and septal annular velocity was used for statistical purposes. LV filling index was determined by the ratio of trans-mitral flow velocity to annular velocity (E/Ei); the index was considered altered for E/Ei ≥ 16 (Citation20).
Statistical analysis
Statistical analysis was performed by SAS System (version 6.12; SAS Institute Inc., Cary, NC, USA) and was mostly descriptive; values are expressed as means ± SD or as percentages. Mean values have been compared by Student's t-test for independent samples and categorical data analyzed by chi-square test or Fisher's exact test when appropriate. The strength of correlation of variables was tested by linear correlation analysis and multiple regression analysis. The candidate explanatory variables were selected on the basis of univariate correlations with the dependent variable. Variables significantly correlated (p < 0.05) with the dependent variable of interest in the univariate analysis were included in the multiple regression models. Models were checked in order to exclude multi-collinearity (cut point for tolerance was 0.10 or variance inflation factor > 10). A p-value < 0.05 was considered statistically significant.
Results
A total of 660 out of 701 hypertensive subjects (mean age 65 ± 13 years, 48% men) fulfilled all study criteria and constituted the study population. Briefly, 91% of the sample was taking antihypertensive drugs, 55% was elderly (≥ 65 years age), 36% obese and 10% had type 2 diabetes mellitus. As for echocardiographic data, 61% fulfilled the criteria for LVH, 18% for LA enlargement and 11% for altered LV filling index, according to non gender-specific thresholds indicated under Methods.
For the present analysis, the patients were divided in two groups according to the age < 65 or ≥ 65 years (i.e. younger vs older hypertensive subjects).
shows clinical and echocardiographic characteristics of the 298 younger hypertensives categorized according to LV geometric patterns. Normal LV geometry was the prevalent pattern (35%), followed by concentric LVH (26%), eccentric LVH (23%) and concentric LV remodeling (16%). Mean age was significantly lower in the normal LV geometry group than in the remaining ones. Clinic SBP and BMI tended to increase from normal geometry to concentric LVH; this was also the case for prevalence rates of male gender, obesity, type 2 diabetes mellitus, antihypertensive treatment. By definition, RWT was markedly greater in LV concentric remodeling and concentric LVH compared with normal geometry and eccentric LVH.
Table I. Clinical and echocardiographic characteristics in younger hypertensive patients categorized by left ventricular geometry.
A graded decline in E/A mitral ratio, Ei velocity and Ei/Ai ratio occurred across the groups, whereas an opposite trend was observed for deceleration time and E/Ei ratio. These differences were in most instances statistically significant.
As reported in , the distribution of LV patterns across the 362 older hypertensive elderly was markedly different from their younger counterparts, being normal LV geometric pattern the less prevalent (14%) and concentric LVH the most frequent one (45%). As for the younger group, BMI, clinic BP, male gender and obesity prevalence increased from normal LV geometry to concentric LVH. Of note, differences in diastolic function indexes across LV geometric patterns were less pronounced in older hypertensives, in whom deceleration time and Ei/Ai differences did not achieve statistical significance, in front of similar LV mass/height2.7 values as in younger subjects of the same group.
Table II. Demographic and clinical characteristics in elderly hypertensive categorized by left ventricular geometry.
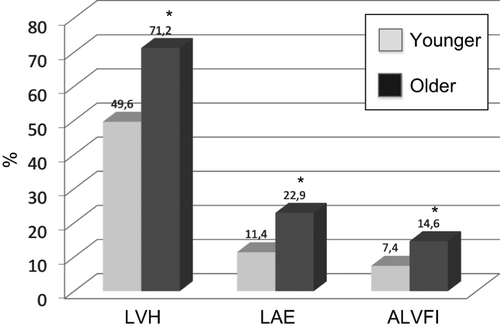
As depicted in , a significant difference in the prevalence of LVH (49.6% vs 71.2%, p < 0.01), LA enlargement (11.4% vs 22.9%, p < 0.01) and abnormal LV filling index (7.4% vs 14.6%, p < 0.01) was found between younger and older subjects.
Figure 1. Prevalence rates of left ventricular hypertrophy (LVH), left atrial enlargement (LAE) and abnormal left ventricular filling index (ALVFI) in younger and in older subjects. *p < 0.01.
When alterations in LAVI as well as LV filling index were separately analyzed in younger and older hypertensives across LV geometric patterns, the following findings were observed: (i) LA enlargement ranged from 4.2% (concentric remodeling) to 19.2% (eccentric LVH) in the former group and from 5.5% (concentric remodeling) to 27.4% (concentric LVH) in the latter group; (ii) patients with concentric LVH had the highest prevalence of abnormal LV filling index in both groups.
Correlation analyses: univariate correlations
In the whole population, LV mass was, in ranking order, positively associated with body size indexes, LAVI, clinic SBP, E/Ei ratio, deceleration time, age and inversely associated with ejection fraction and Eì. Similar findings were observed in younger hypertensives; in older ones LV mass did not correlate either with age or with LV diastolic parameters ().
Table III. Univariate correlation analysis between left ventricular mass and clinical/echocardiographic parameters in the study population as a whole and in the two age groups.
As shown in , RWT was positively correlated with age, E/Ei ratio, deceleration time, clinic SBP and BMI and inversely correlated with E/A ratio and Eì in the total study population. In both age subgroups, E/A and Ei were significantly correlated with RWT. This was not the case for deceleration time and E/Ei, which lost their statistical significance in older and younger hypertensives, respectively.
Table IV. Univariate correlation analysis between relative wall thickness and clinical/echocardiographic parameters in the study population as a whole and in the two age groups.
When significant variables were tested in multiple regression analyses, in the total study population BMI (β = 0.304), LAVI (β = 0.285), Ei (β = 0.480), SBP (β = 0.166) and mitral deceleration time (β = 0.088) turned out to be independently associated with LV mass (p < 0.05 at least for all). While similar results were found in younger hypertensives, in the older subgroup neither Ei velocity nor deceleration time correlated with LV mass.
Age (β = 0.135), Ei velocity (β = 0.107) or E/Ei (β = 0.092) and SBP (β = 0.79) were the only significant correlates of RWT in the whole sample. Again, the association between Ei and RWT persisted significant in younger but not in the older hypertensives.
Discussion
Alterations of diastolic properties in the hypertensive heart may be related to interplay of factors including increased BP itself, structural LV changes (i.e. increased collagen matrix, disorganization of collagen fibers, abnormal collagen type I/III ratio) and coronary microcirculation impairment (Citation6,Citation7,Citation21). The association between LV concentric geometry and diastolic dysfunction has been widely documented either in population-based studies as in hypertensive cohorts (Citation22,Citation23).
The present study offers a new piece of information on the relationship between LV geometry and diastolic function in a large cohort of subjects with preserved systolic function examined in the setting of echocardiographic practice; in particular, we analyzed the strength of this relationship according to an age-based analysis.
Our findings show that: (i) concentric LV geometry in hypertensives older than 65 years was 1.4-fold higher compared with their younger counterparts; this difference was paralleled by a greater prevalence of LA enlargement and altered LV filling, these alterations being 2.0- and 1.9-fold higher in the former group, respectively; (ii) at variance from younger hypertensives, in the older group neither LV mass nor RWT, a continuous index of LV geometry, was independently correlated to LV diastolic parameters.
Several aspects of our work deserve to be mentioned.
Prevalence of cardiac phenotypes, namely LVH, LA enlargement and abnormal LV filling index, may differ according to demographic and clinical characteristics of the study sample, as well as cut-off criteria defining this categorical parameters. A high prevalence of LVH, a cardinal feature of hypertensive heart disease, was found in our population, ranging from 50% in subjects younger than 65 years to 71% in older ones; these figures are consistently higher than those reported in population-based samples and in hypertensive cohorts attending specialist centers (Citation24). Our values, on the other hand, are in line with figures reported in a large Spanish survey carried out in primary care centers (Citation25) and in selected high-risk hypertensive populations with electrocardiographic LVH (Citation26) or resistant hypertension (Citation27). Several factors may explain the high prevalence of LVH in our survey, such as a mean age of 65 years, long duration of hypertension in the majority of participants, less than 10% of whom were untreated, large prevalence of obesity (i.e. 36%) and indexation of LV mass to height to allometric power of 2.7 rather than to body surface area. Although the most appropriate method for normalizing LV mass in adults is still debated, indexation to body surface area has been reported markedly to underestimate the presence of LVH in overweight and obese subjects (Citation28). Alterations in LA size and diastolic function occurred less frequently than LVH, as less than 20% of subjects were found to have LA enlargement and only 10% had an increased LV filling pressure, according to the criteria recommended by current guidelines. An increased LV filling pressure, however, could be suspected in as many as 45% subjects with E/Ei values at tissue Doppler analysis ranging from 9 to 15 of the intermediate (gray) range. Moreover, less conservative LAVI thresholds (≥ 24 ml/m2) (Citation29) than the 29 ml/m2 recommended by ASE guidelines (Citation9), would have substantially increased the prevalence of LA enlargement.
Several studies have consistently documented an increased prevalence of LV concentric geometry with aging either in apparently healthy individuals as in subjects with systemic arterial hypertension (Citation22). A number of clinical investigations indicated that the shift from normal to concentric geometry is a compensatory adaptation to age-related increases in peripheral and central BP and total arterial stiffness. Our data, in keeping with previous reports, show that: (i) LV concentric geometry (LV concentric remodeling and concentric LVH) was the prevalent geometric phenotype in the older group (60% vs 42% in the younger group); (ii) age was the most important independent correlate of RWT.
A direct relationship between LVH severity and diastolic impairment has been observed in different clinical settings such as hypertension (Citation30), hypertrophic cardiomyopathy (Citation31) and aortic stenosis (Citation32). Our results are in line with previous evidence by showing that a progressive worsening in LV diastolic function occurred from normal LV geometry to concentric LVH in the whole study population as well as in both age groups. To this issue, the present study adds the important notion that factors involved in LV diastolic impairment in hypertensive heart disease may differ according to age. In multivariate analyses, LV diastolic parameters in older hypertensives, at variance from their younger counterparts, did not correlate either with LV mass or RWT. This observation suggests that in older hypertensives the relationship between cardiac morphology and diastolic function is affected by a series of aging-associated factors other than the amount of LV cardiomyocytes, such as increased collagen content and impaired coronary microcirculation.
Limitations of the study
Our observations derive from a cohort of Caucasian hypertensive patients with preserved systolic function, in sinus rhythm and without significant valve diseases, and should not be extended to other ethnic groups or different clinical conditions. Furthermore, we had no access to laboratory data such as metabolic and renal parameters, which correlate with diastolic dysfunction. Finally, as our investigation was based on standard echocardiographic methods, circulatory and ultrasonographic markers of myocardial fibrosis as well as parameters of coronary microcirculation were not available.
Conclusions
As clinical perspective, our findings indicate that in elderly hypertensives diastolic dysfunction may occur independently from LV structural and geometric alterations and represent a further argument against the clinical indication of limited echocardiography selectively aimed to measure morphological parameters such as LV mass and RWT in the hypertensive setting (Citation33).
Conflict of interest
None. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, . The heart in hypertension. N Engl J Med. 1992;327:998–;1008.
- Ruilope LM, Schmieder R. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens. 2008;21:500–506.
- Cuspidi C, Meani S, Valerio C, Sala C, Fusi V, Zanchetti A, Mancia G. Age and target organ damage in essential hypertension: Role of the metabolic syndrome. Am J Hypertens. 2007;20:296–303.
- Zanchetti A, Cuspidi C, Comarella L, Agabiti-Rosei E, Ambrosioni E, Chiarello M, . Left ventricular diastolic dysfunction in elderly hypertensives: Results of the APROS-diadys study. J Hypertens. 2007;25:2158–2167.
- Diez J, Frohlich E. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1–8.
- Frohlich E, Gonzales A, Diez J. Hypertensive left ventricular hypertrophy risk: Beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011;29:17–26.
- Weber KT. Cardioreparation in hypertensive heart disease. Hypertension. 2001;38[part 2]:588–591.
- Gonzalez A, Lopez B, Diez J. Fibrosis in hypertensive heart disease role of the angiotensin–aldosterone system. Med Clin N Am. 2004;88:83–97.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- Andren B, Lind L, Hedenstierna G, Lithell H. Left ventricular hypertrophy and geometry in a population sample of elderly males. Eur Heart J. 1996;17:1800–1807.
- Muller-Brunotte R, Kahan T, K Malmqvist, Edner M. Blood pressure and left ventricular geometric pattern determine diastolic function in hypertensive myocardial hypertrophy. J Hum Hypertens. 2003;17:841–849.
- Fox ER, Taylor J, Taylor H, Han H, Sundarshi T, Arnett D, Myerson M. Left ventricular geometric patterns in the Jackson cohort of the atherosclerotic risk in communities (ARIC) study: Clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238–244.
- Nardi E, Palermo A, Mulè G, Cusimano P, Cottone S, Cerasola G. Impact of type 2 diabetes on left ventricular geometry and diastolic function in hypertensive patients with chronic kidney disease. J Hum Hypertens. 2011;25:144–151.
- Dhuper S, Abdullah RA, Weichbrod L, Mahadi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity. 2011;19:128–133.
- Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884.
- Lavie CJ, Milani RV, Ventura HO, Messerli FH. Left ventricular geometry and mortality in patients > 70 years of age with normal ejection fraction. Am J Cardiol. 2006;98:1396–1399.
- 2007 Guidelines for the Management of Arterial Hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007:25:1105–1187.
- Devereux RB, Reickek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Verdecchia P, Angeli F, Gattobigio R, Sardone M, Porcellati C. Asymptomatic left ventricular systolic dysfunction in essential hypertension: Prevalence, determinants, and prognostic value. Hypertension. 2005;45:412–418.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Smiseth OA, Waggoner AD, . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133.
- Galderisi M. Diagnosis and management of left ventricular diastolic dysfunction in the hypertensive patient. Am J Hypertens. 2011;24:507–517.
- Markus MRP, Stritzke J, Lieb W, Mayer B, Luchner A, Doring A, . Implications of persistent prehypertension for ageing-related changes in left ventricular geometry and function: The MONICA/KORA Augsburg study. J Hypertens. 2008;28:2040–2049.
- Li L, Shigematsu L, Hamada M, Hiwada K. Relative wall thickness is an independent predictor of left ventricular systolic and diastolic dysfunctions in essential hypertension. Hypertens Res. 2001;24:493–499.
- Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left ventricular hypertrophy in hypertension: An updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343–349.
- Coca A, Gabriel R, de la Figuera M, Lopez-Sendon JL, Fernandez R, Sagastagoitia JD, . On behalf the VITAE Investigators. The impact of different echocardiographic diagnostic criteria on the prevalence of left ventricular hypertrophy in essential hypertension. J Hypertens. 1999;17:1471–1480.
- Wacthell K, Bella JN, Liebson PR, Gerdts E, Dahlof B, Aalto T, . Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population. The LIFE Study. Hypertension. 2000;35:6–12.
- Castelpoggi CH, Pereira VS, Fiszman R, Cardoso CRL, Muxfeldt ES, Salles GF. A blunted decrease in nocturnal blood pressure is independently associated with increased aortic stiffness in patients with resistant hypertension. Hypertens Res. 2009;32:591–56.
- de Simone G, Wacthell K, Palmieri V, Hille DA, Beevers G, Dahlof B, . Body build and risk of cardiovascular events in hypertension and left ventricular hypertrophy. Circulation. 2005;111:1924–1931.
- Leung DY, Chi C, Allman C, Boyd D, Ng AC, Kadappu KK, . Prognostic implications of left atrial volume index in patients with sinus rhythm. Am J Cardiol. 2010;105:1635–1639.
- Chahal NS, Kim TK, Jain P, Chambers JC, Kooner JS, Senior R. New insights into the relationship of left ventricular geometry and left ventricular mass with cardiac function: A population study of hypertensive subjects. Eur Heart J. 2010;31:588–594.
- Carasso S, Yang H, Woo A, Jamorski M, Wigle ED, Rakowski H. Diastolic myocardial mechanics in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2010;23:164–171.
- Stewart RA, Kerr AJ, Whalley GA, Legget ME, Zeng I, Williams MJ, . Left ventricular systolic and diastolic function assessed by tissue Doppler imaging and outcome in asymptomatic aortic stenosis. Eur Heart J. 2010;31: 2216–2222.
- Leese PJ, Viera AJ, Hinderliter AL, Steams SC. Cost- effectiveness of electrocardiography vs electrocardiography plus limited echocardiography to diagnose LVH in young, newly identified, hypertensives. Am J Hypertens. 2010;23:592–598.
