Abstract
Recent studies suggest that decreased estimated glomerular filtration rate (eGFR) and uric acid (UA) may be independent risk factors for arterial stiffness (AS). As serum UA level is linked to renal function, we hypothesize that decreased eGFR may be not an independent risk factor of AS, but may be related to UA level. In this study, we aimed to validate this hypothesis in a large community-based Chinese population. A total of 13,899 people were selected from the Cardiovascular Risk Survey (CRS) from October 2007 to March 2010. Pulse wave velocity (PWV) was calculated using the established methods. The relationships between eGFR, fasting blood glucose (FBG), UA and PWV were analyzed with multivariate linear regression. We found that PWV was significantly correlated to FBG (r = 0.173, p < 0.001) and UA (r = 0.177, p < 0.001), and inversely correlated to eGFR (r = − 0.161, p < 0.001). A multivariable regression analysis revealed that FBG (β = 0.056, p < 0.001) and UA (β = 0.039, p < 0.001), but not eGFR (β = − 0.011, p = 0.062) were significantly related to elevation of PWV. In women, eGFR was not an independent risk factor of AS with progressively decreasing renal function (all p > 0.05). However, in men, eGFR was associated with PWV in subjects with eGFR < 60 ml/min/1.73 m2. Our results suggest that decreased eGFR is not independently associated with AS in Chinese women.
Introduction
There is a high prevalence of coronary artery disease (CAD) in chronic kidney disease (CKD) patients, which is associated with a decrease in the estimated glomerular filtration rate (eGFR) (Citation1). Several studies have revealed that arterial stiffness (AS) is both a predictor of cardiovascular disease (Citation2–4) and a correlate of reduced glomerular filtration rate (GFR) (Citation5–7). The pulse wave velocity (PWV), one of the most studied parameters of AS, is a strong, independent predictor of cardiovascular events and mortality (Citation8), and is one of the commonly used methods for measuring AS (Citation9,Citation10). It is a useful method in large clinical studies (Citation4,Citation11–13) because it provides a simple measurement that can be performed in primary care settings, without expensive or elaborate equipment, extensive training or experience.
Some studies indicate that AS measured by PWV is associated with renal dysfunction not only in CKD patients (Citation14), but also in hypertensive patients (Citation15,Citation16), even within the normal range of eGFR (Citation17). Accumulated evidence also suggests that serum uric acid (UA) level is associated with PWV and that hyperuricemia is an independent risk factor of AS (Citation18–20). Therefore, we hypothesize that decreased eGFR may be not an independent risk factor of AS, but that its effect on PWV may be related to the increased serum UA level. In this study, we aimed to validate this hypothesis in a large community-based Chinese population.
Methods
Subjects
The Cardiovascular Risk Survey (CRS) study is a multi-ethnic, community-based, cross-sectional study designed to investigate the prevalence and risk factors for cardiovascular diseases and determine the genetic and environmental contributions to atherosclerosis, CAD and cerebral infarction (CI) of Chinese Han, Uygur and Kazakh population in Xinjiang of western China from October 2007 to March 2010. We used a stratified sampling method to select a representative sample of the general population of Chinese Hans, Uygurs and Kazakhs of this area. Seven cities (Urumqi, Kelamayi, Hetian, Zhaosu, Fukang, Tulufan and Fuhai) were chosen and, based on the government record of registered residence, one participant was randomly selected from each household. In this way, a total of 14,618 participants (5757 Hans, 4767 Uygurs and 4094 Kazakhs), were randomly selected from 26 villages of these seven cities and were invited to participate. Those whose data were incomplete were excluded (182 Hans, 332 Uygurs and 205 Kazakhs). Finally, 13,899 individuals (95.08%) were analyzed in the present study.
The present study was conducted in accordance with the Declaration of Helsinki guidelines, and informed consent was obtained from each individual according to a protocol approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University.
PWV measurement
Bilateral brachial-ankle PWV (baPWV) was measured in all subjects using the form ABI/PWV (VP1000; Colin Co., Ltd., Komaki, Japan), which is a device with four cuffs that can simultaneously measure blood pressure levels in both arms and legs, and automatically calculate the ankle brachial pressure index (ABI). In addition, it can also record pulse waves through sensors in the cuffs, store data from the start point of each pulse wave in the right arm and both the legs in memory, record the time difference between transmission time to arm and transmission time to ankle as “transmission time”, calculate the transmission distance from the right arm to each ankle according to body height, and automatically compute and output the baPWV values by transmission time and transmission distance. As there was a significant positive correlation between left and right baPWV, we used the mean right/left baPWV value during our analysis (Citation21,Citation22).
Covariates
We collected information on each subject’s medical history and lifestyle characteristics using standardized questionnaires. Systemic arterial hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or a diastolic blood pressure (DBP) ≥ 90 mmHg (Citation23), on at least two separate occasions, or anti- hypertensive treatment. Hypercholesterolemia was defined as a documented total cholesterol (TC) of ≥ 240 mg/dl (≥ 6.2 mmol/l) or current treatment with cholesterol-lowering medication. Diabetes mellitus was defined as the presence of an active treatment with insulin or an oral antidiabetic agent; for patients on dietary treatment, documentation of an abnormal FBG or glucose tolerance test based on the World Health Organization criteria (Citation24) was required to establish this diagnosis. Smoking status classifications were current smokers, former smokers and never-smokers. All the participants underwent a standardized physical examination performed by experienced research staff. Anthropometric measurements were conducted in light clothing and without shoes. Height was measured to the nearest 0.1 cm and weight was measured with a standard scale in the upright position to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured to the nearest 0.1 cm at the midpoint between the lower border of the rib cage and the upper hip bone (iliac crest) during expiration.
Biochemical analysis
Serum was separated from the samples within 30 min and stored at − 80°C until analysis. We measured the serum concentration of UA, creatinine, triglycerides (TG), TC, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting glucose using equipment for chemical analysis (Dimension AR/AVL Clinical Chemistry System, Newark, NJ, USA) employed by the Clinical Laboratory Department of the First Affiliated Hospital of Xinjiang Medical University. Glomerular filtration rate was estimated by using the following equation: eGFR = 186 × serum creatinine − 1.154 × age − 0.203 × 0.742 (if female) (Citation25).
Statistical analysis
Data analysis was performed using the computer software Statistical Package for Social Sciences-SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL, USA). Demographic and clinical characteristics of the study population were expressed as the mean ± standard deviation or as a ratio based on the renal function categories. The bivariate associations between variables were examined using Pearson's correlation coefficients. All parametric data were analyzed using Student's t-test, analysis of variance (ANOVA) and analysis of covariance (ANCOVA) wherever appropriate. Multivariable linear regression was used to assess whether eGFR and UA were independent predictors of AS. Within each multivariable regression analysis, five models were constructed. In Model 1, the variables included AS risk factors, such as age, SBP, BMI, TC and TG. To assess whether FBG was a significant predictor, in Model 2, we added it to the variables used in Model 1. In Model 3, we added UA to the variables employed in Model 1 to assess whether it was an independent predictor of AS. To assess whether eGFR was an independent predictor of AS, in Model 4, we added it to the variables used in Model 1. Finally, in Model 5, we added FBG, UA and eGFR to the variables employed in Model 1 to assess whether these three factors were independent of each other and other risk factors. We also investigated the interactions between eGFR and age, SBP, BMI, FBG, and UA in the statistical models. Statistical significance was determined at p < 0.05.
Results
The mean age of the participants was 50.84 years, and 46.3% of the participants were men and 29.6% were smokers (). As shown in , PWV was statistically significant and directly correlated to age, SBP, BMI, fasting blood glucose (FBG) and UA, and was inversely correlated to eGFR.
Table I. Participant characteristics (n = 13,899).
Table II. Correlation coefficients among pulse wave velocity (PWV), age, body mass index (BMI), systolic blood pressure (SBP), estimated glomerular filtration rate (eGFR) and fasting blood glucose (FBG).
FBG level in participants with AS (PWV > 1400 cm/s) was significantly higher than that in participants without AS (PWV ≤ 1400 cm/s) [(5.34 ± 1.89 mmol/l) vs (4.92 ± 1.36 mmol/l); p < 0.001; ], and was significantly related to PWV (p < 0.001; ). The UA level in participants with AS was significantly higher than that in participants without AS [(286.64 ± 86.98 mmol/l) vs (260.76 ± 80.80 mmol/l); p < 0.001; ], and was significantly related to PWV (p < 0.001; ). Furthermore, the eGFR level in participants with AS was significantly lower than that in participants without AS [(104.06 ± 34.14 ml/min/1.73 m2) vs (96.37 ± 31.42 ml/min/1.73 m2); p < 0.001; ]; and was significantly related to PWV (p < 0.001; ).
Figure 1. Relationship between fasting blood glucose (FBG), uric acid (UA) and estimated glomerular filtration rate (eGFR) and the presence of arterial stiffness (AS) and pulse wave velocity (PWV) values. (A) Box plots showing the distribution of UA in participants with and without AS (PWV > 1400 cm/s); (B) scatter plot showing the correlation between UA and PWV; (C) box plots showing the distribution of FBG in participants with and without AS (PWV > 1400 cm/s); (D) scatter plot showing the correlation between FBG and PWV; (E) box plots showing the distribution of eGFR in participants with and without AS (PWV > 1400 cm/s); (F) scatter plot showing the correlation between eGFR and PWV.
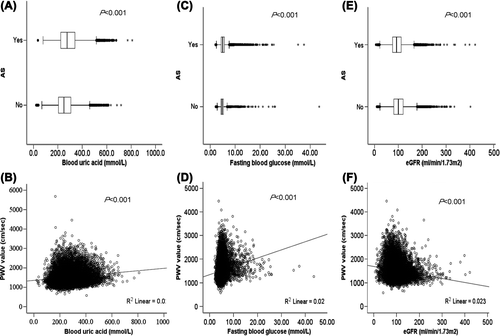
In a multivariable linear regression model, variables significantly associated with PWV included age, BMI, SBP and TG (Model 1, ). After adjustment for these risk factors in Model 1, FBG, UA and eGFR were independently related to PWV (Models 2, 3 and 4, respectively; ). Nevertheless, after adjustment for UA and FBG as well as Model 1, the independent effect of eGFR disappeared (Model 5, , p = 0.062), but the effect of TC was significant (Model 5, , p = 0.012), suggesting that eGFR is not independently related to PWV. In a gender-specific model, we divided these subjects into three groups according to the eGFR categories. As shown in , in women, the eGFR was not independently related to PWV in each category of renal function (all p > 0.05); however, in men, the eGFR was associated with PWV only in subjects with eGFR < 60 ml/min/1.73 m2.
Table III. Association of uric acid (UA), fasting blood glucose (FBG) and estimated glomerular filtration rate (eGFR) with pulse wave velocity (PWV): multivariable linear regression models.
Table IV. Association of estimated glomerular filtration rate (eGFR) with pulse wave velocity (PWV) in subgroups: multivariable linear regression models.
Discussion
In this study, we investigated the relationships between UA, FBG, eGFR and PWV in a large sample of Chinese population. Our main results suggest that elevated UA and FBG levels, but not decreased eGFR level, are independently associated with AS in Chinese women.
Several studies have suggested that AS measured by PWV is associated with renal dysfunction (Citation14–17). However, our results are not in agreement with those findings. Through simple analysis, we observed a significant association of eGFR with PWV, and in a multivariable linear regression model including age, SBP, BMI and TC, this association remained significant; however, in a multivariable linear regression model including UA, besides FBG, age, SBP, BMI and TC, this relationship disappeared. These results suggest that eGFR is not independent of serum UA levels. Both Miyatake et al. (Citation26) and Kawamoto et al. (Citation21), who did not consider serum UA level in the mutivariable model, observed a positive association of eGFR with AS.
UA is the end product of purine metabolism in humans. Sources of purine are either endogenous or exogenous, with the former from de novo synthesis and nucleic acid breakdown, and the latter from dietary purine intake. In the steady state, this daily production and ingestion of approximately 700 mg of UA is balanced by daily elimination of an equal amount of UA from the body, of which approximately 70% (roughly 500 mg daily) needs to be excreted by the kidneys (Citation20). Therefore, elevated UA level is not only associated with diet, but also related to renal function. Recently, two large prospective population studies from Japan examined the relationship between serum UA level and development of kidney disease. Tomita et al. (Citation27) observed a strong association between serum urate level and renal failure, even when adjusted for covariate effects. Similarly, Iseki et al. (Citation28) found that hyperuricemia was associated with a greater risk of end-stage renal disease even after adjustment for comorbidities. Nevertheless, not only serum UA, but also decreased renal function was considered an independent risk factor for cardiovascular event, including CAD, AS and stroke.
Schillaci et al. (Citation17) demonstrated that a mildly reduced kidney function was responsible for increased AS. Interestingly, in a large longitudinal study of patients with essential hypertension, Benetos et al. (Citation29) found that serum creatinine level was a major determinant of accelerated progression of AS. Tsai et al. (Citation18) suggested that UA was an independent predictor of AS in hypertensive patients. Nevertheless, these studies did not consider UA and eGFR simultaneously in the multivariable analysis. In the present study, although an inverse relationship was found between eGFR and AS through simple analysis, in the multivariable regression analysis, the independent effect of eGFR was not observed in the total population.
Although several studies have indicated that eGFR is associated with PWV, the mechanisms linking PWV to renal function are largely unknown. Several studies have indicated that increased AS may be a cause or an effect of reduced renal function, and may result in an increase in SBP leading to high pulse pressure. The high pressure waves generated may cause injury to the renal vascular system, and thus result in lowering of GFR (Citation30,Citation31). In addition, impaired renal function may lead to stiffening of the large arteries (Citation32,Citation33) through several mechanisms, including accumulation of advanced glycosylation end products, increased collagen cross-linking and activation of the renin–angiotensin system (Citation34). Nevertheless, the association between UA and renal function may be an important factor contributing to AS, which was not considered in several previous studies. As elevated UA level not only results from increased intake, but also decreased renal function, serum UA level may be a better predictor of AS than eGFR.
There are several advantages with respect to the inclusion of a large community-based cohort of individuals from the community. We used uniform protocols in groups, including questionnaires, anthropometric measurements, assessment of conventional risk factors and the PWV measure. A potential weakness of this study is the cross-sectional study design, which cannot establish causality relations.
Conclusions
Our results suggest that elevated serum UA and FBG are independent risk factors of AS, and that decreased eGFR is not independently associated with AS in Chinese women.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work has been supported financially by grants from the Natural Science Foundation of Xinjiang (2011211D32).
References
- Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28:354–360.
- Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–1860.
- Laurent S, Boutouyrie P, Asmar R, Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Laurent S, Katsahian S, Fassot C, Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206.
- Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494–501.
- Lacy P, Carr SJ, O’Brien D, Reduced glomerular filtration rate in pre-dialysis non-diabetic chronic kidney disease patients is associated with impaired baroreceptor sensitivity and reduced vascular compliance. Clin Sci (Lond). 2006;110:101–108.
- Briet M, Bozec E, Laurent S, Arterial stiffness and enlargement in mild to moderate chronic kidney disease. Kidney Int. 2006;69:350–357.
- Khoshdel AR, Carney SL, Balakrishnan RN, Gillies A. Better management of cardiovascular diseases by Pulse Wave Velocity: combining clinical practice with clinical research using evidence-based medicine, Clin Med Res. 2007;5:45–52.
- Pannier BM, Avolio AP, Hoeks A, Mancia G, Takazawa K. Methods and devices for measuring arterial compliance in humans. Am J Hypertens. 2002;15:743–753.
- O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444.
- Boutouyrie P, Tropeano AI, Asmar R, Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15.
- Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090.
- Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050.
- Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494–501.
- Mourad JJ, Pannier B, Blacher J, Creatinine clearance, pulse wave velocity, carotid compliance and essential hypertension. Kidney Int. 2001;59:1834–41.
- Kawamoto R, Kohara K, Tabara Y, Miki T, Ohtsuka N, Kusunoki T, An association between decreased estimated glomerular filtration rate and arterial stiffness. Internal Med. 2008;47:593–598.
- Schillaci G, Pirro M, Mannarino MR, Relation between renal function within the normal range and central and peripheral arterial stiffness in hypertension. Hypertension. 2006;48:616–621.
- Tsai WC, Huang YY, Lin CC, Uric acid is an independent predictor of arterial stiffness in hypertensive patients. Heart Vessels. 2009;24:371–375.
- Saijo Y, Utsugi M, Yoshioka E, Relationships of C- reactive protein, uric acid, and glomerular filtration rate to arterial stiffness in Japanese subjects. J Hum Hypertens. 2005;19:907–913.
- Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Clev Clin J Med. 2008, 75(suppl 5):S13–S16.
- Kawamoto R, Kohara K, Tabara Y, An association between decreased estimated glomerular filtration rate and arterial stiffness. Internal Med. 2008;47:593–598.
- Ohnishi H, Isobe T, Saitoh S, Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose. Diabetes care. 2003;26:437–440.
- Guidelines Subcommittee. World Health Organization- International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens. 1999;17:151–183.
- World Health Organization Study Group. Diabetes mellitus. WHO Tech Rep Ser. 1985;727:1–104.
- Massimo C. Evaluation of glomerular filtration rate and of albuminuria/proteinuria. J Nephrol. 2010;23:125–132.
- Miyatake N, Shikata K, Makino H, Numata T. Relation between the Estimated Glomerular Filtration Rate and Pulse Wave Velocity in Japanese. Inter Med. 2010;49:1315–1320.
- Tomita M, Mizuno S, Yamanaka H, Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403–409.
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650.
- Benetos A, Adamopoulos C, Bureau JM, Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207.
- Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res. 2002;90:1316–1324.
- Pedrinelli R, Dell’Omo G, Penno G, Microalbuminuria and pulse pressure in hypertensive and atherosclerotic men. Hypertension. 2000;35:48–54.
- Briet M, Bozec E, Laurent S, Arterial stiffness and enlargement in mild to-moderate chronic kidney disease. Kidney Int. 2006;69:350–357.
- London GM, Blacher J, Pannier B, Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–8.
- Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97.
