Abstract
Prehypertension is characterized by an increased cardiovascular risk and by an increased prevalence of target organ damage compared with the pure normotensive state. The present study was designed to assess in prehypertensive subjects the possible relationships between early left ventricular dysfunction, vascular inflammation and aortic stiffness. The study population consisted of 31 untreated prehypertensive subjects (age: 34 ± 6 years, mean ± SD) and 31 age-matched pure normotensive controls. Left ventricular function was assessed by echocardiography, aortic distensibility parameters were derived from aortic diameters measured by ultrasonography, and high-sensitivity C-reactive protein was assessed by latex-enhanced reagent. Prehypertensive subjects displayed a significantly lower E/A ratio and a significantly greater deceleration time and isovolumetric relaxation time compared with normotensive controls. They also displayed aortic systolic diameter, diastolic diameter and mean aortic stiffness index beta significantly increased while systo-diastolic diameter change, mean aortic distensibility and aortic strain were significantly reduced compared with controls. Values of inflammatory markers were increased. At multiple regression analysis, E/A ratio was significantly related to high-sensitivity C-reactive protein and aortic stiffness index beta, after correction for age, left ventricular mass index and mean blood pressure (β coefficient = −0.49, overall r2 = 0.24, p = 0.01 and β coefficient =−0.46, overall r2 = 0.21, p = 0.02, respectively). Thus, in prehypertension, left ventricular dysfunction is significantly related to vascular inflammation and aortic stiffness, suggesting that early cardiac and vascular alterations may have an increased inflammatory process as a common pathophysiological link.
Introduction
Some years ago, the American Joint National Committee on Hypertension (JNC VII) provided a new category in the definition and classification of the hypertensive states (Citation1). The Committee indeed unified the blood pressure categories previously defined as “normal” and “high-normal” into a single clinical entity defined as “prehypertension”, characterized by systolic blood pressure values between 120 and 139 mmHg and/or diastolic blood pressure between 80 and 89 mmHg. There are several reasons for this decision. First, the evidence from the Framingham Heart Study that in such subjects the risk of developing throughout the incoming months and/or years a “true” hypertensive state is higher than that characterizing the true normotensive state (Citation2). Second, the observation that this condition is frequently associated with subclinical organ damage, such as an increased arterial stiffness and intima-media thickness, an impaired left ventricular diastolic function, an augmented left ventricular mass and may display an enhanced vascular inflammatory process (Citation3–13). Finally, the finding that the prehypertensive state carries an increased risk of vascular events compared with the true normotensive state (Citation14,Citation15), presumably because of the above-mentioned alterations and, more in general, because of the complex pathophysiological background of this condition.
Despite the large number of data collected in the past few years on the structural and functional cardiovascular abnormalities characterizing prehypertension, no information is available on whether and to what extent the early alterations in left ventricular diastolic function seen in prehypertension relate to vascular inflammation and aortic stiffness impairment. The present study, which is part of a joint research project between Milan an Ankara academic centers, was aimed at addressing this issue by evaluating subjects with a documented prehypertensive state but without any concomitant cardiovascular risk factor.
Methods
Study population
The study population included 31 newly diagnosed prehypertensive patients (20 males), aged 34 ± 6 years (mean ± SD) and 31 healthy controls matched for age, sex and body mass index. Patients were recruited from those followed by the outpatient clinic of the Department of Cardiology, Gulhane Military Medical Academy, Ankara, Turkey. Controls were recruited from the individuals who were visited by the same institution for periodic routine examinations. To be enrolled into the study, patients were required to have prehypertension, according to the Seventh Report of the Joint National Committee (JNC 7) criteria (Citation1) and not to be under antihypertensive drugs and/or non-pharmacological interventions. The study was carried out in agreement with the declaration of Helsinki, local ethics committee approval and patients’ written informed consent. Eligible patients underwent standard clinical examination, electrocardiogram (ECG), transthoracic echocardiography, chest radiography and routine laboratory examinations.
Patients with mild-to-moderate hypertension (blood pressure values ≥140/90 mmHg at three different visits spaced each other by an interval of 2–3 weeks), according to the European Society of Hypertension and European Society of Cardiology criteria (Citation16) or secondary hypertension, history of acute infection within the past 30 days, presence of any chronic inflammatory-autoimmune disease, connective tissue disorders, known malignancy, bradycardia (heart rate <60 beats/min), atrio-ventricular block, left bundle branch block, atrial fibrillation or other cardiac arrhythmias, valvular heart disease, coronary artery disease, aortic disease (Marfan's syndrome, coarctation of aorta, aortic aneursym or aortic surgery), myocardial infarction or cerebrovascular accident within 6 months, left ventricular hypertrophy (> 110–125 g/m2 in men and women, respectively), were not eligible. Additional exclusion criteria were acute or chronic renal dysfunction, hepatic dysfunction, asthma or chronic obstructive lung disease, impaired glucose tolerance, diabetes mellitus (fasting plasma glucose >126 mg/dl), metabolic syndrome, obesity (body mass index >30 kg/m2), dyslipidemia, treatment with medications that could affect blood pressure, inappropriate echocardiographic window, pregnancy or nursing women.
Measurements
Blood pressure. Blood pressure was measured by the same investigator three times on the right arm in sitting position, following 10 min resting, using a standard mercury sphygmomanometer and the average of the three measurements was used for the analysis. Phase I and V Korotkoff sounds were employed to assess systolic and diastolic blood pressure, respectively. The diagnosis of prehypertension was based on systolic blood pressure values between 120 and 139 mmHg and/or diastolic blood pressure between 80 and 89 mmHg at three different visits spaced each other by an interval of 2–3 weeks.
Echocardiography. M-mode, two-dimensional color-Doppler echocardiography was performed by using ESAOTE 2.5-MHz probe (ESAOTE, Genova, Italy) at the left lateral decubitus position. Echocardiographic measurements were performed by two cardiologists unaware of the patients’ clinical data. Measurements of the left atrium and the left and right ventricles were obtained from the parasternal long-axis view at a speed of 50 mm/s as recommended by the American Society and the European Association of Echocardiography guidelines (Citation17,Citation18). Five consecutive cardiac cycles were averaged for every echocardiographic measurement. Left ventricular systolic and diastolic diameters (LVIDs, LVIDd), and left atrial systolic diameter (LAd) were calculated. Left ventricular ejection fraction (LVEF) was measured by the software using the Teichholz formulae (Citation17). Left ventricular mass (LVM) was calculated according to Penn convention method and indexed to body surface area (Citation17). Transmitral flow velocity pattern was evaluated from the apical four-chamber view with pulsed-wave Doppler placing the sample volume at the tips of mitral leaflets during diastole. Early (E) and late (A) mitral flow peak velocities, the E/A ratio, deceleration time (DT) and isovolumic relaxation time (IVRT) were used to assess the left ventricular diastolic function (Citation18).
Aortic elasticity. Aortic systolic and diastolic diameters (Aos and Aod) were measured by using M-mode aortic tracing obtained 3 cm above the aortic valve. Aos was determined at the time of the full opening of the aortic valve and Aod was determined at the peak of QRS. All parameters were measured in five consecutive cardiac cycles and averaged. Simultaneously, cuff brachial artery systolic (SBP) and diastolic (DBP) pressures were measured with an aneroid sphygmomanometer. Aortic elasticity was assessed using the following indexes (Citation19):
Aortic strain (%) = 100 × (Aos − Aod)/Aod
Aortic distensibility index (cm−2 dyn−1 10−6) = 2 × aortic strain × (SBP − DBP)
Aortic stiffness index beta = (SBP/DBP)/ Aortic strain
The coefficients of variation for aortic systolic diameter, diastolic diameter, systolic and diastolic blood pressures, all of which are the main components of aortic elasticity calculation, were 4.9%, 6.6%, 3.5% and 7.7%, respectively. Intra- and inter-observer variabilities were found to be 2.8% and 4.2 % for aortic systolic diameter; 2.6% and 4.7% for aortic diastolic diameter; 2.2% and 3.2% for systolic blood pressure; 2.5% and 2.7% for diastolic blood pressure, respectively. These data are in line with those obtained via the gold standard approach to assess arterial elasticity, i.e. the sphygmocor technique (Citation20).
Blood chemistry. Venous blood samples were collected in tubes containing K3 EDTA and were spun at 5000 rpm for 15 min. Plasma and serum samples were stored at −80°C until assays were made. Total plasma cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, uric acid and glucose concentrations were measured with a spectrophotometric method (Olympus AU-2700 autoanalyzer, Hamburg, Germany). Low-density lipoprotein (LDL) cholesterol levels were calculated by Friedwald formula. Serum insulin levels were quantified by the Modular Analytics E 170 System (Roche Diagnostics, Indianapolis, IN, USA). Insulin resistance was assessed using the homeostatic model with the formula: [HOMA-IR] = [Fasting plasma glucose (mg/dl) × immunoreactive insulin (IRI) (IU/ml)]/405 (Citation21). High-sensitivity C-reactive protein measurements were performed with latex-enhanced reagent (Dade Behring Deerfield, IL, USA) using a Behring BN ProSpec analyzer (Dade Behring). Whole blood cell count was measured by Coulter LH 780 Hematology Analyzer (Beckman Coulter, Inc. Fullerton, CA, USA).
Statistical analysis
Statistical analysis was performed by using the SPSS 15.0 Statistical Package Program for Windows (SPSS Inc., Chicago, IL, USA). We used one-sample Kolmogorov–Smirnov and Levene tests to determine the distribution characteristics of variables and variance homogeneity. Results are expressed as the mean ± SD, median and percentages. The differences between groups were tested by chi-square, independent samples t-test and Mann–Whitney U tests. The relationship between variables was analyzed with Spearman correlation test. Intra- and inter-observer variabilities were calculated as a relative error. Linear regression analysis was performed to evaluate the association between left ventricular diastolic functions, markers of vascular inflammation and aortic elasticity parameters. Next, linear regression analysis was done to evaluate the association between left ventricular diastolic parameters and other variables. Then, multiple linear regression analysis was done to clarify the contributions of aortic elasticity and high-sensitivity C-reactive protein adjusting for age, left ventricular mass index (LVMI) and mean blood pressure. Differences were considered statistically significant at a p-value <0.05.
Results
Baseline characteristics of the study population are shown in . Individuals with pre-hypertension displayed significantly greater systo-diastolic blood pressure, pulse pressure, white blood cell count and high-sensitivity C-reactive protein than controls. The echocardiographic parameters () show that prehypertensive and normal participants displayed similar values of left ventricular end-diastolic and end-systolic diameters, ejection fraction, left atrial diameters, LVMI, diastolic and systolic posterior wall thickness and interventricular septum thickness.
Table I. Baseline anagraphic, anthropometric, hemodynamic and biochemical parameters in the study population.
. Echocardiographic findings in the population of the study.
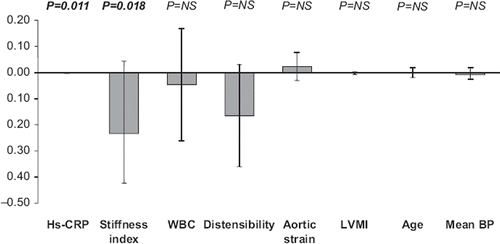
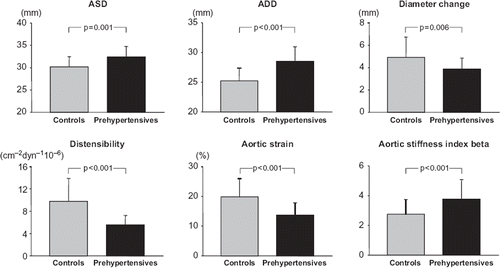
Subjects with prehypertension had a significantly lower E/A ratio and a significantly greater deceleration time and isovolumetric relaxation time compared with normotensive controls (). In prehypertensive subjects, aortic systolic diameter, diastolic diameter and mean aortic stiffness index beta were significantly increased while systo- diastolic diameter change, mean aortic distensibility and aortic strain were significantly reduced compared with controls (). At multiple regression analysis () in the prehypertensive individuals, the E/A ratio was significantly related to high-sensitivity C-reactive protein and aortic stiffness index beta, after correction for age, LVMI, and mean blood pressure (β coefficient = −0.49, overall r2 = 0.24, p = 0.01 and β coefficient = −0.46, overall r2 = 0.21, p = 0.02, respectively). No correlation was found, on the other hand, between E/A and LVMI, aortic distensibility, aortic strain, age and mean blood pressure ().
Figure 1. Mean (± standard deviation) values of E/A ratio, deceleration time and isovolumetric relaxation time (IVRT) in healthy controls and in subjects with prehypertension. Statistical significance between data obtained in the two groups is shown.
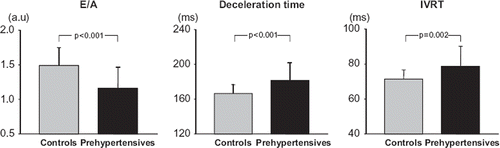
Figure 2. Mean (± standard deviation) values of aortic systolic (ASD) and diastolic (ADD) diameter, diameter change, aortic distensibility, strain and stiffness index beta in healthy controls and in subjects with prehypertension. Statistical significance between data obtained in the two groups is shown.
Discussion
The results of the present study show an alteration of left ventricular diastolic function in subjects with prehypertension compared with healthy true normotensive controls. They also show a significant increase in the value of high-sensitivity C-reactive protein and white blood cells, as markers of inflammation and an impairment in aortic elasticity, documented by a decrease in aortic distensibility and an increase in aortic stiffness index. However, the main and new study finding concerns the evidence of a significant relationship between E/A ratio and both high-sensitivity C-reactive protein and vascular stiffness in the prehypertensive subjects. The multiple regression analysis with E/A ratio as a dependent variable shows a statistical significant correlation between the E/A ratio, high-sensitivity C-reactive protein (beta: −0.002) and aorta stiffness index (beta: −0.233). The association remained significant after adjustment for age, gender and body mass index, suggesting that systemic inflammation and arterial stiffness independently predict the E/A values in subjects with prehypertension.
Previous studies evaluated left ventricular diastolic function in individuals with prehypertension via both conventional echo-Doppler and tissue Doppler echocardiography (Citation5–7,Citation10,Citation12). The results show that, compared with healthy normotensive subjects, patients with prehypertension have a lower peak E wave velocity, a higher isovolumetric relaxation time, a significant decrease of E/A ratio, that was generally >1 and a significant increase in E/E’, indicating the presence of an early impairment in left ventricular diastolic function. In a study (Citation6) the E’/A’ ratio was <1, but only in non-dipper subjects, suggesting that stable high blood pressure values predict the appearance of the diastolic dysfunction. This is in agreement with the results of a large observational report (Citation7) demonstrating a significantly higher prevalence of diastolic dysfunction in subjects with persistent prehypertension compared with age-matched normotensive controls.
In the present study, the E/A ratio was significantly lower while deceleration time and isovolumetric relaxation time values significantly higher than those detectable in control age-matched normal subjects. However, these modifications do not exceed the proposed cut-off for the diagnosis of diastolic dysfunction (Citation18), but, as suggested by another study (Citation12), indicate a mild impairment of left ventricular diastolic relaxation, as an intermediate step between diastolic dysfunction and normal diastolic function. The E/A ratio >1 supports this interpretation, because values of E/A ratio <1 are found when myocardial relaxation is markedly delayed and is associated with a deceleration time value >220 ms (Citation19). Another important finding of our study is represented by the fact that in our prehypertensive patients the significant decrease in aortic elasticity (reduced distensibility and increased stiffness index) was accompanied by the significant increase in inflammatory markers such as high-sensitivity C-reactive protein and white blood cell count. These findings are consistent with previous reports documenting the association between prehypertension and arterial stiffness (Citation6,Citation8–9,Citation11,Citation13) and between prehypertension and inflammation (Citation3,Citation11,Citation14, Citation22).
A close relationship between inflammatory markers and arterial stiffness, assessed via different approaches (measurement of pulse wave velocity, augmentation index, aortic elasticity, pulse pressure), has been reported in subjects with prehypertension (Citation11,Citation14,Citation22,Citation23) and in apparently healthy subjects (Citation24–26), suggesting the role of inflammation as pathophysiological mechanism potentially responsible for the arterial stiffening process. While the association between prehypertension with arterial stiffness, as well with inflammation, has been reported previously, little information is available on the relationships between left ventricular diastolic function, inflammation and arterial stiffness, simultaneously assessed in the same subjects. At best of our knowledge, three studies (Citation8,Citation9,Citation27) have shown a significant correlation between impaired diastolic function and arterial stiffness, but only in two (Citation8,Citation9) have the levels of high-sensitivity C-reactive protein been evaluated. In one (Citation8), high-sensitivity C-reactive protein was high but not statistically different from the controls, whereas in the other (Citation9), the levels of high-sensitivity C-reactive protein were higher compared with normotensive subjects, and correlated with the vascular stiffness and diastolic parameters. However, this study was performed in young and adolescents, most of whom with obesity and type 2 diabetes. Therefore, the present study is the only one available so far showing in the same adult subjects with prehypertension, without cardiovascular risk factors, a significant correlation between impaired left ventricular diastolic relaxation, arterial stiffness and inflammation. Our study was not aimed at dissecting the pathophysiological mechanisms linking inflammation, arterial stiffness and diastolic dysfunction. However, since inflammation impairs endothelial function by decreasing nitric oxide (Citation28), which in turn is involved in the functional regulation of both large artery elasticity and diastolic compliance (Citation29), inflammation may represent one possible pathophysiological mechanism linking arterial stiffness and diastolic impairment in prehypertension.
Our study has some limitations but also a clinical implication. The limitations refer to relatively small sample size, the absence of Valsava maneuver and the diastolic assessment only via the echo Doppler technique, an approach which is less sensitive than the tissue Doppler one (Citation18). However, we have to take into account that (i) the Valsava maneuver is not standardized and thus the test performance is characterized by a large inter-individual variability of the responses (Citation30) and (ii) the echo Doppler approach, although with limitations, still stands as an acceptable tool to assess left ventricular diastolic function (Citation18). The clinical implication is that, since the early cardiac and vascular alterations occurring in prehypertension may have as common pathophysiological link an increased inflammatory process, an early therapeutic intervention should be aimed at reducing inflammation. Future studies are thus needed to determine the effects of therapeutic interventions capable of reducing inflammation on left ventricular function and arterial stiffness.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chobanian AV, Bakris GL, Black HR Cushman WC, Green LA, Izzo JL Jr, . National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–2572.
- Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: A cohort study. Lancet. 2001;358:1682–1686.
- Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573.
- Abhayaratna WP, Marwich TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: An echocardiographic survey. Heart. 2006;92: 1259–1264.
- Drukteinis JS, Roman MJ, Fabsitz RR, Lee ET, Best LG, Russell M, . Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescent and young adults: The Strong Heart Study Circulation. 2007;115:221–227.
- Erdogan D, Caliskan M, Yildirim I, Gullu H. Effect of normal blood pressure, prehypertension and hypertension on left ventricular diastolic function and aortic elastic properties. Blood Pressure. 2007;16:114–131.
- Markus MR, Strtzke J, Lieb W, Mayer B, Luchner A, Döring A, Implications of persistent prehypertension for ageing-related changes in left ventricular geometry and function: The Monica/Kore Augsburg study. J Hypertens. 2008;26: 2040–2049.
- Heffernan KS, Karas RH, Kuvin JT, Jae SY, Vieira VJ, Fernhall B. Carotid artery stiffness, high-density lipoprotein cholesterol and inflammation in men with pre-hypertension. J Hum Hypertens. 2009;23:590–596.
- Manios E, Tsivgoulis G, Koroboki E, Stamatelopoulos K, Papamichael C, Toumanidis S, . Impact of prehypertension on common carotid artery intima-media thickness and left ventricular mass. Stroke. 2009;40:1515–1518.
- Yeter E, Akçay M, Keles T, Durmaz T, Bayram NA, Ozdemir L, . The association of diastolic dysfunction and circadian variation of blood pressure in prehypertension. J Am Soc Echocardiogr. 2009;22:726–731.
- Myredal A Gan LM, Osika W. Increased intima thickness of the radial artery in individuals with prehypertension and hypertension. Atherosclerosis. 2010;209:147–151.
- Di Bello V, Talini E, Dell’Omo G, Giannini C. Delle Donne Mg, Canale ML, . Early left ventricular mechanics abnormalities in pre-hypertension: A two-dimensional strain echocardiography study. Am J Hypertens. 2010;23:504–512.
- Urbina, Em, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre- hypertension in youth. J Clin Hypertens. 2011;13:332–342.
- Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000.Arch Intern Med.2004;164:2113–2118.
- Gupta AK, McGlone M, Greenway FL, Johnson WD. Prehypertension in disease-free adults: A marker for an adverse cardiometabolic risk profile. Hypertens Res. 2010;33:905–910.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . Guidelines for the management of arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, . Recommendations for the evaluation of left ventricular diastolic function by echocardiography Eur J Echocardiography. 2009;10:165–193.
- Lacombe F, Dart A, Dewar E, Jennings G, Cameron J, Laufer E. Arterial elastic properties in man: A comparison of echo-Doppler indices of aortic stiffness. Eur Heart J. 1992;13: 1040–1045.
- Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol. 2011:57:1511–1522.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28: 412–419.
- Fung MM, Rao F, Poddar S, Mahata M, Khandrika S, Mahata SK, . Early inflammatory and metabolic changes in association with AGTR1 polymorphism in prehypertensive subjects. Am J Hypertens. 2001;24:225–233.
- Toikka JO, Niemi P, Ahotupa M, Niinikoski H, Rönnemaa T, Viikari JS, . Decreased large artery distensibility in borderline hypertension is related to increased in vivo low-density lipoprotein oxidation. Scand J Clin Lab Invest. 2002;62: 301–306.
- Kampus R, Kals J, Ristimðe T, Fischer K, Zilmer M, Teesalu R. High-sensitivity C-reactive protein affects central hemodynamics and augmentation index in apparently healthy persons. J Hypertens. 2005;22:1133–1139.
- Duprez DA, Somasundaram PE, Sigurdsson G, Hoke L,Relationship between C-reactive protein and arterial stiffness in an asymptomatic population. J Hum Hypertens. 2005;19: 515–519.
- Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974.
- Kim SH, Cho GY, Baik I, Lim SY, Choi CU, Lim HE, . Early abnormalities of cardiovascular structure and function in middle aged Korean adults with prehypertension: The Korean Genome Epidemiology study. Am J Hypertens. 2011;24:218–224.
- Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, . Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999.
- Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217.
- Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–297.