Abstract
Background. To investigate the effects of a single dose of metformin (MF) on endothelium-dependent vasodilatation and serum antioxidant and free fatty acid levels in patients with primary hypertension (PH) after an acute glucose load. Materials and methods. Patients with untreated PH were randomized to a no-metformin group (PH, n = 34) and a metformin group (PH+ MF, n = 28) who received a single dose of 500 mg metformin before testing. Healthy volunteers (n = 31) served as a control group. Brachial artery endothelium-dependent flow-mediated vasodilatation (FMD) was determined at 0, 1, 2 and 3 h after glucose load. Levels of serum superoxide dismutase (SOD), total antioxidant capacity (T-AOC), anti-superoxide anion free radical (AntiO2) and free fatty acids (FFA) were measured. Results. The FMD in the PH group decreased significantly 1 h after glucose load (PH: 10.9 ± 2.9% vs 13.67 ± 3.42% before glucose load). Metformin inhibited the effects of glucose load on FMD. At 1 h after acute glucose load, the concentrations of SOD, T-AOC and AntiO2 in the PH group decreased significantly compared with their fasting levels, and metformin inhibited the acute glucose load-induced decline in SOD and T-AOC levels. Conclusions. Metformin can prevent transient endothelial dysfunction caused by acute glucose load in patients with PH.
Introduction
Vascular endothelial dysfunction has been shown to be an underlying factor in the development of atherosclerosis (Citation1,Citation2). Hypertension (HTN), hyperglycemia and dyslipidemia are the three major risk factors of vascular endothelial dysfunction, and interactions of these three factors expedite the occurrence and development of atherosclerosis (Citation3). The postprandial phase has been shown to be important in the development of atherosclerosis (Citation4,Citation5), and numerous studies have shown that dietary glycemic load is associated with coronary heart disease and risk of hemorrhagic stroke (Citation6–9). Additionally, it has been shown that high postprandial triglyceride levels may lead to alterations of vascular endothelial function (Citation10,Citation11), and insulin resistance (IR) may be the primary mechanism (Citation3,Citation5).
Elevated blood glucose may damage vascular endothelial function through a number of different mechanisms (Citation12,Citation13): (i) increasing superoxide anions and glycosylated end products; (ii) auto-oxidation of glucose; (iii) abnormal metabolism of arachidonic acid; (iv) activation of protein kinase C; (v) reduction of NOS cofactor; and (vi) activation of the aldose reductase pathway. Increased production of intracellular reactive oxygen species (ROS) in target cells is thought to function as the link between high blood glucose levels and the activation of all the aforementioned mechanisms. A study by Gallo et al. (Citation14) showed that high blood glucose levels increased ROS in endothelial cells, and that the effect was ameliorated by metformin. Increased ROS production and activity is thought to result from an alteration of the oxidation and anti-oxidation balance in vivo by high blood glucose. This leads to decreased production of nitric oxide (NO) and reduction of biological activity, thereby impairing endothelial-dependent flow-mediated vasodilatation (FMD).
Metformin, a drug widely used to treat type II diabetes, lowers glucose levels by increasing glucose uptake in muscles and by decreasing the production of glucose in the liver (Citation15). While the exact mechanism of action of metformin is not clearly understood, studies have found that it prevents endothelial cell damage and death (Citation16–18). However, it remains unclear whether metformin can alleviate acute glucose load-induced transient damage to endothelial cells in patients with HTN. Thus, the purpose of this study was to examine the effect of metformin in reducing the transient damage through monitoring the FMD and the levels of antioxidants and FFAs in the blood.
Patients and methods
Subjects
This study was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University and all the patients signed informed consent. A total of 62 patients with untreated primary hypertension (PH; grade 1–2 based on the Chinese guidelines for hypertension diagnosis and treatment of 2005) (Citation19) were enrolled. The diagnosis of PH was determined by blood pressure monitoring in the clinic and/or 24-h ambulatory blood pressure monitoring. Eligible patients were randomly divided into two groups: a non-treatment (PH) group that did not receive any antihypertension medications and a metformin group (PH+ MF group) who received a single 500 mg dose of metformin before testing. Normotensive healthy volunteers without a family history of HTN were recruited to serve as a control group (NT).
All the participants met the following conditions: (i) coronary heart disease was ruled out by Holter monitoring, treadmill exercise ECG, SPECT myocardial perfusion imaging, or coronary angiography; diabetes mellitus and impaired glucose tolerance were ruled out by measurement of fasting and 2-h postprandial blood glucose; thyroid, liver, kidney, and pancreas diseases were ruled out by biochemical testing and imaging examinations; and left ventricular hypertrophy was ruled out by echocardiography; (ii) no chronic consumptive disease or malignancy; (iii) had never received any antihypertensive drugs, hypoglycemic drugs, or lipid-regulating drugs, and they stopped the use of any medications 5–7 daus before the study; (iv) were non-smokers and refrained from alcohol consumption and a high-fat diet within 12 h before the study; (v) postmenopausal women did not take estrogen; and (vi) fasting serum total cholesterol (TC) < 5.2 mmol/l, triglyceride (TG) < 1.7 mmol/l, high-density lipoprotein cholesterol (HDL-C) > 0.9 mmol/l and low-density lipoprotein cholesterol (LDL-C) < 3.4 mmol/l.
Oral glucose tolerance test (OGTT)
All subjects fasted for 12 h. Seventy-five grams of anhydrous glucose was dissolved in 250 ml of warm water, and subjects finished drinking the glucose solution within 5 min. Venous blood was drawn before drinking the glucose solution and 1, 2 and 3 h after drinking the glucose solution for the measurement of plasma glucose level. A Hitachi 2170A automatic biochemical analyzer (Hitachi Tokyo Japan) was used to measure the plasma glucose level via the oxidase method.
Measurement of endothelium-dependent FMD
Endothelium-dependent FMD was detected using a previously described method (Citation1,Citation20). The test was carried out in the early morning after a 12-h fast using an Acuson 128X110 color Doppler ultrasound system (Acuson, Mountain View, CA, USA) and 7.0-MHz linear array probe. The probing depth was 4 cm, and the room temperature was controlled between 22° and 23°C. The examination was performed with the patient in the supine position, and the target vessel was the 5-cm segment of the right brachial artery above the elbow. At the same time, a cuff with a width of 12.5 cm was wrapped around the arm with its lower edge 5 cm above the elbow and it was connected to a Hokanson E20 rapid cuff inflator (Bellevue, WA, USA). After the subject rested for 10 min, the brachial artery diameter was measured twice during the resting state and the average of the two values was considered the baseline value. The cuff was rapidly inflated to 200 mmHg (1 mmHg = 0.133 kPa), the pressure was maintained for 5 min, and then the cuff was rapidly deflated to 0 mmHg. The brachial artery lumen diameter was measured immediately, and after 60 s. The percentage change in the lumen diameter was defined as: (lumen diameter 60 s after deflation− baseline lumen diameter)/baseline lumen diameter, and was used to reflect the endothelium-dependent vasodilatation. During the measurement process, when the arterial intima of the anterior and posterior walls were shown clearly, the gain was adjusted until the lumen interface could be identified to a satisfactory extent and then the images were magnified until the line width on the screen was 0.065 mm. The measurement was carried out at the end of diastole, i.e. when the R-wave appeared on the ECG. Measurements were taken three times, and the average value was calculated. The same experienced professional technician performed all of the measurements in the study. For determination of intra-observer variation, two examinations were carried out in 15 patients within 1 week. The test procedure was single blind, i.e. the examiner was blinded to test results. The technical error was defined as: (measured value the first time− measured value the second time)/(measured value the first time+ measured value the second time)/2, and intra-observer variation = 1.57%. For determination of inter-observer variation, two double-blind observers measured the same patient at different time points during the same day, and the inter-observer variation was 2.46%.
Detection of serum anti-oxidant levels
A 5-ml sample of peripheral venous blood was collected before subjects drank the 75 g glucose solution (0 h), and at 1, 2 and 3 h after drinking the glucose solution. The samples were placed in a water bath at 37°C for 45 min, and then centrifuged at 3500 rpm at 4°C for 10 min to separate the serum. The serum was used for measuring the levels of superoxide dismutase (SOD), total antioxidant capacity (T-AOC), anti-superoxide anion free radical (AntiO2) and free fatty acid (FFA). All the samples were stored at 20°C, and measurements were performed in batches. SOD (Cat# K-P169), T-AOC (Cat# A015), AntiO2 (Cat# A052) and FFA (Cat# A024) ELISA kits were purchased from Jiancheng Biological Engineering (Nanjing, China).
Statistical analysis
Baseline characteristic were expressed as mean and standard deviation (SD) for continuous variables and count for categorical variables. Comparability of baseline characteristic between the three groups was analyzed using one-way analysis of variance (ANOVA) for continuous variables and chi-square for categorical variables. All five variables (FMD, SOD, T-AOC, Anti-O2 and FFA) were analyzed using one-way ANOVA to identify the difference at baseline between the three groups. When a significant difference among the three groups was apparent, multiple comparisons of means were performed using the Bonferroni procedure. Comparisons of differences of all five variables before and after taking glucose among the three groups were analyzed using one-way ANOVA, and the Bonferroni procedure was used for multiple comparisons. Furthermore, differences over time in the five variables among the three groups were analyzed by repeated measures ANOVA and the Bonferroni procedure was used in multiple comparisons of means between two groups. We excluded outliers in order to address their potential effect on the results. Outliers are defined as values that are three standard deviations away from the mean. All statistic assessments were evaluated at the 0.05 level of significance. Statistic analyses were performed using SAS 9.2 statistics software (SAS Institute Inc., Cary, NC, USA).
Results
Participant characteristics
A total of 105 subjects were initially enrolled into this study, with 35 subjects in each group. However, only 93 patients completed the study. In the PH group, one patient left the study due to intolerance to multiple venous punctures required by the measurements of OGTT. In the PH+ MF groups, a total of seven patients left the study (three patients developed psychological resistance to taking metformin after performance of FMD, and were allowed to leave the study; two patients left the study due to intolerance to multiple venous punctures required by the measurements of OGTT; two patients failed to show up for the study and could not be contacted subsequently). In the NT group, four patients left the study due to intolerance to multiple venous punctures required by the measurements of OGTT. The baseline characteristics of the groups are shown in . The groups were comparable with respect to gender, age, fasting plasma glucose (FPG), lipid profile and body mass index (BMI). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were comparable between the two PH groups, but were significantly greater than in the normotensive control group (all, p < 0.001). Importantly, there was no significant difference in FPG levels between the healthy controls and hypertensive patients.
Table I. Participant characteristics.
Change in FMD after oral glucose load
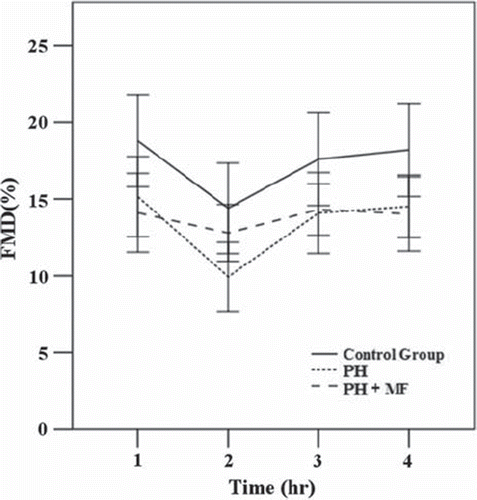
To examine the effect of acute glucose load on the functions of endothelial cells, we first measured the FMD of all three groups before and after glucose intake for 1, 2 and 3 h. The results are shown in . These results indicate that baseline FMDs of patients with primary hypertension (PH and PH + MF) were significantly reduced when compared with control group (Control). We also showed that the FMD decreased 1 h after the glucose load, and it was restored 2 h after the glucose load for all three groups. This suggests that the endothelial effect of a glucose load occurs primarily during the first hour after glucose intake.
Figure 1. Changes in flow-mediated vasodilatation (FMD) after high glucose intake at different time points in three groups The difference in FMD among the three groups were statistically significant at each time point (all, p < 0.05). After adjustment using the Bonferroni method, control group vs primary hypertension (PH) and control group vs PH+ metformin (MF) were significantly different at all four time points (all, p < 0.05). PH vs PH+ MF was statistically significant at 1-h time point only (p < 0.05). Data were expressed as mean ± SD. Outliers were excluded.
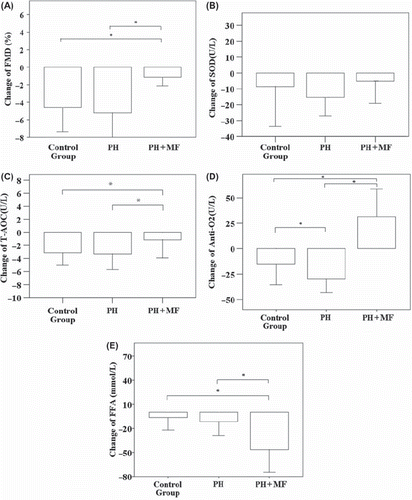
shows a comparison of the changes in FMD, T-AOC, AntiO2, SOD and FFA values in fasting samples and samples drawn 1 h after the oral glucose load. Subjects in the PH+ MF group took a single dose of metformin immediately before the test. The change of FMD, T-AOC, AntiO2 and FFA were found to be significantly different between the control group and the PH+ MF group, as well as between the PH group and PH+ MF (all, p < 0.001). The change of AntiO2 was also found to be significantly different between the control group and PH group (p < 0.001). The results suggest that administration of metformin immediately before glucose intake was able to alleviate reduction in endothelial function, and metformin is associated with an increase of antioxidant activity in the blood.
Figure 2. The effects of metformin (MF) after glucose load. The changes in (A) flow-mediated vasodilatation (FMD) and the changes in the serum levels of (B) superoxide dismutase (SOD), (C) total antioxidant capacity (T-AOC), (D) anti-superoxide anion free radical (AntiO2), and (E) free fatty acids (FFA) before and 1 h after administration of the glucose load were compared among the three groups. (A) There were significant differences in FMD between the control group and primary hypertension (PH)+ MF group, and the PH group and PH+ MF group (both, p < 0.001). (B) No significant difference in SOD was found between any two groups (all, p > 0.05). (C) There were significant differences in T-AOC between the control group and PH+ MF group, and between the PH group and the PH+ MF group (both, p < 0.001). (D) There were significant differences in AntiO2 between any two groups (all, p < 0.001). (E) There were significant differences in FFA between the control group and PH+ MF group, and between the PH group and PH+ MF group (both, p < 0.001). Data were expressed by mean ± SD. *Indicated there was significant difference. Outliers were excluded.
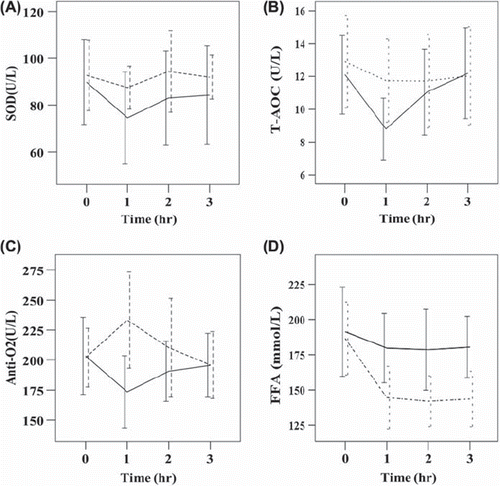
Changes of SOD, T-AOC, AntiO2 and FFA with time after oral glucose load
shows a comparison of the serum levels SOD, T-AOC, AntiO2 and FFA immediately before and 1, 2 and 3 h after the glucose load in the PH group and the PH+ MF group. The results show that both the serum level of T-AOC and AntiO2 returned to the pre-glucose load level 2 h after administration of the glucose load in the NT group. In the MF group, the serum level of AntiO2 increased significantly in the first hour then decreased to the pre-glucose load level in the hour, suggesting the effect of metformin on the level of AntiO2 is only transient. However, the level of FFA in the PH+ MF group remained low through the third hour, suggesting the mechanism of metformin on the reduction of FFA maybe different from that of increasing AntiO2 in the serum.
Figure 3. The effect of metformin (MF) on serum antioxidant and free fatty acids (FFA) levels over time in the patients with primary hypertension. The serum levels of (A) superoxide dismutase (SOD), (B) total antioxidant capacity (T-AOC), (C) anti-superoxide anion free radical (AntiO2), and (D) FFA at four time points before and after the oral glucose load were compared between primary hypertension (PH) and PH+ MF groups. Dashed line, PH+ MF group; solid line, PH group. There were significant differences in SOD (p < 0.001), T-AOC (p = 0.005), AntiO2 (p < 0.001), and FFA (p < 0.001) between the two groups over four time points. Data were expressed by mean ± SD. Outliers were excluded.
Discussion
In this study, we evaluated the effect of a single dose of metformin on endothelial function in non-diabetic, hypertensive subjects. We showed that a single dose of metformin prevented endothelial cell dysfunction resulting from a glucose load in hypertensive patients, and the mechanism of action may be related to the recovery of antioxidant capacity and a decline in the levels of FFAs. Our results were consistent with previous studies showing reduced endothelium-dependent relaxation in fructose-fed rats (Citation21). Our data also agreed with previous results showing that metformin inhibits the blood pressure increase seen in fructose-fed rats (Citation22–24), suggesting that metformin-mediated enhancement of insulin and decrease of the insulin-induced sympathetic tone by metformin may play a role in the response. It is important to note that our patient population was classified as non-diabetic hypertensive, since there was no significant difference in FPG between the healthy controls and hypertensive patients. The hypertensive patients in our study did not have the problems associated with hypoinsulinemia or IR seen in diabetic patients.
FMD was previously shown to be significantly reduced in hypertensive patients when compared with normotensive patients (Citation25,Citation26). In this study, we also showed a decrease in baseline endothelial functions of patients with PH compared with normotensive controls. We suggest that this could be due to increased vascular wall tension as a result of long-term elevated blood pressure and increased blood flow shear stress. Vascular endothelial cell damage, resulting in reduced release of vasoactive substances especially NO, is thought to lead to impaired endothelial-dependent vasodilatation (Citation13).
A number of reports have shown an association between increased glycemic load and coronary heart disease (Citation6–8). Glycemic load has also been shown to be associated with endothelial cell injury (Citation12,Citation13,Citation27), although the mechanism underlying the role of glucose on impairment of endothelial function was unclear. However, FMD was previously shown to correlate inversely with serum HbA1c levels in women (Citation28). Pioglitazone was also previously shown to improve endothelial function in non-diabetic, hypertensive patients who were insulin-resistant. Pioglitazone-mediated improvement of FMD in these subjects was suggested to be via amelioration of their IR (Citation29). Our data were in agreement with these studies, showing a significant decrease in FMD of normal as well as hypertensive patients, within 1 h of glycemic load.
Metformin lowers glucose levels by increasing glucose uptake in muscles and by decreasing the production of glucose in the liver (Citation15), presumably by stimulation of 5′-AMP-activated protein kinase (AMPK) and subsequent activation of atypical protein kinase C (aPKC) (Citation30). Metformin is also thought to prevent endothelial cell damage and death (Citation16,Citation17). Hou et al. (Citation17) reported a metformin-mediated reduction in intracellular ROS via upregulation of thioredoxin expression. Guigas et al. (Citation18) performed an in vitro study, which showed that metformin inhibited mitochondrial permeability transition and cell death. Detaille et al. (Citation16) also reported that metformin prevents glucose-induced cell death through a mitochondrial permeability transition dependent process. Our results show that metformin inhibited the glucose-induced damage to vascular endothelial function agreed with previous studies reporting the beneficial effects of metformin on endothelial function in women with PCOS (Citation31,Citation32). The metformin-mediated effect on vascular endothelial function in women with PCOS has been attributed to improve IR (Citation32).
Metformin has previously been shown to reduce oxidative stress by inhibiting the generation of hyperglycemia-induced free radicals (Citation33). A similar study showed that metformin attenuated diabetic nephropathy by modulating the expression of genes involved in oxidative stress (Citation34). Our results were consistent with these studies and showed that a glucose load induced a significant reduction in the levels of serum antioxidants including SOD, T-AOC and AntiO2. This suggested that high blood glucose-induced oxidation activity resulted in a decreased antioxidant capacity in vivo and subsequent impairment of vascular endothelial function. We also showed that metformin significantly improved glucose-induced impairment of endothelial function via restoration of antioxidant capacity.
Our data were consistent with previous results showing that elevation of serum FFA levels impaired insulin-mediated vasodilatation and NO production (Citation35). Endothelial dysfunction is an independent component of IR, and IR is closely related to the occurrence and development of HTN. PH complicated with IR and endothelial cell dysfunction is one of the most important factors increasing the risk of cardiovascular disease (Citation3), presumably via elevation of blood FFAs (Citation35). Our results showed an increasing trend of serum FFA levels in patients with PH compared with normal controls. However, the level of FFAs was significantly decreased after taking metformin, a result also noted in prior studies (Citation16,Citation36). This may in part be due to the effect of metformin on decreasing the uptake and oxidation of FFAs via increased phosphorylation of AMPK-α1 in skeletal muscle cells (Citation37), or via promoting AMPK activation in hepatocytes (Citation38). To this end, it will be interesting to investigate the mechanisms underlying metformin-induced decrease in FFA levels in PH patients in the future.
There are some limitations to this study that should be considered. First, glycemic load may cause dysfunction of cardiovascular endothelial cells. Also, based on previous research showing that oral intake of glucose had no effect on the nitroglycerin mediated vascular dilation, we did not measure endothelial independent vascular dilation, which is another limitation of this study. In this study, we did not measure the peak concentration of serum metformin after administration of a single 500-mg dose. We aim to address this issue in future studies. Importantly, since this is a single dose study, care must be exercised in extrapolating our results to steady-state and chronic medication conditions.
Conclusions
A single dose of metformin prevented transient endothelial dysfunction caused by acute glucose load in patients with HTN. This effect might be related to restoration of antioxidant capacity and reduction of the free fatty acid levels. Thus, metformin may be a valuable option for treating endothelial dysfunction in patients with HTN.
Funding
Key Project of Science and Technology Department of Fujian Province of China (2010Y0021).
Conflict of interest: The authors declare no conflict of interest.
References
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, . Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115.
- Funada J, Sekiya M, Hamada M, Hiwada K. Postprandial elevation of remnant lipoprotein leads to endothelial dysfunction. Circ J. 2002;66:127–132.
- van Oostrom AJ, Cabezas MC, Rabelink TJ. Insulin resistance and vessel endothelial function. J R Soc Med. 2002;95 Suppl 42:54–61.
- Parks EJ. Recent findings in the study of postprandial lipemia. Curr Atheroscler Rep. 2001;3:462–470.
- Kolovou GD, Anagnostopoulou KK, Daskalopoulou SS, Mikhailidis DP, Cokkinos DV. Clinical relevance of postprandial lipaemia. Curr Med Chem. 2005;12:1931–1945.
- Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75: 492–498.
- Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, . A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461.
- Mursu J, Virtanen JK, Rissanen TH, Tuomainen TP, Nykanen I, Laukkanen JA, . Glycemic index, glycemic load, and the risk of acute myocardial infarction in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Nutr Metab Cardiovasc Dis. 2011;21:144–149.
- Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr. 2007;85:1521–1526.
- Wilmink HW, Twickler MB, Banga JD, Dallinga-Thie GM, Eeltink H, Erkelens DW, . Effect of statin versus fibrate on postprandial endothelial dysfunction: Role of remnant-like particles. Cardiovasc Res. 2001;50:577–582.
- Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354.
- Lee IK, Kim HS, Bae JH. Endothelial dysfunction: Its relationship with acute hyperglycaemia and hyperlipidemia. Int J Clin Pract Suppl. 2002;(129):59–64.
- Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol. 2007;46:229–237.
- Gallo A, Ceolotto G, Pinton P, Iori E, Murphy E, Rutter GA, . Metformin prevents glucose-induced protein kinase C-beta2 activation in human umbilical vein endothelial cells through an antioxidant mechanism. Diabetes. 2005;54: 1123–1131.
- Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: An update. Ann Intern Med. 2002;137:25–33.
- Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, . Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187.
- Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, . Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010;396:199–205.
- Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, . Metformin inhibits mitochondrial permeability transition and cell death: A pharmacological in vitrostudy. Biochem J. 2004;382(Pt 3):877–884.
- Hypertension CfRoCGfPaToPw. Chinese guidelines for prevention and treatment of patients with hypertension. Chinese J Hypertension. 2005;134:39.
- Corretti MC, Plotnick GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. Am J Physiol. 1995;268(4 Pt 2):H1397–H1404.
- Verma S, Bhanot S, Yao L, McNeill JH. Defective endothelium-dependent relaxation in fructose-hypertensive rats. Am J Hypertens. 1996;9(4 Pt 1):370–376.
- Verma S, Bhanot S, McNeill JH. Decreased vascular reactivity in metformin-treated fructose-hypertensive rats. Metabolism. 1996;45:1053–1055.
- Verma S, Bhanot S, McNeill JH. Antihypertensive effects of metformin in fructose-fed hyperinsulinemic, hypertensive rats. J Pharmacol Exp Ther. 1994;271:1334–1337.
- Verma S, Bhanot S, McNeill JH. Metformin decreases plasma insulin levels and systolic blood pressure in spontaneously hypertensive rats. Am J Physiol. 1994;267(4 Pt 2): H1250–H1253.
- Gokce N, Holbrook M, Duffy SJ, Demissie S, Cupples LA, Biegelsen E, . Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension. 2001;38:1349–1354.
- Rubira MC, Consolim-Colombo FM, Rabelo ER, Yugar-Toledo JC, Casarini D, Coimbra SR, . Venous or arterial endothelium evaluation for early cardiovascular dysfunction in hypertensive patients?J Clin Hypertens (Greenwich). 2007;9:859–865.
- Dengel DR, Kelly AS, Steinberger J, Sinaiko AR. Effect of oral glucose loading on endothelial function in normal-weight and overweight children. Clin Sci (Lond). 2007;112:493–498.
- Lorbeer R, Empen K, Dorr M, Arndt M, Schipf S, Nauck M, . Association between glycosylated haemoglobin A(1c) and endothelial function in an adult non-diabetic population. Atherosclerosis. 2011;217:358–363.
- Horio T, Suzuki M, Takamisawa I, Suzuki K, Hiuge A, Yoshimasa Y, . Pioglitazone-induced insulin sensitization improves vascular endothelial function in nondiabetic patients with essential hypertension. Am J Hypertens. 2005;18(12 Pt 1):1626–1630.
- Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, . Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127.
- Orio F, Jr., Palomba S, Cascella T, De Simone B, Manguso F, Savastano S, . Improvement in endothelial structure and function after metformin treatment in young normal-weight women with polycystic ovary syndrome: Results of a 6-month study. J Clin Endocrinol Metab. 2005;90:6072–6076.
- Naka KK, Kalantaridou SN, Kravariti M, Bechlioulis A, Kazakos N, Calis KA, . Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: A prospective randomized study. Fertil Steril. 2011;95:203–209.
- Attia SM, Helal GK, Alhaider AA. Assessment of genomic instability in normal and diabetic rats treated with metformin. Chem Biol Interact. 2009;180:296–304.
- Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192:233–242.
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000; 49:1231–1238.
- Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–1350.
- Bogachus LD, Turcotte LP. Genetic downregulation of AMPK-alpha isoforms uncovers the mechanism by which metformin decreases FA uptake and oxidation in skeletal muscle cells. Am J Physiol Cell Physiol. 2010;299:C1549–C1561.
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, . Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174.
