Abstract
Background. Recent studies have shown a strong relationship between testosterone levels and vasomotor actions. The aim of this study is to compare the elastic properties of the aorta in male patients with hypogonadism and eugonadal healthy control subjects. Method. A total of 22 male with hypogonadism (mean age: 35.2 ± 9.5 years, mean disease duration: 5.3 ± 1.8 years) and 25 age-, sex- and weight-matched eugonadal healthy subjects (mean age: 34.5 ± 8.2 years) were enrolled in the study. Aortic stiffness (β) index, aortic strain (AoS) and aortic distensibility (AoD) were calculated from the aortic diameters measured by transthoracic echocardiography and blood pressure obtained by sphygmomanometer. Results. The routinely performed echocardiographic parameters were similar between patient and control groups. There were significant differences between the control and patient groups in β index (1.75 ± 0.44 vs 2.68 ± 1.72, p < 0.001), AoS (18.52 ± 6.44 vs 12.35 ± 3.88%, p < 0.001) and AoD (7.56 ± 2.86 vs 3.96 ± 1.24, 10−6 cm2/dyn, p < 0.001). There were statistically significant positive correlations between the serum total testosterone level and AoD (r = 0.539, p < 0.001) and AoS (r = 0.372, p = 0.036); moreover, there was a negative correlation between the serum total testosterone level and β index (r = − 0.462, p = 0.001). In multivariate analysis, serum total testosterone level was significantly related with AoD, AoS and β index (respectively, RR = 2.88, p = 0.004; RR = 3.45, p = 0.001; RR = 2.64, p = 0.01). Conclusion. The study results showed that aortic elasticity was impaired in patients with hypogonadism. We also have demonstrated a statistically significant correlation between aortic elastic properties and the serum total testosterone level.
Introduction
Sexual disparities in a variety of cardiovascular disorders (CVDs) have been demonstrated in many epidemiological and clinical studies. In some of those studies, a direct effect on vascular function by both female and male sex hormones has been suggested (Citation1). In addition to many metabolic roles, testosterone may be involved directly in the regulation of vascular tonus. Testosterone has been shown to dilate aortic, brachial and coronary vascular systems by both endothelial-dependent and independent mechanisms (Citation2–7). These observations suggest that testosterone may be an important regulator of vascular compliance in large and medium-sized arteries. Reduced vascular compliance due to impaired endothelial release of mediators contributes to arterial stiffening (Citation8,Citation9). In addition to being a marker for degenerative physical changes, increased arterial stiffness has important hemodynamic consequences, and is an independent marker of cardiovascular risk (Citation10–12).
The role of sex steroid hormones in modulating vascular function in men is of great importance, given that a reduction in plasma testosterone is strongly associated with several conditions including obesity, metabolic syndrome, dyslipidemia, endothelial cell dysfunction, diabetes, vascular disease, insulin resistance and arterial stiffness (Citation13–16).
Aortic elasticity can be investigated non-invasively by transthoracic echocardiography as formulating the pulsatile changes in diameter of the ascending aorta. In previous studies, non-invasive estimation of aortic distensibility (AoD) by evaluation of aortic diameters with the help of echocardiography and blood pressure data showed almost the same accuracy as invasive methods measure (Citation17). Aortic elastic properties reflect the aortic stiffness, which is associated with subclinical atherosclerosis and predicts cardiovascular morbidity and mortality (Citation18). Aortic stiffness has been shown to be in association with a variety of hormonal disturbances like hypogonadism (Citation19,Citation20).
In the present study, we aimed to compare the elastic properties of the aorta in male patients with hypogonadism and eugonadal healthy subjects. Also we investigated the relationship between aortic elastic properties [β index, aortic strain (AoS) and AoD] and the sex hormone levels in male patients with hypogonadism.
Methods
Study population
The study population included 22 consecutive male patients with adult-onset idiopathic hypogonadotrophic hypogonadism who were referred from our Endocrinology and Metabolism Unit of the Internal Medicine Department (mean age, 35.2 ± 9.5 years, and mean disease duration: 5.3 ± 1.8 years) and 25 healthy male subjects as controls (mean age: 34.5 ± 8.2 years). The diagnosis of hypogonadism was established according to the Endocrine Society Clinical Practice Guideline (Citation21). Hypogonadism was defined by symptoms of androgen deficiency and total morning serum testosterone levels less than 300 ng/dl. Age, gender, body mass index (BMI) and biochemical measurements were recorded. The baseline demographical characteristics and clinical features of the patients and the control subjects are given in .
Table I. Baseline clinical and laboratory characteristics of the hypogonadal and control groups.
The eugonadal control subjects had no cardiovascular or any other organ system disease and with normal physical examination, chest X-ray, electrocardiogram, and 2D and Doppler echocardiography. Patients with hypertension, renal failure, diabetes, heart failure, valvular heart disease, coronary artery disease, chronic obstructive pulmonary disease and arrhythmia like atrial fibrillation were excluded from the study. Other exclusion criteria were the presence of any kind of pituitary tumor or any other hypothalamic–pituitary diseases. Patients with other hormone deficiencies were also excluded due to possible confounding effects. Two patients with poor echocardiographic image quality were also excluded before enrollment to the study. This study, consistent with the Declaration of Helsinki, was approved by the Ethics Committee of Hacettepe University Faculty of Medicine, and informed consent was obtained from all participants.
Blood sample analysis
Serum samples were obtained from an antecubital vein between 08:00 and 10:00 h, for measuring serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), total/free testosterone levels and biochemistry panel including lipid panel. Serum total and free testosterone, FSH and LH levels were measured by radioimmunoassay methods from fresh serum samples [Immulite 2000 Total Testosterone, Chemiluminescent Enzyme Immunoassay (Diagnostic Product Corp., Los Angeles, CA, USA); Architest FSH, Architest LH, Chemiluminescent Microparticle Immunoassay (Abbott Laboratories, USA)]. The intra-assay and inter-assay coefficients of variation were 2.5% and 3.6% for the LH assay, 2.9% and 3.7% for the FSH assay, 3.5% and 5.0% for the total testosterone assay, respectively.
Echocardiography
All participants underwent standard echocardiographic imaging in the left lateral decubitus position using a commercially available system (Vingmed System Five GE ultrasound, Horten, Norway). Images were obtained using a 2.5–3.5 MHz transducer in the parasternal and apical views. Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) diameters were determined with M-mode echocardiography under 2D guidance in the parasternal long-axis view, according to the recommendations of the American Society of Echocardiography (Citation22). Left ventricular ejection fraction was calculated from apical four-chamber view, according to the modified Simpson's rule. All echocardiographic measurements were carried out by two experienced cardiologists who were unaware of the clinical data.
Pulsed-wave (PW) Doppler was performed in the apical four-chamber view to obtain mitral inflow indices to assess LV filling according to the recommendations of the American Society of Echocardiography (Citation23). Measurements of mitral inflow include the peak early filling (E-wave) and late diastolic filling (A-wave) velocities, the E/A ratio, deceleration time (DT) of early filling velocity, and the IVRT, derived by placing the cursor of CW Doppler in the LV outflow tract to simultaneously display the end of aortic ejection and the onset of mitral inflow.
Pulmonary systolic arterial pressure was estimated by continuous wave Doppler as peak regurgitation velocity plus an assumed right atrial pressure in relation to the size and respiratory excursion of inferior cava vein visualized in subcostal view (Citation22).
Measurement of elastic properties of the aorta
AoD was calculated non-invasively based on the relationship between changes in aortic diameter and pressure with each cardiac cycle (Citation24). Ascending aorta was recorded at a level 3 cm above the aortic valve in the M-mode tracing guided by the 2D echocardiography in the parasternal long-axis view. Systolic diameter (SysD) was measured as the maximal anterior motion of the aorta and diastolic diameter (DysD) at the peak of the QRS complex of the simultaneously recorded electrocardiogram. Five consecutive cardiac beats were measured routinely and average of the values was recorded. The following aortic elastic indices were calculated (Citation25):
AoS = (SysD− DysD)/DysD
β index = ln (SBP/DBP)/[(SysD− DysD)/DysD]
where SBP and DBP are the systolic and diastolic blood pressures, and “ln” is the natural logarithm.
AoD = 2×(SysD− DysD)/[(SBP− DBP)× DysD]× 10−6 cm2/dyn
Intraobserver and interobserver variability for the systolic and diastolic dimensions ranged from 4.4% to 5.1%.
Statistical analysis
All statistical analyses were performed using statistical software (SPSS, version 17.0 for Windows; SPSS, Chicago, IL, USA). Continuous variables were given as mean± SD; categorical variables were defined as percentage. For numerical variables, an independent sample t-test and Mann–Whitney U test were used for inter-group comparisons. Chi-square test and Fisher's exact chi-square test were used for comparisons of categorical variables. Two-tailed p < 0.05 was considered significant. Correlation analyses were performed using the Pearson coefficient of correlation. To assess the relationship between sex hormone levels with aortic elastic properties, multivariate logistic regression analysis was performed, and results are shown as an odds ratio (OR) with 95% confidence intervals (CIs).
Results
The baseline characteristics of the 47 study participants (22 hypogonad, 25 control) were given in . The mean age of the hypogonadal patients at entry was 35.2 ± 9.5 years, and that of the control subjects 34.5 ± 8.2 years (p = NS). As shown, BMI, low-density lipoprotein cholestrol (LDL-C), high-density lipoprotein cholestrol (HDL-C), triglycerides and fasting plasma glucose (FPG) of the hypogonadal and eugonadal healthy control men were similar. By design, the two groups differed in their circulating testosterone and free testosterone concentrations. All subjects were normotensives and any significant difference in systolic or diastolic blood pressures, pulse pressure and pulse rate was not observed between the two groups (p = NS).
Comparison of the baseline echocardiographic values among patients with hypogonadism and the control subjects revealed no difference between the two groups regarding to LV systolic and diastolic diameters, ejection fraction and LV diastolic filling parameters.
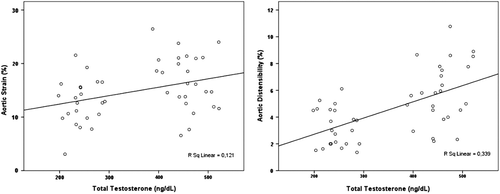
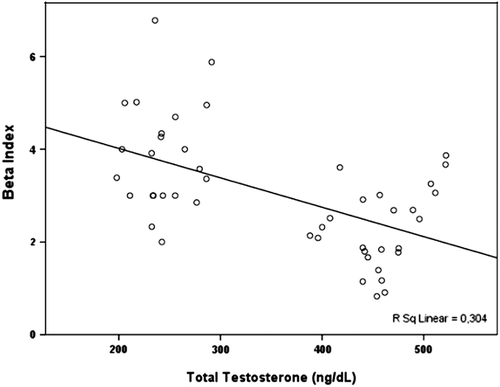
Aortic elasticity parameters are also shown in . Aortic systolic and diastolic diameters were similar among patients and control subjects. Furthermore, AoS (12.4 ± 3.9% vs 16.6 ± 4.2%, p < 0.001) and AoD (5.4 ± 1.9 vs 6.9 ± 2.3, 10 −6 cm2/dyn, p < 0.001) were significantly decreased in the patient group. Additionally, β index was increased in hypogonadism group compared with control subjects (3.1 ± 1.1 vs 2.3 ± 0.9, p = 0.002). There was no correlation between the aortic elastic properties and the LV systolic and diastolic functions. There were statistically significant positive correlations between the serum total testosterone level and AoD (r = 0.539, p < 0.001) and AoS (r = 0.372, p = 0.036) (), moreover there was a negative correlation between the serum testosterone level and β index (r = − 0.462, p = 0.001) (). In multivariate analysis, serum total testosterone level was significantly related with AoD, AoS and β index (respectively, relative risk, RR = 2.88, p = 0.004; RR = 3.45, p = 0.001; RR = 2.64, p = 0.01).
Table II. Aortic elastic properties in the hypogonadal and eugonadal control men.
Discussion
In this study, we compared the aortic elastic properties in patients with hypogonadism and eugonadal healthy subjects, and whether a simple association between total testosterone level and the aortic stiffness. Our data showed that patients with hypogonadism have increased arterial stiffness, as measured by AoS, AoD and β index, and there was a significant correlation between elastic properties of the aorta and the total testosterone level.
Aortic elastic properties (aortic stiffness index, AoS and AoD), which were calculated from pulsatile changes in ascending aorta, may be used for measuring large arterial stiffness (Citation26). Several reports have shown that large arterial stiffness is an independent predictor of cardiovascular morbidity and mortality (Citation18,Citation25,Citation27). However, few studies have investigated the association between testosterone level and arterial stiffness (Citation19,Citation28). Previous studies have demonstrated significant correlations between aortic stiffness parameters, which were calculated from the ascending aorta and parameters derived from aortography and pulse wave analysis methods (Citation29). In the present study, we used conventional parameters of aortic elasticity (AoS, AoD and β index), which were derived from the pulsatile change in aortic diameter during echocardiographic evaluation.
Several lines of evidence support the statement that serum testosterone influences arterial vasculature dynamics. There is an inverse relationship between serum testosterone and blood pressure (BP) (Citation30). Furthermore, in a cross-sectional study of 55 older men, Dockery et al. (Citation31) reported that pulse wave velocity (PWV) was inversely related to serum free testosterone index. In the Baltimore Longitudinal Study of Aging, an arterial stiffness index calculated from peak systolic and end diastolic diameters of the common carotid artery and simultaneous brachial artery BP correlated negatively with serum testosterone, after adjustment for confounding factors such as age, pulse pressure, FPG and BMI (Citation16). In that study, serum testosterone obtained 5 years prior to the assessment of arterial properties, predicted the arterial stiffness index, suggested a cause and effect relationship between low testosterone and the evolution of subsequent increased arterial wall rigidity. Our findings differ from the previous studies in regard to patient age groups. We showed impaired aortic elastic properties in younger patient group.
Androgen receptors have been demonstrated within aortic, peripheral vascular and ventricular mammalian cells (Citation32), and more recently in normal male and female left ventricles (Citation33). In addition, testosterone has direct influences on vascular hemodynamics, although the exact mechanisms remain unclear. In vitro, testosterone induces relaxation of rabbit coronary arteries and aorta through an endothelial-independent mechanism (Citation3). However, testosterone produced dilatation of canine coronaries via a nitric oxide-dependent pathway in vivo (Citation2), and in males with coronary artery disease, high dose testosterone enhanced flow-mediated dilatation of the brachial artery (Citation5), suggesting an endothelium-dependent mechanism. Similarly, in men with established coronary disease, testosterone, administered by intracoronary infusion and at physiological concentrations, dilated coronary arteries and increased blood flow (Citation4). These observations suggest a direct role for testosterone in modulating blood flow and vascular resistance in medium and large sized arteries. In the present study, hypotestosteronemia may therefore have resulted in impaired vasodilatory function of conduit arteries, thus reducing aortic compliance.
As discussed above, testosterone also has been shown to modulate each of the mechanisms that contribute to aging-associated endothelial dysfunction. However, whether decreased testosterone levels are directly associated with the endothelial dysfunction and increased incidence of CVDs remains controversial. It should be in mind that also aging related vascular changes in eugonadal men could result in decreased testosterone levels (Citation34). In the meta-analysis of published studies to evaluate the association between endogenous testosterone and mortality, Araujo et al. (Citation35) showed that low endogenous testosterone levels were associated with increased risk of all-cause and CVD death in community-based studies of older men, but there was high between-study heterogeneity in age groups. So, we need longitudinal studies that will assess coronary artery changes to help us attain a better understanding of the effects of testosterone-replacement therapy on the CV system in aging hypogonadal men. Because of our study group was composed of adult-onset hypogonadism patients, vascular aging may be less than those studies in the literature in our patients.
The present study should be interpreted in the light of some limitations. First, the patients and control subjects did not undergo 24-h ambulatory blood pressure monitoring. Second, we performed a cross-sectional study. Large-scale, prospective studies may be performed in the future to detect the exact pathophysiology of the aortic stiffness in this patient group. The administration of the testosterone replacement therapy and the effects on aortic stiffness may be more helpful. Also, other methods like PWV, augmentation index, etc., may be more useful and objective than conventional methods.
In conclusion, hypogonadism in men is associated with increased arterial stiffness as reflected in increased β-index and, possibly, in decreased AoS and AoD. Large-scale, prospective, randomized and controlled studies would be useful for detecting prognostic significance of aortic stiffness in patients with hypogonadism and the effect of hormone replacement therapy on aortic elasticity parameters may be highlighted.
Conflict of disclosure statement: There is no conflict of interest related with our manuscript and was not supported by any grant or institution.
References
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340.
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, . Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619.
- Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91:1154–1160.
- Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–1696.
- Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol. 2000;85:269–272.
- Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE. Testosterone-induced relaxation of coronary arteries: Activation of BKCa channels via the cGMP- dependent protein kinase. Am J Physiol Heart Circ Physiol. 2012;302:H115–H123.
- Perusquia M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol. 2010;298:H1301–H1307.
- McVeigh G, Brennan G, Hayes R, Cohn J, Finkelstein S, Johnston D. Vascular abnormalities in non-insulin-dependent diabetes mellitus identified by arterial waveform analysis. Am J Med. 1993;95:424–430.
- McVeigh GE, Brennan GM, Cohn JN, Finkelstein SM, Hayes RJ, Johnston GD. Fish oil improves arterial compliance in non-insulin-dependent diabetes mellitus. Arterioscler Thromb. 1994;14:1425–1429.
- Arnett DK, Evans GW, Riley WA. Arterial stiffness: A new cardiovascular risk factor?Am J Epidemiol. 1994; 140:669–682.
- Glasser SP, Arnett DK, McVeigh GE, Finkelstein SM, Bank AJ, Morgan DJ, . Vascular compliance and cardiovascular disease: A risk factor or a marker?Am J Hypertens. 1997;10:1175–1189.
- Traish AM, Kypreos KE. Testosterone and cardiovascular disease: An old idea with modern clinical implications. Atherosclerosis. 2011;214:244–248.
- Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30:477–494.
- Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22.
- Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30:23–32.
- Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, . Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. 2006;290:E234–E242.
- Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Yaron M, Greenman Y, Rosenfeld JB, Izkhakov E, Limor R, Osher E, . Effect of testosterone replacement therapy on arterial stiffness in older hypogonadal men. Eur J Endocrinol. 2009;160:839–846.
- Kyriazis J, Tzanakis I, Stylianou K, Katsipi I, Moisiadis D, Papadaki A, . Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant. 2011;26:2971–2977.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, . Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536–2559.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133.
- Tavil Y, Kanbay A, Sen N, Ulukavak Ciftçi T, Abaci A, Yalçin MR, . The relationship between aortic stiffness and cardiac function in patients with obstructive sleep apnea, independently from systemic hypertension. J Am Soc Echocardiogr. 2007;20:366–372.
- Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: Comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–996.
- Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, . Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802.
- Stefanadis C, Dernellis J, Tsiamis E, Stratos C, Diamantopoulos L, Michaelides A, . Aortic stiffness as a risk factor for recurrent acute coronary events in patients with ischaemic heart disease. Eur Heart J. 2000;21:390–396.
- Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, . The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267.
- O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658.
- Fogari R, Preti P, Zoppi A, Fogari E, Rinaldi A, Corradi L, . Serum testosterone levels and arterial blood pressure in the elderly. Hypertens Res. 2005;28:625–630.
- Dockery F, Bulpitt CJ, Donaldson M, Fernandez S, Rajkumar C. The relationship between androgens and arterial stiffness in older men. J Am Geriatr Soc. 2003;51:1627–1632.
- McGill HC, Jr., Sheridan PJ. Nuclear uptake of sex steroid hormones in the cardiovascular system of the baboon. Circ Res. 1981;48:238–244.
- Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98: 256–261.
- Lopes RA, Neves KB, Carneiro FS, Tostes RC. Testosterone and vascular function in aging. Front Physiol. 2012;3:89.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96: 3007–3019.