Abstract
Objectives. The circadian rhythm (CR) of arterial blood pressure (ABP) in Alzheimer disease (AD) patients was examined in a case–control clinical study. Methods. This study was constructed using the case–control method and investigates non-hypertensive AD patients, compared with normotensive controls from a primary care setting. Twenty-four-hour ABP was measured with an automatic oscillometric device and recorded every 30 min throughout the day and night. Extreme dipper, dipper, non-dipper and reverse-dipper patterns were defined as those individuals with > 20%, 10–20%, < 10% and no fall in nocturnal ABP relative to daytime values. Results. There were significant differences in ABP dipper status between cases and controls (cases – 16.15%, 60.00%, 17.70% and 6.15% vs controls – 3.19%, 31.9 2%, 42.02% and 22.88% for reverse dipper, non-dipper, dipper and extreme dipper, respectively, df = 3, χ2 = 56.76, p < 0.001). Compared with normal controls, AD patients had significantly higher 24-h mean blood pressure, 24-h mean systolic blood pressure (SBP), night mean SBP, night mean pulse pressure (PP) and 24-h mean PP. There were no significant differences in 24-h mean diastolic blood pressure (DBP), daytime mean DBP or night-time mean DBP, and no significant differences in daytime mean SBP. Conclusions. The circadian rhythm of ABP in AD patents differed from normal controls, perhaps from higher night SBP in AD patents.
Introduction
Arterial blood pressure (ABP), particularly systolic pressure (SBP), rises with age, leading to a high prevalence of hypertension in older people (Citation1). The prevalence and incidence of both hypertension and Alzheimer disease (AD) also rises with age, suggesting that hypertension might be a risk factor for AD (Citation2). In a recent meta-analysis associating hypertension and AD, the incidence of AD with hypertension or antihypertensive medication use was examined; results demonstrated no association of risk for AD with hypertension. These results might be strong evidence that hypertension is not a risk factor for AD (Citation3,Citation4). There are studies on the association of low ABP with incidence of AD (Citation5), but these do not reach a definite link between the two. Although ABP levels might be risk factors for AD, this theory is still being researched (Citation3,Citation5–9).
With advancing age, a progressive deterioration of circadian rhythms occurs, compared with the healthy (Citation10,Citation11). Often, changes in the sleep–wake cycle are seen manifested by reductions in sleep quality and impairment in cognitive performance (Citation12,Citation13). Moreover, AD patients show exaggeration of such age-related changes, affecting as many as a quarter of patients during some stage of their illness (Citation14–16). Daytime agitation, night-time insomnia and restlessness are some of the common behavioral changes presenting in AD (Citation14,Citation15). Nocturnal sleep disturbances often accompany daytime naps, frequently in direct association with the extent of AD (Citation16,Citation17). Circadian rhythm disturbances (CRDs) in AD often presented dramatically (Citation14) and are associated with shorter survival in long-term care residents, in addition to being causes of physical and psychological burden for caregivers (Citation18,Citation19).
The normal ABP circadian rhythm in the general population is well studied (Citation20–24). Aging leads to a multitude of changes in the cardiovascular system, including increased vascular stiffness, increased incidence of ABP (Citation25), and progressive deterioration of circadian rhythms (Citation10,Citation11). CRDs are commonly in AD (Citation2,Citation14). From these, it is presumed that circadian rhythm in patients with AD might be different from that in subjects without AD.
Patients and methods
From January 2010 to September 2011, AD patients were recruited by geriatrics specialists according to NINCDS-ADRDA criteria from a primary care setting. The primary care setting was a section of the geriatrics department of a hospital in a city in west-south China, which carried out medical examinations on the elderly (Citation26). Cases included 70 men and 60 women, with mean age of 76.25 ± 4.15 years, ranging from 65 to 85 years. Normal controls (with the similar age, gender, daily activities, sleep quality and health status, except AD) were selected. Daily activities were reported by participants and their family members, and sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI). In addition, health status was evaluated according to medical records. The normal controls included 188 subjects, with a mean age of 75.89 ± 3.76 years, ranging from 65 to 85 years, including 101 men and 87 women. A prior diagnosis of cardiovascular, cerebrovascular, peripheral vascular disease, sleep apnea syndrome or chronic renal failure were excluded, as were a prior diagnosis of hypertension or antihypertensive medication use. Informed consents were obtained from all participants (as well as their legal proxies). The Research Ethics Committee of the Sichuan University approved the study.
Ambulatory ABP monitoring
A 24-h ambulatory ABP monitoring was performed using with an automatic oscillometric device (Takeda TM-2430) and data were analyzed with Sigma 2000 software (Citation27). Patients were instructed to keep their habitual routine and present a report with activities done in their primary care setting. The recorder was calibrated with a mercury sphygmomanometer and set to take readings at 30-min intervals from 06:00 to 22:00 h, and every 30 min from 22:00 to 06:00 h. All patients were encouraged to carry out their normal daily awake and sleep periods in the primary care setting. The recordings were analyzed to obtain 24-h daytime and night-time mean SBP and diastolic blood pressure (DBP). The 24-h mean BP = 1/2(24-h mean SBP + 24-h mean DBP) (Citation28,Citation29).
Classification of dipper status
Dippers were defined as those individuals with a 10–20% fall in nocturnal ABP (night-time mean SBP or night-time mean DBP) relative to daytime values. Extreme dippers were defined as a greater than 20% fall in nocturnal ABP. Non-dippers were defined as a less than 10% nocturnal fall, and those with no fall in ABP were defined as reverse-dippers (Citation30–32).
Statistical analysis
ABP characteristics were compared between control and case using an unpaired Student's t-test for continuous variable and Pearson chi-square or Fisher's exact test (where an expected cell count was < 5) for categorical variables.
Results
Baseline characteristics of cases and controls
The ages of cases and controls were 76.25 ± 4.15 and 75.89 ± 3.76 years, respectively. There was no significant difference in age between the two cohorts (t = 0.670, p = 0.991) and 54% of patients were men in each of the groups. There was no significant difference in heart ratio (HR) (including 24-h mean HR, daytime mean HR, night-time mean HR) between cases and controls ().
Table I: Blood pressure characteristics between case and control.
ABP levels in case and control groups
Compared with normal controls, AD patients had significantly higher 24-h mean BP (97.32 ± 5.24 vs 95.02 ± 5.13 mmHg, t = 3.896, p < 0.001), 24-h mean SBP (120.79 ± 7.00 vs 116.20 ± 7.33 mmHg, t = 5.597, p < 0.001), 24-h mean PP (46.81 ± 12.33 vs 42.33 ± 11.40 mmHg, t = 3.335, p = 0.001), night-time mean SBP (116.58 ± 9.82 vs 107.22 ± 12.01 mmHg, t = 7.341, p < 0.001) and night-time mean PP (45.05 ± 13.40 vs 37.26 ± 14.57 mmHg, t = 4.842, p < 0.001). None of the differences in other parameters between cases and controls were significant (p > 0.05) ().
BP dipper status in cases and controls
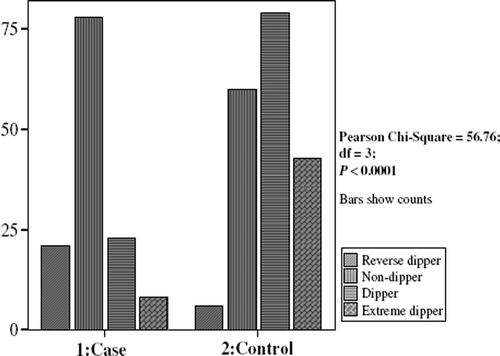
Compared with normal controls, AD patents demonstrated a higher percentage of reverse dipper status (16.15% vs 3.19%), more non-dipper status (60.00% vs 31.92%), less dipper status (17.70% vs 42.02%) and less extreme dipper status (6.15% vs 22.88%) (df = 3, χ2 = 56.76, p < 0.001) ().
Discussion
In this case–control study of AD patients, both cases and controls showed a clear circadian rhythm of ABP. However, AD patients were more likely to be reverse dippers and non-dippers. In the elderly controls, a dipper or extreme dipper pattern was more likely. Furthermore, in AD patients, circadian rhythm of ABP (higher BP during the day than during the night) was attenuated. In this case–control study, subjects with reverse dipper or non-dipper were significantly at higher risk of AD than those without.
Among the various biological rhythms contributing to normal bodily functions, disturbance in the 24-h day/night activity/rest cycle is considered an important chronobiological factor associated with several medical conditions (Citation33,Citation34). In most healthy people, the ABP biological rhythm was characterized by people exhibiting the highest levels of ABP from late morning to middle afternoon. A physiological decline in ABP of at least 10% from daytime values occurred during sleep (Citation33). However, in the AD patients, 70% did not demonstrate a normal ABP biological rhythm.
The suprachiasmatic nucleus (SCN) of the anterior hypothalamus is considered a master clock for controlling circadian rhythm in mammals (Citation35). This nucleus controls melatonin secretion via a multi-synaptic pathway influencing the biological clock, ABP, core body temperature and sleep. The CRD of ABP in AD patients might be explained by the fact that light inputs from the retina in AD patients could not stimulate SCN, which may lead to abnormal melatonin secretion.
Additionally, ABP may also be influenced by several factors such as daily activities, eating habits, and so on. When cases and controls were included, the differences in these factors were controlled for as far as possible.
In the present study, none of the AD or control subjects had hypertension. The subjects with AD had higher night-time mean SBP, 24-h mean BP and 24-h mean SBP than those without. There was no difference in 24-h mean DBP, daytime mean SBP, daytime mean DBP, daytime mean PP or night-time mean DBP, so the differences in BP dipper status resulted from the higher night-time mean SBP in AD patients. In AD patients without hypertension, the higher night-time mean SBP led to CRD of ABP in AD patients. However, since this is a case–control study, a causal association between AD and ABP circadian rhythm could not be established.
This study had some limitations that deserve to be mentioned. First, in this case–control study, controls were selected to assure that they closely matched the cases (including daily activities and sleep quality), but selection bias could be an issue. However, daily activities and sleep quality could influence the circadian rhythm of ABP and it was therefore important to remove such influence on circadian ABP rhythm. Second, some AD cases were treated with drugs that could influence ABP, while none of the controls were using drugs.
In conclusion, in the present study, we found that in AD patients without hypertension, these were important disturbances of the circadian rhythm of ABP in AD patients, which were largely dependent from the higher night-time SBP. The causal association between high night-time SBP and risk of AD should be further explored.
NOTICE OF CORRECTION
The version of this article published online ahead of print on 16 November 2012 contained errors in the list of authors. The list of authors have been corrected for this version.
Acknowledgements
The authors thank the staff of the Key Laboratory of Chronobiology of Health Ministry in Basic and Forensic School of Sichuan University, and the Department of Geriatrics of the third hospital of Mianyang, and all study participants (as well as their legal proxies) for their great contributions.
Disclosure: The authors wish to extend their full confidence that there are no conflicts of interest in this research article and that only the fullest integrity was practiced in its composition.
This work was supported by the Science and Technology Graveness Project of Sichuan Province (2010FZ0061) and from the Illustrious Youth Specialist Project of Sichuan Province (2012JQ0050).
References
- Wagner A, Sadoun A, Dallongeville J, Ferrières J, Amouyel P, Ruidavets JB, Arveiler D. High blood pressure prevalence and control in a middle-aged French population and their associated factors: the MONA LISA study. J Hypertens. 2011;29:43–50.
- McDowell I. Alzheimer's disease: insights from epidemiology. Aging (Milano). 2001;13:143–162.
- Guan JW, Huang CQ, Li YH, Wan CM, You C, Wang ZR, . No association between hypertension and risk for Alzheimer's disease: A meta-analysis of longitudinal studies. J Alzheimers Dis. 2011;27:799–807.
- Chang-Quan Huang, Hui Wang, Chao-Min W, Zheng-Rong W, Jun-Wen G, Yong-Hong L. The association of antihypertensive medication use with risk of cognitive decline and dementia: A meta-analysis of longitudinal studies. Int J Clin Pract. 2011;65:1295–305.
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:1667–1672.
- Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: A systematic review and meta-analysis. Epidemiology. 2011;22:646–59.
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, . Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137: 149–155.
- Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. 2001;58:1640–1646.
- Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23:116–124.
- Urbanski HF, Sorwell KG. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age (Dordr). 2011 Dec 25. [Epub ahead of print].
- Cayetanot F, Nygård M, Perret M, Kristensson K, Aujard F. Plasma levels of interferon-gamma correlate with age-related disturbances of circadian rhythms and survival in a non-human primate. Chronobiol Int. 2009;26:1587–1601.
- Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89.
- Naismith SL, Lewis SJ, Rogers NL. Sleep-wake changes and cognition in neurodegenerative disease. Prog Brain Res. 2011;190:21–52.
- Yesavage JA, Noda A, Hernandez B, Friedman L, Cheng JJ, Tinklenberg JR, . Alzheimer's Disease Neuroimaging Initiative. 5. Circadian clock gene polymorphisms and sleep-wake disturbance in Alzheimer disease. Am J Geriatr Psychiatry. 2011;19:635–643.
- Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33:211–223.
- Roberts LM, Pattison H, Roalfe A, Franklyn J, Wilson S, Hobbs FD, . Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction?Ann Intern Med. 2006;145:573–581.
- Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology. 2002;58:1055–1061.
- Bianchetti A, Scuratti A, Zanetti O, Binetti G, Frisoni GB, Magni E, . Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia. 1995;6:108–112.
- Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The timing of activity rhythms in patients with dementia is related to survival. J Gerontol A Biol Sci Med Sci. 2004;59:1050–1055.
- Cugini P, Gori MC, Petrangeli CM, Tisei P, Giubilei F. Preserved blood pressure and heart rate circadian rhythm in early stage Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 1999;54:M304–M308.
- Hermida RC, Ayala DE, Iglesias M. Circadian rhythm of blood pressure challenges office values as the “gold standard” in the diagnosis of gestational hypertension. Chronobiol Int. 2003;20:135–156.
- Goto N, Uchida K, Morozumi K, Ueki T, Matsuoka S, Katayama A, . Circadian blood pressure rhythm is disturbed by nephrectomy. Hypertens Res. 2005;28:301–306.
- Soylu A, Yazici M, Duzenli MA, Tokac M, Ozdemir K, Gok H. Relation between abnormalities in circadian blood pressure rhythm and target organ damage in normotensives. Circ J. 2009;73:899–904.
- Shiotani H, Umegaki Y, Tanaka M, Kimura M, Ando H. Effects of aerobic exercise on the circadian rhythm of heart rate and blood pressure. Chronobiol Int. 2009;26:1636–1646.
- Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585.
- Aprahamian I, Martinelli JE, Cecato J, Yassuda MS. Screening for Alzheimer's disease among illiterate elderly: Accuracy analysis for multiple instruments. J Alzheimers Dis. 2011;26:221–229.
- O’Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: Recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536.
- Routledge FS, McFetridge-Durdle JA, Dean CR. Stress, menopausal status and nocturnal blood pressure dipping patterns among hypertensive women. Can J Cardiol. 2009;25:e157–e163.
- Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104:783–789.
- Jerrard-Dunne P, Mahmud A, Feely J. Circadian blood pressure variation: Relationship between dipper status and measures of arterial stiffness. J Hypertens. 2007;25:1233–1239.
- Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, . Reproducibility of nocturnal blood pressure fall in early phases of untreated essential hypertension: A prospective observational study. J Hum Hypertens. 2004;18: 503–509.
- Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha-adrenergic blocker, doxazosin: Results from results from the HALT study. Hypertension. 2000;35: 787–794.
- Moniwa N, Varagic J, Ahmad S, Voncannon JL, Ferrario CM. Restoration of the blood pressure circadian rhythm by direct renin inhibition and blockade of angiotensin II receptors in mRen2.Lewis hypertensive rats. Ther Adv Cardiovasc Dis. 2012;6:15–29.
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599.
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495.