Abstract
The results of existing controlled clinical trials were synthesized to determine effects of aerobic exercise training on resting systolic (SBP) and diastolic blood pressure (DBP) among previously sedentary older adults, to quantify the magnitude of observed changes, and to examine the influence of the associated interventional variables on these changes. Studies were identified via a systematic computer database search, hand searching, and cross-referencing of previously located articles. All potentially eligible articles were carefully reviewed and examined with the established inclusion criteria. Twenty-three studies, representing a total of 1226 older subjects, were included in the final analysis. Robust statistically significant effects were found in terms of the pooled standardized effect size of − 0.33 ± 0.06 (p < 0.0001) in SBP and − 0.39 ± 0.09 (p < 0.0001) in DBP. When compared with the control group, net decreases in both SBP (− 5.39 ± 1.21 mmHg, p < 0.0001) and DBP (−3.68 ± 0.83 mmHg, p < 0.0001) were observed in older exercisers, representing a 3.9% and a 4.5% reduction, respectively. This meta-analytic study provides robust quantitative data to support the efficacy and effectiveness of controlled endurance exercise training in decreasing resting SBP and DBP among previously sedentary older adults.
Introduction
The resting blood pressure (RBP) increases progressively with advancing age. This increase probably reflects stiffening of the blood vessels and augmented vascular resistance (Citation1). These age-related RBP changes can result in health problems especially for normotensive individuals who have a 90% lifetime risk for developing hypertension after age 55 or older (Citation2). The risk of cardiovascular disease beginning at 115/75 mmHg doubles with each increment of 20 mmHg in systolic blood pressure (SBP) or 10 mmHg in diastolic blood pressure (DBP) (Citation3). The relative risks, which contribute to developing and ultimately dying from many chronic diseases such as cardiovascular disease and hypertension, increase with advancing age (Citation4,Citation5). Unfortunately, the prevalence of hypertension increases with advancing age even further, to the point where more than half of people 60–69 years of age and approximately three-fourths of those 70 years of age and older are affected (Citation6). This particular population is also the one with the lowest rates of the elevated blood pressure control (Citation7) unless broad and effective preventive measures are implemented.
Physical inactivity appears to play a role in the RBP increase and deterioration of blood vessels; on the contrary, regular aerobic exercise reduces RBP in both normotensive and hypertensive individuals (Citation4,Citation8). Discrepant data regarding the specific quantity and quality of aerobic exercise training and RBP changes, however, have been presented in published studies (Citation8). The results on the exercise-induced change of both resting SBP and/or DBP from existing controlled clinical trials and meta-analytic studies vary significantly; and data in those studies demonstrating positive RBP reductions consequent to aerobic exercise have been presented with a greater variation of the magnitude in such changes (Citation8–12). This is the case particularly for older adults. Randomized controlled trials have led to conflicting or inconclusive results regarding the effects of chronic aerobic exercise on resting SBP and RBP in normotensive and hypertensive adults 50 years of age and older (Citation8). Specific data with regard to the direction and magnitude of RBP changes among older adults in response to controlled aerobic training remain to be further evaluated and established. Furthermore, RBP response differences among the individual studies could be incompletely explained by the characteristics of the studies and interventional exercise programs. Importantly, the study design and quality, including sample size and participants’ age ranges in the studies, are other factors that likely contribute to the controversy and thus preclude drawing conclusions concerning these issues.
As a result, these disagreements and controversies have stimulated a research need regarding controlled chronic aerobic exercise and responding RBP changes in older adults. Accordingly, the primary purpose of the present study was to determine the effects of controlled aerobic exercise on RBP among previously sedentary older adults, to quantify the magnitude of observed changes, and to examine the influence of relevant variables on the RBP changes. To address this aim, we applied a meta-analytic approach to synthesize the results of controlled clinical trials. Meta-analysis may increase statistical power for primary end points and for subgroups, resolve uncertainty when reports disagree, improve estimates of effect size, answer questions not posed by the authors of individual trials, and highlight and qualify areas of weakness in the original literature (Citation13). We reason that the synthesized results of a large population approach may provide new, broad and deeper insight into these controversial issues relevant to RBP response to aerobic exercise in the older population.
Methods
Data search and identification
A standard literature search was performed following outlines and recommendations for conducting meta-analysis (Citation13–15). We searched through electronic databases including MEDLINE-PubMed, SPORTDiscus, PsycINFO, HealthSTAR, EMBASE and Dissertation Online. A specialized reference librarian and an expert on exercise-induced physiological changes in aging were consulted. A broad rather than a more specific search was applied to identify relevant articles. The search strategy included using keywords either alone or in various combinations. Extensive manual searches were conducted. Cross-referencing was performed from the bibliographies of published reviews and included articles.
Study selection
This meta-analysis included randomized and non-randomized controlled trials with a comparative placebo or non-intervention control group, in which aerobic exercise and/or conditioning was the only exercise intervention or treatment. Exercise training was ≥ 2 weeks and, the frequency, intensity, and duration of the training regimen were reported in quantifiable terms. The included studies were published and/or indexed as journal articles after 1980 in English-language, in which older subjects (mean age ≥ 60 years) were previously sedentary and a reported measure of RBP changes could be calculated. An intervention with a mixed treatment was not included. Relevant abstracts from publications, conference proceedings or dissertations were checked but excluded. All included studies were reassessed to ensure independency of each study's data.
Data abstraction and quality assessment
An abstraction form was used to extract data during the coding process. The coded variables included study design, subject characteristics, RBP measurement characteristics, exercise characteristics and primary outcomes. The calculation of multiple study treatment effects was adopted. If a study contained more than one experimental group, then each group mean was calculated independently and treated as a single data point. Data were independently abstracted by researchers and discrepancies were resolved by re-reviewing, double-checking and discussing the relevant information. Alterations were also made based on the results of reliability checks.
The publication bias was explored by plotting net changes in RBP against sample size for each study group (Citation16). The Q-statistic was used to examine homogeneity of results for the standardized effect sizes (Citation17,Citation18). The I2 tests were incorporated to assess inconsistency across the trial results (Citation19). Study quality was assessed using a validated and reliable questionnaire that a 5-point scale ranges from a low of 0 to a high of 5 with the higher score representing higher quality (Citation20).
Statistical analysis
The standardized effect size was calculated according to standard procedures (Citation18). Computations and statistics related to effect sizes were undertaken using Comprehensive Meta Analysis (Biostat Inc, USA). Net changes in RBP outcomes were calculated as the difference (exercise minus control) of the changes (pre- minus post-intervention) in these mean values. Pooled estimates of effect sizes were calculated by using both fixed-effect and random-effect models (Citation17,Citation18). Ninety-five percent confidence intervals were calculated for both individual effect sizes and pooled results. Subgroup analyses of overall net changes and combined effect sizes in resting SBP and DBP were performed according to study design, subject physical characteristics and exercise training characteristics. Specifically, these analyses included: study year, country, randomization, within-study mean age of subjects, subject age span or range within the sample, sample size of study, initial RBP status, and exercise regiments. Insufficient data were available to perform the analyses of SBP and DBP outcomes for gender, ethnicity, health status, and medication. The SPSS (SPSS Inc, USA) was used for all other statistical analyses. A p-value of ≤ 0.05 was considered significant.
Results
Study selection and sample size
After a trial flow of assessment for exclusion, twenty-five studies were identified that met the initial inclusion criteria. Two studies were subsequently excluded, because of missing outcome data or including other treatments. Thus, 23 studies (Citation9,Citation10,Citation21–41) were included in the final analysis for assessment. These studies generated a total of 1226 subjects in 26 exercise groups and 23 control groups with 765 and 461 subjects, respectively.
Study characteristics and quality
The included studies were published in 15 different peer-reviewed journals. Approximately 87% of the primary authors worked in academia and 13% in clinical settings. Fifteen studies were implemented in the USA. About 83% of the investigations were conducted in universities or colleges. Approximately 74% of the studies reported written informed consent from subjects. Twenty-two studies reported the subject adherence, ranged from 63% to 100% in the exercise groups and from 69% to 100% in the control groups. The average number of subjects who completed each study ranged from 10 to 166 in exercisers and six to 81 in the controls. No significant differences were observed between the exercise and control groups in relation to the number of subjects and percentage of dropouts. Fourteen studies were randomized trials (61%) while the other nine were non-randomized (39%). Twelve studies reported no treatment to control groups, seven used matching procedures, three provided wait list, two indicated as attention or other, while six did not report the control status. Only one study applied an intention-to-treat approach to analyze data. All others appeared to use a per-protocol approach. Study quality analysis ranged from 1 to 3 (mean ± SD = 2 ± 1).
Subject description
Approximately 87% of the studies did not report race/ethnicity of the subjects. Subjects in eight studies were female while only one study was limited to males. For the interested outcomes, 61% of the studies presented mixed-gender results. Most of the studies (91%) provided information on co- morbidities with reporting all subjects healthy. Some subjects in four studies were reported having elevated RBP, but our subgroup analyses did not find statistically significant differences in exercise induced RBP changes when the data were partitioned according to hypertensive and normotensive status. About half of the studies reported that none of the subjects was taking medication during the study, 7% reported some subjects taking medication, and 43% did not specify the medication status. Initial physical characteristics of the subjects are described in . Initial resting SBP ranged from 119 to 153 mmHg (mean ± SD = 135.83 ± 10.63 mmHg) in the control groups and from 120 to 161 mmHg (mean ± SD = 137.11 ± 11.90 mmHg) in the exercise groups. For resting DBP, initial values in the controls ranged from 74 to 97 mmHg (mean ± SD = 81.02 ± 5.80 mmHg), and in the exercisers from 72 to 100 mmHg (mean ± SD = 81.67 ± 7.06 mmHg). Neither initial resting BP nor other variables were found to be statistically different between the two groups.
Table I. Initial physical characteristics of subjects.
Description of exercise intervention
Mode. About 89% of the exercise groups used walking and/or jogging as the primary training modality. Many exercise programs included one or more of the following: walking, jogging, running, cycling, stair-climbing, aerobic dance, outdoor aerobic performance, and aerobic games. Intensity. Exercise intensity expressed as %HRmax, %VO2max, %VO2R or %HRR, maximal heart rate per minute, etc., with the use of %HRmax, %VO2max, and %HRR as the most common. Those common intensities varied ranging at 50–90% HRmax, 40–80% VO2max or 30% − 85% HRR. Duration. Time of each training session ranged from 19 to 60 min. The mean duration for most groups lasted 30–50 min. Frequency. Most programs trained subjects approximately 3 days/week and the mean frequency ranged from 1 to 5.2 times per week. Time. Length of exercise intervention varied from 8 to 42 weeks, with more than the half lasting between 20 and 30 weeks. Others. The training sessions were supervised in 13 studies. Three studies were outpatient and/or self-reported. The remaining seven studies did not report such information. The primary methods for intensity monitoring included one or more of the following: subjects self-palpation of radial or carotid pulses, monitored by heart rate device, RPE, ECG, and by investigators and/or study staff. The majority of studies used heart rate measurements to monitor exercise intensity levels.
Synthesized outcomes and subgroup analysis
shows results of pre- and post-intervention RBP for each group. The net changes in resting SBP and DBP was statistically significant (p < 0.0001) and represented a net decrease of −5.39 ± 1.21 mmHg (mean ± SEM, 95% CI = − 7.82 to −2.95) in SBP and − 3.68 ± 0.83 mmHg (mean ± SEM, 95% CI = −5.35 to − 2.00) in DBP. These changes were equivalent to reductions of approximately 3.9% in SBP and 4.5% in DBP.
Table II. Resting systolic and diastolic blood pressure (mmHg) results from the included studies.
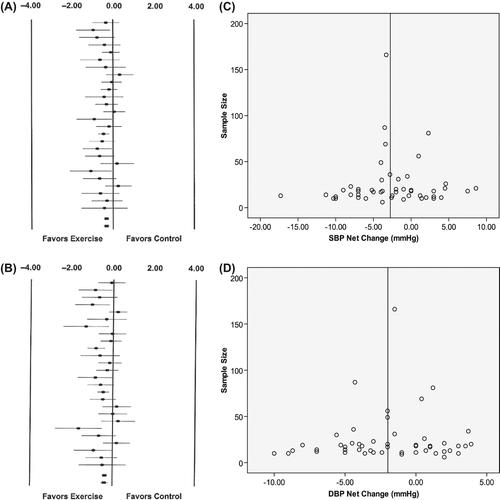
Results of the individual and combined effect sizes in resting SBP and DBP are presented in (A and B). Across all designs and categories, the pooled effect sizes from both models were very close to unity, and the two effects for the net changes of SBP and DBP reductions were statistically significant (both p < 0.0001). There was an average effect size of − 0.33 ± 0.06 (mean ± SEM, 95% CI = − 0.45 to −0.21) from a fixed-model in SBP, with no statistically significant heterogeneity observed (Q = 28.39, p = 0.290). The pooled effect size of DBP was −0.39 ± 0.09 (mean ± SEM, 95% CI = −0.56 to −0.23) by a random-model, as a significant heterogeneity was found (Q = 44.65, p = 0.009). I2 was 11.9% for SBP, presenting little variability between studies. The I2 value for DBP was 44.0%, indicating inconsistency is moderate. Visual inspection of the funnel plots in (C and D) shows that net changes in SBP and DBP are symmetrical around the weighted mean net changes line, suggesting absence of publication bias.
Figure 1. (A) and (B) present Ladder Plots of standardized effect sizes for changes in resting systolic blood pressure (SBP) and in resting diastolic blood pressure (DBP). The black circles represent standardized effect size of the change outcome for each group; the bars that pass through the circles represent the 95% confidence intervals (CIs) for each standardized effect size of the change outcome. The rectangular symbols represent the pooled effect sizes of the mean change outcomes by fixed or random models for resting blood pressure (BP); the bars that pass through the rectangle represent the 95% confidence intervals (CIs) of the pooled effect sizes of the mean change outcome. (C) and (D) present Funnel Plots of net changes in SBP (C) and DBP (D) vs study group sample size. The vertical line represents the mean BP change, weighted for the number of participants in the trials.
Statistically significant differences were found when subgroup analyses were performed for effect size and net changes in resting SBP and DBP. Smaller sample size trials (< 30 subjects) had a statistically significant larger reduction and/or effect size in SBP and DBP than larger sample size (> 30 subjects): the SBP mean net change was − 8.02 ± 4.82 mmHg vs −3.13 ± 5.25 mmHg (p = 0.021) and the SBP effect size was −0.57 ± 0.37 vs 0.25 ± 0.32 (p = 0.027); the DBP mean net change was −5.61 ± 3.56 mmHg vs − 2.02 ± 3.21 mmHg (p = 0.012) and the DBP effect size was −0.64 ± 0.53 vs 0.32 ± 0.42 but not significant (p = 0.10). A statistically significant greater effect size in resting SBP was detected in the subjects’ age span more than 15 years. For resting DBP, a greater and statistically significant effect size was revealed when the mean age of subjects was older than 70 years, as well as when training duration was less than approximately 45 minutes. No other statistically significant associations were found for effect size and net changes in RBP and selected variables, such as study year, randomization, study country, and initial RBP status.
Discussion
The main findings of the current meta-analysis of controlled clinical trials on chronic aerobic exercise training and RBP effects are: (i) that training significantly reduces both resting SBP and DBP in sedentary older adults, and the revealed evidence was derived from a large sample size consisting of 1226 older individuals with a narrow mean age range (68 ± 6.6 years); (ii) that effects of this training-induced reduction are robust and statistically significant: when compared with the control groups, the net change of approximately −5.4 mmHg in resting SBP and − 3.7 mmHg in resting DBP could be expected, representing 3.9% and 4.5% reductions, respectively; (iii) that a clinical trial with a small sample size would elicit a large but probably less accurate magnitude of the RBP reduction; (iv) that a spread age span or range and/or a greater mean age of subjects in an investigation would result in a larger but possibly exaggerated RBP decrease; (v) that training regimens for the attainment of the RBP reduction in most of the included studies show an aerobic exercise of 30–50 min per session at the intensity between 50–90% HRmax, 40–80% VO2max or 30–85% HRR with ˜3 days/week for about 20–30 weeks.
The outcome of the aerobic exercise-induced resting SBP and DBP net changes, whether it is effective enough or to which extend it can be reduced in older adults, can have important clinical implication and merit in health and medical field. The incidence of hypertension increases in both men and women as a function of age (Citation3). Older individuals are also vulnerable to the development of isolated systolic hypertension, a clinical entity associated with significant increases in cardiovascular disease. With advanced age, the percentage of patients with controlled hypertension declines progressively (Citation42). It has been estimated that for normotensive and hypertensive adults, a 5-mmHg reduction in SBP reduces mortality rates of 4% and 9% from coronary heart disease, 6% and 14% from strokes, and 3% and 7% from all causes, respectively (Citation43). A reduction of 5 mmHg in DBP has also been associated with a 34% decrease in stroke and a 21% decrease in coronary heart disease for hypertensives (Citation42). The current study provides evidence of the capacity of controlled aerobic exercise to meaningfully influence RBP. From a clinical perspective, these reductions are important for public health, especially for the sedentary older population. In addition, our data may be beneficial to clinical applications for older hypertensive patients to manage RBP by coupling with aerobic exercise training.
Irrespective of age, our findings of exercise- induced RBP reductions are consistent with reports from many published studies (Citation8,Citation11,Citation12,Citation44–48). However, there was a wide age span or range of subjects in these studies, for example, 21–79 years (Citation45), 18–79 years (Citation46), 17–88 years (Citation47) or 18 years and above (Citation48). This greater age variation may be a confounding variable or even problematic when attempting to derive appropriate conclusions about the effectiveness and estimated magnitude of the RBP response to aerobic exercise training for older individuals. Age can be an important variable affecting RBP. It is well documented that both resting SBP and DBP increase with advanced age. In the general population, the prevalence of hypertension increases dramatically with ageing (Citation6,Citation49,Citation50). Age may influence the magnitude of RBP changes following aerobic exercise. The discrepant results about the exercise-induced RBP reductions in older adults have been presented in those published studies. A meta-analysis reported that the overall weighted net decrease was − 3.0 mmHg in SBP and − 2.4 mmHg in DBP among all subjects with mean age ranged from 21 to 83 years (Citation51). Whelton et al. found that the overall pooled net effect of aerobic exercise on SBP and DBP was −3.84 mmHg and −2.58 mmHg, respectively (Citation48). A narrative review by Hagberg et al. revealed a weighted average reduction in SBP and DBP (− 7.6/− 8.8 mmHg) among hypertensive adults aged 60 years and older (Citation11). However, a meta-analysis that included seven randomized trials with subjects’ average age of 50 found net reductions of approximately −2 mmHg (2%) in SBP and −1 mmHg (1%) in DBP, but only the SBP change was statistically significant (Citation12). In our study, the magnitude of aerobic training-induced decreases is approximately − 5.4 mmHg and −3.7 mmHg in resting SBP and DBP, or 3.9% and 4.5% reductions, respectively. When data were partitioned according to subject mean age (< 65 vs > 65 years and < 70 vs > 70 years), younger older group had a smaller reduction in SBP net change and effect size than older group, though it was statistically non-significant. More reductions in DBP net change were observed in older group than in younger older group, with statistically significant larger effect sizes found in subjects with the mean age older than 70 years. In addition, a statistically significant larger effect size was observed in subjects with the age span more than 15 years. These findings imply that age and/or age span or range could be an influenced factor when the researches on older adults assess the effectiveness of aerobic exercise on RBP and determine the magnitude of RBP net changes consequent to such training. Unlike many previous studies, this meta-analysis was specifically focus on older adults. The mean age of 1226 older subjects in the study was relatively narrow, ranging from approximately 61 to 83 years. Reasonably, our results could be more representative of the aerobic training effects on resting SBP and DBP for a specific segment of older population. The presented magnitude of RBP reductions as a result of controlled aerobic exercise may also be considered a valid and closer estimate of the population effect for individuals whose average age is ≥ 60 years. Accordingly, these findings could be beneficial to future prospective research on this area and to professional practice such as exercise prescriptions for older adults to promote their cardiovascular health.
This meta-analysis found that studies with a smaller sample size produce significant larger RBP reductions. In the above mentioned narrative review (Citation11), the larger SBP and DBP decrease was derived from a relatively small sample size with 164 subjects in SBP and 55 in DBP. There is a rule known as the law of larger numbers related to sample size (Citation52). Specifically, a larger sample should be more accurate than a smaller sample. Thus, a larger sample size provides more representative information about the defined population, decreases the error between the sample mean and the population mean, and increases the power of making a more precise estimate. Therefore, our findings can be reasonably considered more representative of accurate information about the effects of aerobic exercise on resting SBP and DBP in older adults, given the fact that the present study included a larger study sample size, larger number of subjects from the selected studies, and a relatively narrow age range of the subjects.
The results of the current study have to be interpreted with caution due to limitations. This meta-analysis synthesized the results of controlled clinical studies from randomized and non-randomized trials. However, no statistically significant differences were detected for the net changes and effect sizes in both SBP and DBP. We did not contact authors of some studies with missing information or data. However, our experience has shown considerable time investment with a low response rate and little, if any, additional information (Citation53). This study was restricted to only studies published in English; and it might introduce language and publication bias (Citation54). To reduce publication bias and improve the quality of future meta-analytic works, the pooling of studies from other countries and languages other than English is warranted. Another limitation is that unpublished studies were not included. Although we conducted a thorough literature search, the possibility of missing other useful sources from unpublished studies may exist. Those studies, however, may be of lower methodological quality than those published in peer-reviewed journals. Importantly, we did search and review dissertations and relevant reports to minimize potential publication bias. Furthermore, as suggested, we examined funnel plots to detect the presence of bias in meta-analysis and the results appeared to show the absence of bias. Although potential sources provide a very limited amount of additional information, future meta-analyses may consider unpublished studies to fully reflect existing evidence.
Our meta-analytic investigation provides some new information to the body of knowledge specifically for previously sedentary older adults ages 61 to 83 in terms of the efficacy and effectiveness of controlled aerobic exercise training to decrease resting SBP and DBP. This training-induced RBP adaptation is clinically important for the older population and has protective benefits to promoting healthy cardiovascular aging, decreasing cardiovascular risk factors, and providing clinical applications to prevent or treat cardiovascular disease. Future prospective clinical studies should include a larger sample size of at least 30 or more subjects. Distinguished baseline blood pressure of subjects (e.g. male or female, hypertensive or normotensive older individuals) needs to be considered. The age span of subjects in future studies is recommended being as narrow as possible. Information of the exercise regimens in most of the included studies is useful to exercise prescriptions but the appropriate level of aerobic training required for RBP health benefit may be different for older men and women and for older individuals in different age segments.
Conflicts of interest
The authors have no conflict of interest to declare.
Disclosure of funding
No funding was received for this work.
References
- Burt VL, Whelton D, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population: Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313.
- Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913.
- American College of Sports Medicine. Position stand: Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530.
- Rigaud AS, Forette B. Hypertension in older adults. J Gerentol Med Sci. 2001;56A:M217–M225.
- The Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [JNC 7]. U.S. Department of Health and Human Services, Institutes of Health, National Heart, Lung, and Blood Institute, and National High Blood Pressure Education Program. NIH Publication No. 04–5230, 2004. p. 8–10.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–486.
- American College of Sports Medicine. Position stand: Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553.
- Braith RW, Pollock ML, Lowenthal DT, Graves JE, Limacher MC. Moderate- and high-intensity exercise lowers blood pressure in normotensive subjects 60–79 years of age. Am J Cardiol. 1994;73:1124–1128.
- Hagberg JM, Montain SJ, Martin WH, Ehsani AA. Effect of exercise training in 60- to 69-year-old persons with essential hypertension. Am J Cardiol. 1989;64:348–353.
- Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension. Sports Med. 2000;30:193–206.
- Kelley GA, Kelley KS. Aerobic exercise and resting blood pressure in older adults: A meta-analysis review of randomized controlled trials. J Gerontol Med Sci. 2001;56A:M298–M303.
- Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analysis of randomized controlled trials. New Engl J Med. 1987;316:450–455.
- Egger M, Smith GD, Phillips AN. Meta-analysis: Principles and procedures. BMJ. 1997;315:1533–1537.
- Glass GV, MeGaw B, Smith ML. Meta-analysis in social research. Beverly Hills, CA: Sage; 1981. p. 57–69.
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- DerSimonian, R., Laird, N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88.
- Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. p. 122–8.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clin Trials. 1996;17:1–12.
- Binder EF, Birge SJ, Kohrt WM. Effects of endurance exercise and hormone replacement therapy on serum lipids in older women. J Am Geriatr Soc. 1996;44:231–236.
- Cononie CC, Graves JE, Pollock ML, Phillips MI, Sumners C, Hagberg JM. Effect of exercise training on blood pressure in 70- to 79-yr-old men and women. Med Sci Sports Exerc. 1991;23:505–511.
- Gillett PA, White AT, Caaserta MS. Effect of exercise and/or fitness education on fitness in older, sedentary, obese women. J Aging Phys Act. 1996;4:42–55.
- Hamdorf PA, Penhall RK. Walking with its training effects on the fitness and activity patterns zofz. Aust NZ J Med. 1999;29:22–28.
- Hamdorf PA, Withers RT, Penhall RK, Haslam MV. Physical training effects on the fitness and habitual activity patterns of elderly women. Arch Phys Med Rehabil. 1992;73:603–608.
- Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. J Gerontol. 1993;48:P12–17.
- Jessup JV, Lowenthal DT, Pollock ML, Turner T. The effects of endurance exercise training on ambulatory blood pressure in normotensive older adults. Geriatr Nephrol Urol. 1998; 8:103–109.
- Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–1921.
- Kohrt WM, Obert KA, Holloszy JO. Exercise training improves fat distribution patterns in 60- to 70-year-old men and women. J Gerontol. 1992;47:M99–105.
- Motoyama M, Sunami Y, Kinoshita F, Kiyonaga A, Tanaka H, Shindo M, et al. Blood pressure lowering effect of low intensity aerobic training in elderly hypertensive patients. Med Sci Sports Exerc. 1998;30:818–823.
- Okumiya K, Matsubayashi K, Wada T, Kimura S, Doi Y, Ozawa T.. Effects of exercise on neurobehavioral function in community-dwelling older people more than 75 years of age. J Am Geriatr Soc. 1996;44:569–572.
- Posner, J.D., Gorman, K.M., Windsor-Landsberg, Larsen J, Bleiman M, Shaw C, et al. Low to moderate intensity endurance training in healthy older adults: Physiological responses after four months. J Am Geriatr Soc. 1996;40:1–7.
- Puggaard L, Larsen JB, Stovring H, Jeune B. Maximal oxygen uptake, muscle strength and walking speed in 85-year-old women: Effects of increased physical activity. Aging (Milano). 2000;12:180–189.
- Ready AE, Naimark B, Ducas J, Sawatzky JV, Boreskie SL, Drinkwater DT, et al. Influence of walking volume on health benefits in women post-menopause. Med Sci Sports Exerc. 1996;28:1097–1105.
- Seals DR, Hurley BF, Hagberg JM, Schultz J, Linder BJ, Natter L, et al. Effects of training on systolic time intervals at rest and during isometric exercise in men and women 61 to 64. years old. Am J Cardiol. 1985;55:797–800.
- Seals DR, Reiling MJ. Effect of regular exercise on 24-hour arterial pressure in older hypertensive humans. Hypertension. 1991;18:583–592.
- Shin Y-H. The effects of a walking exercise program on physical function and emotional state of elderly Korean women. Public Health Nurs. 1999;16:146–154.
- Sunami Y, Motoyama M, Kinoshita F, Mizooka Y, Sueta K, Matsunaga A, et al. Effects of low-intensity aerobic training on the high-density lipoprotein cholesterol concentration in healthy elderly subjects. Metabolism. 1999;48:984–988.
- Tanaka H, Reiling MJ, Seals DR. Regular walking increases peak limb vasodilatory capacity of older hypertensive humans: Implications for arterial structure. J Hypertens. 1998;16:423–428.
- Whitehurst M, Menendez E. Endurance training in older women: Lipid and lipoprotein responses. Phys Sportsmed. 1991;19:95–98.
- Wood RH, Reyes R, Welsch MA, Favaloro-Sabatier J, Sabatier M, Matthew Lee C, et al. Concurrent cardiovascular and resistance training in healthy older adults. Med Sci Sports Exerc. 2001;33:1715–1758.
- Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA. 2005;294:466–472.
- Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14:570–577.
- Fagard RH, Tipton CM. Physical activity, fitness, and hypertension. In: Bouchard C, Shephard R., Stephens T, editors. Physical activity, fitness, and health. Champaign, IL: Human Kinetics; 1994. p. 633–655.
- Fagard RH. Physical activity in the prevention and treatment of hypertension in the obese. Med Sci Sports Exerc. 1999;11:S624–S630.
- Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA, Andrews GR. The effectiveness of exercise training in lowering blood pressure: A meta-analysis of randomized controlled trials of 4 weeks or longer. J Hum Hypertens. 1997;11: 641–649.
- Kelly G, Tran ZV. Aerobic exercise and normotensive adults: A meta-analysis. Med Sci Sports Exerc. 1995;27:1371–1377.
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503.
- National High Blood Pressure Education Program Working Group. National high blood pressure education program working group report on hypertension in the elderly. Hypertension. 1994;23:275–285.
- Schoenberger JA. Epidemiology of systolic and diastolic systemic blood pressure elevated in the elderly. Am J Cardiol. 1986;57:45C–51C.
- Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675.
- Gravetter FJ, Wallnau LB. Statistics for the behavioral sciences. 5th ed. Wadsworth/Thomson Learning; 2000. p. 219–276.
- Gibson CA, Bailey BW, Carper MJ, Lecheminant JD, Kirk EP, Huang G, et al. Author contacts for retrieval of data for a meta-analysis on exercise and diet restriction. Int J Technol Assess Health Care. 2006;22:267–270.
- Moher, D, Fortin, P, Jadad, AR, Jüni P, Klassen T, Le Lorier J, et al. Completeness of reporting of trials published in languages other than English: Implications for conduct and reporting of systematic reviews. Lancet. 1996;347:363–366.
