Abstract
Blood pressure (BP) is characterized by marked fluctuations occurring within the 24 h as a result of complex interactions between behavioral, environmental, humoral, and neural central or reflex influences. Significant BP variations also occur over more prolonged periods of time (i.e. between days, weeks, months, seasons and even years), not as a random phenomenon but as a result of several interacting factors yet not completely identified. Depending on the method and time interval considered for measurement, the clinical significance and prognostic implications of different types of BP variability (BPV) may substantially differ. Either in the short or in the long term, BPV has been associated with development, progression and severity of cardiac, vascular and renal organ damage and with an increased risk of cardiovascular events and mortality, independently adding to cardiovascular risk, over and above the contribution of elevated mean BP levels. The present paper provides a review on the main methods currently employed for assessment of BPV as well as on the mechanisms, clinical interpretation and prognostic significance of different types of BPV, addressing the question on whether BPV should be a target for antihypertensive treatment for the current prevention of cardiovascular disease.
Introduction
Blood pressure (BP) is characterized by significant short-term fluctuations occurring within 24 h (i.e. from beat-to-beat, min-to-min, hour-to-hour and from day-to-night) as a result of complex interactions between behavioral, environmental, humoral, and neural central or reflex influences. Important BP variations have also been shown to occur over more prolonged periods of time (i.e. between days, weeks, months, seasons and even years), which appear to be not a random phenomenon (Citation1) but rather the result of several interacting factors and mechanisms yet not completely identified. Although BP variability (BPV) is often used as a generic term, its clinical and physiological significance may change depending on the time interval considered and the method employed for its measurement. Although introduced more than 30 years ago, the concept of BPV has gained interest in recent years on the background of the evidence provided by observational studies and post hoc analyses of clinical trials indicating that the adverse cardiovascular (CV) consequences of high BP may not only depend on absolute BP values but also on BPV. When assessed either in the short or in the long term and independently of mean BP levels, an increasing BPV has been shown to be associated with development, progression and severity of cardiac, vascular and renal organ damage and with an increased risk of CV events and mortality. Recently, post hoc analyses of large intervention trials in hypertension have shown visit-to-visit BPV to bear a strong prognostic value for CV morbidity, further motivating the discussion on whether antihypertensive treatment should be targeted to stabilize BPV in addition to control of mean BP values in order to achieve the highest CV protection. The purpose of the present paper is to provide a review on the main methods currently employed for assessment of different types of BPV as well as its mechanisms and determinants. Emphasis is given to the clinical interpretation and prognostic relevance of different types of BPV, addressing the question of whether BPV should be a target for antihypertensive treatment for the current prevention of CV disease.
Assessment of BPV
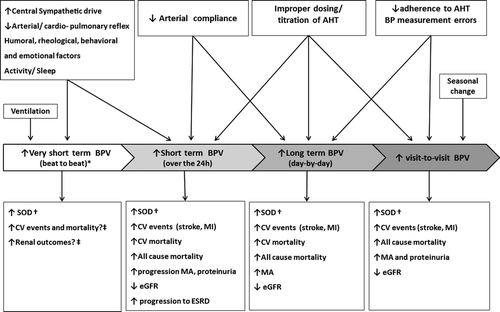
Measures of BPV can be obtained through different methods, i.e. continuous beat-to-beat BP recordings, repeated conventional office BP (OBP) measures, 24-h ambulatory BP monitoring (ABPM) or home BP monitoring (HBPM) over longer time windows. BP fluctuations can also be assessed over different time intervals i.e. in the very short term (beat-by-beat), in the short term (within the 24-h period) or in the long term (day-by-day, visit-to-visit or between seasons). Thus, depending on the time interval and the method employed for its measurement, the clinical significance and prognostic implications of a measure of BPV may substantially differ as well as the mechanisms and determinants influencing each type of BPV(Citation2) ().
Figure 1. Different types of blood pressure variability (BPV), their determinants and prognostic relevance for cardiovascular (CV) and renal outcomes. AHT, antihypertensive treatment; BP, blood pressure; SOD, subclinical organ damage; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; MA, microalbuminuria; MI, myocardial infarction. *Assessed in laboratory conditions; †cardiac, vascular and renal SOD; ‡BPV on a beat-by-beat basis has not been routinely measured in population studies. Taken from reference (Citation2) by permission.
Assessment of BPV in the very short term from beat-to-beat BP recordings
A proper analysis of the fast and short-lasting changes in BP, in particular when focusing on the different frequency components of BP spectra contributing to overall BPV, requires a continuous recording of BP on a beat-by-beat basis (Citation3). For years, the so called intra-arterial Oxford method (Citation4) was the only available method for continuous ambulatory beat-to-beat BP monitoring over the 24 h, and it was by using this technique that different components of BPV could first be identified. However, the convergence of escalating concerns over its invasive nature and methodological difficulties led to the development of the vascular unloading technique (Citation5), which paved the way to ensemble non-invasive devices for continuous BP measurement, based on the Penaz method (Citation6). Through the use of finger cuffs equipped with an infrared photoplethysmograph and sophisticated technology for quantification of finger BP, these devices allow measuring BP levels in a beat-to-beat basis, thus giving the possibility of tracking fast variations in BP values either spontaneously occurring or during stimulated conditions in the laboratory setting (Citation7). Analysis of BPV from continuous recordings not only allows assessment of BPV in terms of its standard deviation (SD), but it also offers the possibility to separately assess its oscillatory components characterized by a different oscillation frequency, through the application of frequency domain analysis (Citation8). It is thus possible to estimate variance or “power” of BP in the high frequency (HF, 0.15–0.5 Hz), low frequency (LF, 0.05–0.15 Hz) and very low frequency (VLF, 0.025–0.05 Hz) regions of BP spectrum, allowing a more in-depth analysis of the mechanisms of autonomic CV modulation underlying overall BP variation as well as the autonomic adjustments in response to antihypertensive therapy (Citation8).
Assessment of short-term BPV within the 24 h with ABPM
It was through the use of intra-arterial BP monitoring in ambulant subjects that the dynamic behavior of BP values over the 24-h period was first shown (Citation9–11). These recordings allowed identification of both beat-by-beat and day–night BP variations (Citation9). Although an accurate assessment of short-term BPV within the 24 h requires continuous BP recordings, it is also possible (although less precisely) through the use of intermittent, non-invasive 24-h ABPM. From these recordings, it is possible to perform the calculation of SD of average systolic, diastolic, and mean arterial pressure values over the 24-h period, or during the daytime and night-time sub-periods (Citation12). More recently, the calculation of the “weighted” SD of the 24-h mean value (i.e. the average of daytime and night-time BP SD, each weighted for the duration of the day and night periods, respectively) has been proposed in order to exclude day–night BP changes from the quantification of overall 24 h SD (Citation13). Other measures of BPV are the calculation of the “residual BPV” remaining after exclusion of the slower components of the 24-h BP profile through spectral analysis (Citation14); and the average of the absolute differences between consecutive measurements (“average real variability”) (Citation15). These parameters, which focus on short-term BP changes and are not affected by the dipping phenomenon, have been shown to be better predictors of organ damage and CV risk than the conventional 24-h SD (Citation13,Citation15,Citation16).
Assessment of long-term BPV
Recent meta-analyses and post hoc interpretations of clinical trials on antihypertensive treatment have shown the clinical relevance of visit-to-visit BPV either in OBP or in average 24-h ambulatory BP (ABP) values, especially in predicting cerebrovascular events (Citation17). However, in the clinical setting, obtaining BP measurements over a consistent number of visits to achieve a meaningful estimate of visit-to-visit BPV is usually difficult. Moreover, OBP readings obtained in the clinic may not provide information on BP during subjects’ usual activities and over a long period, being thus unable to provide a representative measure of patients’ actual BP burden. OBP is thus an imperfect indicator of BP control and is far from being an ideal means to assess visit-to-visit BPV. ABPM provides extensive information on BP levels within the 24 h but it cannot be repeated frequently, and thus it cannot be routinely applied to assess “visit-to-visit BPV”. Although measures performed by patients at home using HBPM may not provide information on 24-h BP profiles, they seem to be an appropriate alternative approach for the assessment of long-term BPV. HBPM allows obtaining day-by-day BP measures in a relatively short period (several days), in fairly standardized conditions (treatment regimen remains stable and significant physiological changes are unlikely to occur) and without the influence of subject's activity (Citation18). Thus, HBPM appears more appropriate for the long-term assessment of BPV and BP control than repeated OBP or ABP measurements.
Mechanisms and determinants of BPV in the short and in the very short term
The results of several studies trying to disentangle the precise contribution of humoral, neural and environmental factors to BPV have indicated that these factors are often inextricably woven together. Separating out these factors makes scientific sense but can be pointless in the clinical setting. BP variations in the very short and in the short term (i.e. within the 24 h) mainly reflect the influences of central and reflex autonomic modulation (i.e. an increased central sympathetic drive and reduced arterial and cardio-pulmonary reflexes) (Citation8,Citation19,Citation20); elastic properties of arteries (i.e. a reduced arterial compliance) (Citation21–23); and the effects of humoral (insulin, angiotensin II, bradykinin, endothelin-1, nitric oxide), rheological (i.e. blood viscosity) and emotional factors of diverse nature and duration (i.e. psychological stress). Although behavioral influences (i.e. physical activity, sleep, postural changes) may induce marked variations in BP over the 24 h, spontaneous and rhythmic BP fluctuations at different frequencies also occur independently on behavior throughout the day and night, presumably because of influences originating in the central nervous system (Citation24). In addition, BP fluctuations also occur in response to the mechanical forces generated by ventilation. Finally, BP variations caused by neural or non-neural influences, are opposed throughout the 24 h by arterial and cardio-pulmonary reflexes, whose reduced efficacy may thus result in an increased BPV. BP variations occurring within the 24 h also include slower BP fluctuations occurring between day and night, which are significantly influenced both by the subject's level of activity during daytime and by the sleep/wakefulness cycle. In the general population, BP falls on average by 10–20% of daytime values during sleep, a phenomenon referred to as dipping. However in some individuals nocturnal decrease in BP is blunted (non-dippers, with a fall in night-time systolic and diastolic BP during night-time < 10% of daytime BP) or even increases (so called risers or “inverted dippers”). Dippers exhibiting night-time BP fall > 20% are known as extreme dippers (Citation25). Remarkably, non-dipping profile of BP is frequently accompanied by increased nocturnal mean BP levels (i.e. night-time BP > 125/75 mmHg) (Citation25). Proposed mechanisms for non-dipping pattern of BP and nocturnal hypertension include an increased sympathetic activity during night-time (Citation26), a decreased renal sodium excretory ability (Citation27), salt sensitivity (Citation28), altered breathing patterns during sleep (i.e. obstructive sleep apnea), leptin and insulin resistance (Citation29), endothelial dysfunction and glucocorticoid use.
Mechanisms and determinants of long-term BPV
Information on the factors involved in long-term BP variations is still limited and incomplete. Behavioral changes are considered to exert a major role on day-to-day BP variations, as indicated by the significant changes observed in ambulatory BP values between working days and the weekend (Citation30). Although long-term BPV (i.e. day-by-day, visit-to-visit or seasonal BP variations) has shown to be a reproducible and not a random phenomenon (Citation1), little is known, on the other hand, about the factors responsible for the BP difference that has been observed between visits spaced by months or years in observational studies and antihypertensive drug trials (Citation17,Citation31,Citation32). It might not entirely consist of spontaneous BP variations, or reflect the same physiological CV control mechanisms of short-term BP fluctuations, but it may also be the result of imperfect stability of BP control in treated subjects (in particular visit-to-visit BP variations during follow-up) or reflect the inconstant accuracy of OBP readings () (Citation33).
Thus, factors influencing the degree of BP control (i.e. patient's adherence to treatment and proper dosing/titration of antihypertensive treatment) or errors in BP measurement may in due course influence day-by-day but specially visit-to-visit BPV. In particular, a poor patients’ compliance with the prescribed therapeutic regimen may influence long-term BPV as dose omission or delay in drug intake during the follow-up period may also contribute to an increased day-by-day and visit-to-visit BPV. Finally, long-term BPV has been reported to occur as a consequence of seasonal climatic changes. Either when considering OBP values, the average of self-BP measurements performed by subjects at home or the mean of 24-h BP values collected by ABPM, systolic and diastolic BP levels have been reported to be lower during summer and higher during winter (Citation34) mainly as a result of changes in outdoor temperature (Citation35). In addition, it has also been reported that in treated hypertensives an improper downward titration of antihypertensive drugs performed on the basis of variations of clinic BP during summer time may reduce the extension of 24-h BP coverage, and contribute to the increase in night-time BP levels reported during hot weather in some studies (Citation35).
Prognostic significance of very short-term and short-term BPV (assessed from 24-h ambulatory beat-to-beat BP recordings)
Early studies using intra-arterial ambulatory BP recordings over the 24 h, showed that BPV (quantified as the SD of the 24 h, day, and night mean values) increases from normotensive to hypertensive subjects, the increase in BP SD being proportional to the increase in mean BP, with, no change in the coefficient of variation, i.e. the SD divided by mean BP levels and multiplied by 100 (Citation9). The few studies in hypertension implementing intra-arterial beat-to-beat BP recordings have shown that an increase in short-term BPV is directly related to severity of target-organ damage in hypertension (Citation10,Citation36). Indeed, for nearly any level of 24-h mean BP, subjects in whom the 24-h BP variability was low had a lower prevalence and severity of target organ damage than those in whom the 24-h BP variability was high. Interestingly, after 7.4 years of follow-up, BPV at the initial evaluation was a significant predictor of target-organ damage indicating that the CV complications of hypertension might depend on the degree of 24-h BPV (Citation36). It should be mentioned, however, that measurement of BPV on a beat-by-beat basis has not been routinely implemented in population studies, thus preventing the determination of the prognostic role of very short-term BP variability, including its different frequency components, in particular for CV mortality.
Prognostic significance of short-term BPV (assessed from 24-h ABPM)
Although most evidence has confirmed the prevailing prognostic value of mean BP levels over BPV, evidence from longitudinal and observational studies has also indicated that short-term BPV within the 24 h may have a non-marginal contribution to CV risk. Either by using intra-arterial or non-invasive BP monitoring, several studies have shown that cardiac, vascular and renal organ damage for a given 24-h BP mean value is more prevalent and severe as 24-h BPV increases (Citation37–41). Most importantly, prospective studies have provided evidence that initial increasing values of BPV within the 24 h are independent predictors of progression of structural cardiac and vascular alterations (i.e. increased left ventricular mass index or carotid-intima media thickness) (Citation42) and of CV events (Citation14,Citation16,Citation42–48), and CV mortality (Citation14,Citation16,Citation48,Citation49). Overall, this evidence supports the concept that the adverse CV consequences of high BP not only depend on absolute BP values but also on BPV, which may independently add to CV risk, over and above the contribution of elevated mean BP levels.
Several studies have also assessed the prognostic relevance of nocturnal BP levels and reduced night-time BP dipping. When considering general populations or hypertensive patients only, observational and longitudinal studies have demonstrated elevated nocturnal BP to be prognostically superior to awake or 24-h BP means in predicting CV morbidity and mortality (Citation50–55), the development of CV events (Citation50,Citation51,Citation56–58) and overall mortality (Citation50–52, Citation57,Citation59,Citation60). This is not surprising given the fact that a patient's nocturnal BP level, without the pressor effects of physical activity, emotional stress and other environmental factors that are usually occurring during the day, may be more reproducibly representative of patient's true BP status. Not only the prognostic role of night-time BP levels, but also the relevance of a “non-dipping” pattern of BP has been explored in several studies. Subjects in whom nocturnal decrease in BP is blunted have been reported to have a higher prevalence of subclinical organ damage (Citation42,Citation61) and an increased risk of CV events (Citation62) and mortality (Citation55), which is even higher in patients in whom BP increases rather than decreases at night (so called risers or “inverted dippers”).
Prognostic relevance of day-by-day BPV
Although most studies on the prognostic relevance of BPV have focused on short-term BP changes assessed from 24-h ABPM, recent evidence has indicated that also an increased day-by-day BPV identified by HBPM is significantly associated with the prevalence and severity of cardiac, vascular and renal organ damage (Citation63) and may be an independent predictor of CV events (Citation64,Citation65). A recent cross-sectional analysis in a population of never-treated hypertensives showed an increased day-by-day HBP variability to be associated with the severity of cardiac (i.e. left ventricular mass index), macrovascular (i.e. increased carotid intima-media thickness) and microvascular (i.e. urinary albumin/creatinine ratio) organ damage (Citation63). In the Ohasama study, an increased day-by-day systolic HBP variability was indeed associated with an increased risk of a composite of cardiac and stroke mortality. However, when these conditions were separately considered, HBP variability remained a significant predictor of stroke mortality but not of cardiac mortality (Citation64). More recently, evidence on the prognostic value of day-by-day HBP variability was provided in a cohort of adults from the general population in the frame of the Finn-Home Study (Citation65). After 7.8 years of follow-up, increasing levels of morning-evening and morning day-by-day HBP variability were found to be significant and independent predictors of CV events.
Prognostic relevance of visit-to-visit BPV
Compared with indices of BPV derived from 24-h ABPM, day-by-day and visit-to-visit measures of BPV spaced by months or even years have been shown to have a stronger prognostic value in observational studies and post hoc analyses of antihypertensive drug trials (Citation17,Citation31,Citation32). This should not be surprising if considered that while a single 24-h ABPM may reflect the BP coverage by antihypertensive medication over the 24 h, visit-to-visit BPV may reflect the degree of BP control and the BP burden for the CV system in the long term. Increasing values of visit-to-visit BPV have been found to be associated with cardiac (i.e. diastolic dysfunction) (Citation66), macrovascular (increased intima-media thickness and stiffness) (Citation67), microvascular (development of micro- and macro-albuminuria, and renal vascular atherosclerosis) (Citation68, Citation69) and cerebral (white matter hyperintensity volume and presence of brain infarctions) (Citation70) organ damage as well as with endothelial dysfunction (Citation71). Recent longitudinal studies and post hoc analyses of clinical trials in hypertension have found an increased visit-to-visit BPV within a given patient, to be predictive of cerebrovascular events (Citation17,Citation72), acute myocardial infarction (Citation73) and all-cause mortality (Citation31), independently of mean BP levels. Interestingly, in the hypertensive population with a history of coronary disease of the INVEST study (International Verapamil–Trandolapril Study), the incidence of fatal and non-fatal CV events increased progressively as the percentage of on-treatment visits with BP controlled (i.e. BP < 140/90 mmHg) increased throughout the treatment period (Citation32). Thus, one may infer that factors affecting the degree of BP control (i.e. patient's adherence to treatment and proper dosing/titration of antihypertensive treatment) may importantly influence visit-to-visit BPV (as assessed with SD or coefficient of variation), and in due course the risk of CV outcomes.
Effects of antihypertensive treatment on very short-term and short-term BPV (assessed from beat-to-beat BP recordings)
Most evidence on the effects of antihypertensive treatment on short-term BPV assessed from beat-to-beat BP recordings in relation to regression/progression of target organ damage (OD) comes from studies in mice. These studies have indicated that specific classes of antihypertensive drugs, alone or in combination, may confer cardiac, renal and brain organ protection mainly through their ability to stabilize BPV independently of their BP lowering effects (Citation74). A study in spontaneously hypertensive rats (SHR), assessing the long-term effects of treatment with nitrendipine vs hydralazine on beat-to-beat BPV and organ damage showed that despite similar reductions in mean BP levels with both drugs, only nitrendipine produced significant reductions in BPV and regression of organ damage (Citation74). The absence of correlation between mean BP levels and organ damage in the group or rats treated with nitrendipine suggested that the beneficial effect of this drug in organ protection could likely be explained by its ability to stabilize BPV (Citation74). Subsequent studies in SHR provided evidence that long-term combination therapy (i.e. atenolol–amlodipine or hydrochlorothiazide–enalapril vs monotheraphy) may be more effective in reducing short-term beat-to-beat BPV and in reducing incidence/progression of organ damage after 18 months of active treatment (Citation75–77). In a further study in stroke-prone spontaneously hypertensive rats (SHR-SP), whereas atenolol and amlodipine alone or in combination were associated with similar reductions in mean BP levels, only combination strategy was able to stabilize BPV (Citation78). Remarkably, combination strategy was also associated with the highest reduction in the incidence of stroke at follow-up suggesting a possible synergism between atenolol and amlodipine for stroke prevention in hypertension. Interestingly, some of these studies have suggested plausible putative mechanisms for these benefits such as restoration of baroreflex sensitivity (BRS) (Citation75,Citation76).
Although several studies in physiological and clinical research have applied analysis of BPV using continuous BP recordings, only few have addressed the effects of BP lowering or specific classes of antihypertensive drugs on indices of short-term BPV derived from beat-to-beat BP recordings in humans. In recent years, a study was conducted to assess the effects of beta-blockade on BRS and BPV in a group of essential hypertensives by performing 24-h intra-arterial BP recordings before and after 1 month of dosing with either acebutolol or labetalol (Citation79). Before treatment, a reduced BRS was associated with a higher BPV, and directly correlated with pulse interval (PI), reinforcing previously reported data from animal studies on the role of the arterial baroreflex in buffering BP variations by adjusting PI (Citation80). The persistent inverse correlation between BRS and BPV after beta-blockade (which significantly increased BRS, p < 0.01), and the absence of correlation for BRS and PI, led to suggesting a stabilizing effect of beta-blockers on BP, more via reflex modulation of peripheral vascular resistance than through their effects on reflex modulation of HR. A subsequent study assessed the effects of lacidipine, a long-acting calcium antagonist, on 24-h mean BP levels, BPV and BRS in hypertensive patients with type II diabetes mellitus (Citation81). Non-invasive beat-to-beat BP monitoring (Portapress™) was performed after a 3-week pre-treatment with placebo and after 4 weeks of active treatment with lacidipine (4 mg/daily) or placebo. Compared with placebo, lacidipine reduced mean BP levels, and 24-h, daytime and night-time BPV, demonstrating its ability to stabilize BPV in addition to its BP lowering effects.
Effects of antihypertensive treatment on short-term BPV as assessed from 24-h ABPM: a target of antihypertensive treatment for CV prevention?
Studies in humans using non-invasive 24-h ABPM have shown that BPV within the 24 h is decreased proportionally to the reduction in mean BP values by a variety of antihypertensive treatments (Citation82–89), thus suggesting that the effects of antihypertensive treatment on BPV are the result of BP lowering per se. Thus, additional longitudinal evidence based on an accurate quantification of BPV is still needed in order to define whether some drugs or treatment strategies have greater effects on within 24-h BP variations than others. Evidence is also needed from longitudinal outcome studies on the possibility that a treatment-induced reduction in short-term BPV might also reduce the development/progression of organ damage and the risk of CV events independently of reductions in mean BP levels (Citation21).
Should long-term BPV be a target of antihypertensive treatment for CV prevention?
The favorable outcomes observed in most controlled trials using different antihypertensive drug classes as an active regimen have strongly supported the preponderant role of BP reduction in achieving CV protection (Citation90,Citation91). However, post hoc analyses and interpretations of clinical trials have raised the hypothesis that part of the benefit may be due to specific properties of some drug classes based on the different rates of outcomes that occurred despite no or little mean BP difference. Overall, these studies have called attention to the additive or alternative role of long-term BPV during treatment. In particular, a series of post hoc analyses of large intervention trials conducted by Rothwell et al. have found intraindividual visit-to-visit variability (i.e. variability of an individual's BP from visit to visit) and interindividual variability (i.e. BP variations from visit-to-visit in independent groups of subjects) to predict incidence of stroke to a much greater extent than average BP (Citation17,Citation92–94). In addition, when outcome trials comparing two antihypertensive regimens were analyzed, the regimen associated with lower intraindividual or interindividual variabilities was also associated with a lower incidence of stroke (Citation17,Citation92,Citation93). However, a major limitation of inter-individual BPV (or group BPV) is that it cannot accurately reflect BP variations from visit-to-visit in individual subjects. More reliable measurements of “real” BPV from day-to-day probably through the use of HBPM, over long-term treatment must be implemented and investigated in upcoming clinical trials for a better understanding of the phenomena underlying the current variable definitions of BPV as well as the benefits of antihypertensive treatment with different drug classes on BPV and CV outcomes.
Based on the prognostic relevance of visit-to-visit BPV one could infer that consistency of BP control represents an additional important goal of antihypertensive treatment. Indeed, several studies have shown that an increase in the proportion of visits with BP control is accompanied by a progressive reduction in the risk of CV events independently of mean BP levels during treatment (Citation32). However, it should be considered that current evidence on visit-to-visit BPV has been obtained using the post hoc approach and quantification of BPV has not always been performed using a sufficient number of measurements (i.e. as it could be the case performed with HBPM) nor using standardized methodologies (i.e. OBP visit-to-visit BPV has only a very limited relationship with 24-h BPV from visit-to-visit) (Citation95), which means that consistency of clinic BP control may not reflect that of 24-h BP control, i.e. a measure of recognized prognostic importance (Citation50,Citation51,Citation59).
Conclusions
Current knowledge on short-term BPV within the 24-h period has important limitations, which makes future studies in this area necessary for a better understanding of the mechanisms and factors involved (e.g., arterial stiffness, genetic variation). There is still much to be learned regarding the factors responsible for long-term BPV, especially from visit-to-visit during antihypertensive treatment. Although the role of neuro-humoral and structural characteristics of the CV system cannot be excluded, it will be important to determine the contribution of factors influencing visit-to-visit BPV such as the timing of BP measurements at different visits in relation to drug administration and the patient's adherence to antihypertensive treatment.
Mounting evidence has indicated that both short- and long-term BPV are associated with development, progression and severity of cardiac, vascular and renal organ damage, and with an increased risk of CV events and mortality, independently adding to CV risk, over and above the contribution of elevated mean BP levels. However, compared with long-term BPV (i.e. day-by-day or visit-to-visit BPV), short-term BPV is far less associated with outcome. However, because 24-h ambulatory BP has not been routinely used in large-scale trials on antihypertensive treatment, the protective effect of treatment-induced changes in 24-h BPV with respect to the concomitant changes in BP mean should be properly documented. Ideally this should be done by quantifying the magnitude of 24-h BPV through BP readings obtained at intervals of ≤ 15 or 20 min, because, beyond these intervals, quantification of the 24-h BP SD can be inaccurate (Citation96).
Although it has been suggested that in order to achieve the highest CV protection in hypertensive patients, antihypertensive treatment should be targeted at normalizing 24-h BPV in addition to reduce absolute 24-h BP levels, evidence is still limited regarding the targets of BPV to achieve with antihypertensive treatment. Moreover, little is known on the possibility that specific drug classes or combinations might lower BPV independently of mean BP reduction.
As recently shown by large meta-analyses of clinical trials on hypertension, an increased visit-to-visit BPV or lack of BP control at any given visit may have adverse prognostic consequences, calling attention to the importance of consistency of BP control over time. In practical terms, assessment of long-term BPV, ideally on a day-by-day basis by means of HBPM might help the practicing physician to optimize antihypertensive treatment at every clinic visit, thus improving stabilization of BP levels in the long term. Finally, it should be emphasized that before being recommended as a target for antihypertensive treatment in daily clinical practice, further prospective outcome studies should be conducted to support that a treatment-induced reduction in BPV is accompanied by a corresponding reduction in CV risk, and to clarify the precise additional contribution to events rate provided by an increased BPV over and above an increase in mean BP levels (Citation48).
Conflicts of interest
Nothing to declare.
References
- Muntner P, Joyce C, Levitan EB, Holt E, Shimbo D, Webber LS, et al. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J Hypertens. 2011;29:2332–2338.
- Parati G, Ochoa JE, Bilo G. Blood Pressure Variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012.
- Mancia G PG, di Rienzo M, Zanchetti A. Blood pressure variability. In: Mancia G ZA, editor. Handbook of hypertension: Pathophysiology of hypertension. Amsterdam: Elsevier Science; 1997. p. 117–169.
- Bevan AT HA, Stott FH. Portable recorder for continuous arterial pressure measurement in man. J Physiol. 1966;38: 186–190.
- Penaz J. [Current photoelectric recording of blood flow through the finger]. Cesk Fysiol. 1975;24:349–352. Prubezny fotoelektricky zaznam prutoku krve prstem.
- Smith NT, Wesseling KH, de Wit B. Evaluation of two prototype devices producing noninvasive, pulsatile, calibrated blood pressure measurement from a finger. J Clin Monit. 1985;1:17–29.
- Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13(6 Pt 1):647–655.
- Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276–1286.
- Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104.
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98.
- Parati G, Omboni S, Rizzoni D, Agabiti-Rosei E, Mancia G. The smoothness index: A new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens. 1998;16:1685–1691.
- Mancia G, Di Rienzo M, Parati G. Ambulatory blood pressure monitoring use in hypertension research and clinical practice. Hypertension. 1993;21:510–524.
- Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058–2066.
- Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, et al. Long-term prognostic value of blood pressure variability in the general population: Results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–1270.
- Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511.
- Stolarz-Skrzypek K, Thijs L, Richart T, Li Y, Hansen TW, Boggia J, et al. Blood pressure variability in relation to outcome in the international database of ambulatory blood pressure in relation to cardiovascular outcome. Hypertens Res. 2010;33:757–766.
- Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905.
- Stergiou GS, Nasothimiou EG. Home monitoring is the optimal method for assessing blood pressure variability. Hypertens Res. 2011;34:1246–1248.
- Mancia G, Parati G, Pomidossi G, Casadei R, Di Rienzo M, Zanchetti A. Arterial baroreflexes and blood pressure and heart rate variabilities in humans. Hypertension. 1986;8:147–153.
- Conway J, Boon N, Davies C, Jones JV, Sleight P. Neural and humoral mechanisms involved in blood pressure variability. J Hypertens. 1984;2:203–208.
- Parati G, Faini A, Valentini M. Blood pressure variability: Its measurement and significance in hypertension. Curr Hypertens Rep. 2006;8:199–204.
- Kotsis V, Stabouli S, Karafillis I, Papakatsika S, Rizos Z, Miyakis S, et al. Arterial stiffness and 24 h ambulatory blood pressure monitoring in young healthy volunteers: The early vascular ageing Aristotle University Thessaloniki Study (EVA-ARIS Study). Atherosclerosis. 2011;219:194–199.
- Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: Findings from 2 large databases. Hypertension. 2012;60:369–377.
- Parati G, Castiglioni P, Di Rienzo M, Omboni S, Pedotti A, Mancia G. Sequential spectral analysis of 24-hour blood pressure and pulse interval in humans. Hypertension. 1990;16:414–421.
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161.
- Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, et al. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension. 2002;39:168–172.
- Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, et al. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis. 1999;33:29–35.
- Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, et al. Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation. 1993;88:986–992.
- Haynes WG. Role of leptin in obesity-related hypertension. Exp Physiol. 2005;90:683–688.
- Murakami S, Otsuka K, Kubo Y, Shinagawa M, Matsuoka O, Yamanaka T, et al. Weekly variation of home and ambulatory blood pressure and relation between arterial stiffness and blood pressure measurements in community-dwelling hypertensives. Clin Exp Hypertens. 2005;27:231–239.
- Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166.
- Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. 2007;50:299–305.
- Parati G, Bilo G. Calcium antagonist added to angiotensin receptor blocker: A recipe for reducing blood pressure variability?: Evidence from day-by-day home blood pressure monitoring. Hypertension. 2012;59:1091–1093.
- Sega R, Cesana G, Bombelli M, Grassi G, Stella ML, Zanchetti A, et al. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. Pressione Arteriose Monitorate E Loro Associazioni. J Hypertens. 1998;16:1585–1592.
- Modesti PA, Morabito M, Bertolozzi I, Massetti L, Panci G, Lumachi C, et al. Weather-related changes in 24-hour blood pressure profile: Effects of age and implications for hypertension management. Hypertension. 2006;47:155–161.
- Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11:1133–1137.
- Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, et al. Relation between blood pressure variability and carotid artery damage in hypertension: Baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19:1981–1989.
- Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens Suppl. 2003;21:S17–S23.
- Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, et al. Blood pressure variability and organ damage in a general population: Results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension. 2002;39(2 Pt 2):710–714.
- Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–332.
- Manios E, Tsagalis G, Tsivgoulis G, Barlas G, Koroboki E, Michas F, et al. Time rate of blood pressure variation is associated with impaired renal function in hypertensive patients. J Hypertens. 2009;27:2244–2248.
- Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation. 2000;102:1536–1541.
- Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke?Stroke. 2000;31:463–468.
- Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257.
- Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: A prospective study. Circulation. 2003;107: 1401–1406.
- Kario K, Ishikawa J, Pickering TG, Hoshide S, Eguchi K, Morinari M, et al. Morning hypertension: The strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res. 2006;29:581–587.
- Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. American J Hypertens. 2007;20:154–161.
- Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic value of reading- to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55: 1049–1057.
- Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities: The Ohasama Study. Hypertension. 2000;36:901–906.
- Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546.
- Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: Follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1183.
- Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: The Ohasama Study. Hypertension. 2005;45:240–245.
- Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61.
- Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet. 2007;370:1219–1229.
- Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10.
- Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415.
- Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Human Hypertens. 2005;19:801–807.
- Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: A prospective study. Hypertension. 1998;31:712–718.
- Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension. 2005;46:156–161.
- Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and mortality: A population-based study. Hypertension. 2005;45:499–504.
- Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805.
- Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: The Ohasama Study. Hypertension. 2006;47:149–154.
- Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K. Maximum value of home blood pressure: A novel indicator of target organ damage in hypertension. Hypertension. 2011;57:1087–1093.
- Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: The Ohasama Study. Hypertension. 2008;52:1045–1050.
- Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: The Finn–Home Study. Hypertension. 2012; 59:212–218.
- Masugata H, Senda S, Murao K, Inukai M, Hosomi N, Iwado Y, et al. Visit-to-visit variability in blood pressure over a 1-year period is a marker of left ventricular diastolic dysfunction in treated hypertensive patients. Hypertens Res. 2011;34:846–850.
- Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: New independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens. 2011; 5:184–192.
- Kilpatrick ES, Rigby AS, Atkin SL. The role of blood pressure variability in the development of nephropathy in type 1 diabetes. Diabetes Care. 2010;33:2442–2447.
- Kawai T, Ohishi M, Kamide K, Onishi M, Takeya Y, Tatara Y, et al. The impact of visit-to-visit variability in blood pressure on renal function. Hypertens Res. 2012;35:239–243.
- Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569.
- Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35: 55–61.
- Hata Y, Kimura Y, Muratani H, Fukiyama K, Kawano Y, Ashida T, et al. Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. Hypertens Res. 2000;23:553–560.
- Hata Y, Muratani H, Kimura Y, Fukiyama K, Kawano Y, Ashida T, et al. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Human Hypertens. 2002;16: 141–146.
- Liu JG, Xu LP, Chu ZX, Miao CY, Su DF. Contribution of blood pressure variability to the effect of nitrendipine on end-organ damage in spontaneously hypertensive rats. J Hypertens. 2003;21:1961–1967.
- Xie HH, Miao CY, Jiang YY, Su DF. Synergism of atenolol and nitrendipine on hemodynamic amelioration and organ protection in hypertensive rats. J Hypertens. 2005;23: 193–201.
- Xie HH, Shen FM, Xu LP, Han P, Miao CY, Su DF. Reduction of blood pressure variability by combination therapy in spontaneously hypertensive rats. J Hypertens. 2007;25:2334–2344.
- Han P, Shen FM, Xie HH, Chen YY, Miao CY, Mehta JL, et al. The combination of atenolol and amlodipine is better than their monotherapy for preventing end-organ damage in different types of hypertension in rats. J Cell Mol Med. 2009;13:726–734.
- Ling G, Liu AJ, Shen FM, Cai GJ, Liu JG, Su DF. Effects of combination therapy with atenolol and amlodipine on blood pressure control and stroke prevention in stroke-prone spontaneously hypertensive rats. Acta Pharmacol Sin. 2007;28: 1755–1760.
- Parati G, Mutti E, Frattola A, Castiglioni P, di Rienzo M, Mancia G. Beta-adrenergic blocking treatment and 24-hour baroreflex sensitivity in essential hypertensive patients. Hypertension. 1994;23(6 Pt 2):992–996.
- Cowley AW, Jr., Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576.
- Frattola A, Parati G, Castiglioni P, Paleari F, Ulian L, Rovaris G, et al. Lacidipine and blood pressure variability in diabetic hypertensive patients. Hypertension. 2000;36: 622–628.
- Ferrari A, Buccino N, Di Rienzo M, Pedotti A, Mancia G, Zanchetti A. Labetalol and 24-hour monitoring of arterial blood pressure in hypertensive patients. J Cardiovasc Pharmacol. 1981;3 Suppl 1:S42–S52.
- Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Grassi G, et al. Evaluation of a slow-release clonidine preparation by direct continuous blood pressure recording in essential hypertensive patients. J Cardiovasc Pharmacol. 1981;3:1193–1202.
- Mancia G, Ferrari A, Pomidossi G, Parati G, Bertinieri G, Grassi G, et al. Twenty-four-hour blood pressure profile and blood pressure variability in untreated hypertension and during antihypertensive treatment by once-a-day nadolol. Am Heart J. 1984;108(4 Pt 2):1078–1083.
- Pomidossi G, Parati G, Motolese M, Mancia G, Zanchetti A. Hemodynamic effects of once a day administration of combined chlorthalidone and metoprolol slow-release in essential hypertension. Int J Clin Pharmacol Ther Toxicol. 1984;22: 665–671.
- Pomidossi G, Parati G, Malaspina D, Camesasca C, Motolese M, Zanchetti A, et al. Antihypertensive effect of a new formulation of slow release oxprenolol in essential hypertension. J Cardiovasc Pharmacol. 1987;10:593–598.
- Parati G, Pomidossi G, Casadei R, Ravogli A, Trazzi S, Mutti E, et al. 24-h ambulatory non-invasive blood pressure monitoring in the assessment of the antihypertensive action of celiprolol. J Int Med Res. 1988;16 Suppl 1:52A–61A.
- Mancia G, De Cesaris R, Fogari R, Lattuada S, Montemurro G, Palombo C, et al. Evaluation of the antihypertensive effect of once-a-day trandolapril by 24-hour ambulatory blood pressure monitoring. The Italian Trandolapril Study Group. Am J Cardiol. 1992;70:60D–66D.
- Mancia G, Omboni S, Ravogli A, Parati G, Zanchetti A. Ambulatory blood pressure monitoring in the evaluation of antihypertensive treatment: Additional information from a large data base. Blood Press. 1995;4:148–156.
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838.
- Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535.
- Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet. 2010;375:906–915.
- Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480.
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948.
- Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability in the European Lacidipine Study on Atherosclerosis: Methodological aspects and effects of antihypertensive treatment. J Hypertens. 2012;30:1241–1251.
- di Rienzo M, Grassi G, Pedotti A, Mancia G. Continuous vs intermittent blood pressure measurements in estimating 24-hour average blood pressure. Hypertension. 1983;5: 264–269.
