Abstract
SURGE 2, a large-scale, practice-based study in 10 countries, evaluated the effects of telmisartan alone or with hydrochlorothiazide (HCTZ) on morning (06:00–11:59) home blood pressure (HBP) control. Hypertensive patients (clinic blood pressure [BP] ≥ 140/90 mmHg) received telmisartan 40 or 80 mg either alone or in combination with HCTZ 12.5 mg for 8 weeks. Treatment could be adjusted if clinic BP remained ≥ 140/90 mmHg. Clinic BP was measured in the morning prior to medication, and seated HBP monitoring was performed, three times per day, 2 days per week. A total of 25,882 patients were included (71% were previously using antihypertensives). There was a statistically significant (all p < 0.001) reduction in mean morning, lunchtime and evening HBP following treatment with telmisartan/telmisartan plus HCTZ, and morning HBP control increased from 10.6–19.8% to 51.1–64.6%. Similar improvements were observed for lunchtime (from 20.6–26.0% to 57.7–70.5%) and evening (from 21.3–31.4% to 59.0–68.8%). The morning HBP response ranged from 62.6–67.5% (systolic BP) and from 81.4–87.0% (diastolic BP). Adverse events were reported by 1.2% of patients. Telmisartan alone or with HCTZ improved morning HBP control and maintained a smooth HBP profile throughout the day in a real-life setting.
Introduction
Ambulatory and home blood pressure monitoring (HBPM) have enabled physicians to determine whether patients with treated hypertension are achieving target blood pressure (BP) goals throughout the 24-h period. In the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study, the 12-year rate of cardiovascular (CV) death in a population of 2051 patients was highest when the home, ambulatory and clinic BP were all elevated, which stresses the importance of out-of-office BP control (Citation1). Home and ambulatory measures have also revealed that the peak incidence in vascular events correlates with a morning blood pressure surge (MBPS) (Citation2–5). Although the MBPS is largely caused by overactivity of the sympathetic nervous system, a sharp increase in the activity of the renin–angiotensin system (RAS) may also be a contributory factor (Citation6). Agents that act on the RAS could therefore have a favourable effect on morning BP control, and may reduce the incidence of MBPS-associated vascular events.
To date, a number of surveys have reported that control of non-clinic BP is low (Citation7–10). In the Japan Home Versus Office Measurement Evaluation (J-HOME) study, which evaluated 3303 treated hypertensive patients, 24.6% were found to have uncontrolled morning BP (Citation9). The AmloDipine Versus AngiotensiN II receptor blocker; Control of blood pressure Evaluation trial in Diabetics (ADVANCED-J) study showed that systolic morning home BP, but not systolic clinic BP, was significantly correlated with urinary albumin creatinine excretion rate in 316 hypertensive patients with type 2 diabetes (Citation11).
Against this background, SURGE 2 (Study of hypertensive population Under treatment with telmisartan in Real clinical conditions with the Goal to control the Early morning blood pressure rise 2) will report the degree of morning home and clinic BP control after 8 weeks of treatment with telmisartan (a RAS blocker) either alone or in combination with hydrochlorothiazide (HCTZ; a diuretic). The elimination half-life for telmisartan, at around 24 h, is the longest of any of the angiotensin II receptor blockers. The long plasma half-life of telmisartan contributes to its prolonged duration of action, as evidenced by the 24-h BP control demonstrated in different clinical trials with telmisartan. Additionally, telmisartan has been shown to reduce target organ damage in a number of clinical studies (Citation12–14).
Materials and Methods
Study design
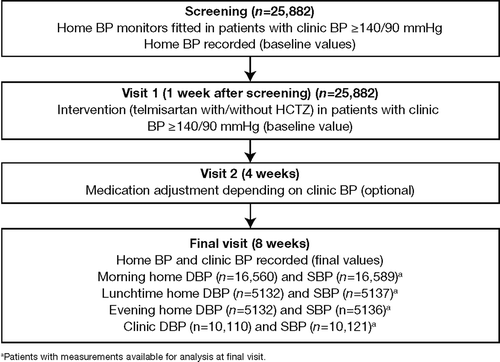
SURGE 2 was an 8-week, prospective, open-label, phase IV, practice-based study conducted in centres across 10 countries (Belgium, Canada, Colombia, Czech Republic, Indonesia, Jordan, Lebanon, Mexico, Venezuela and Yemen). The main aim of this observational study was to assess the degree of morning home BP in patients treated with telmisartan or telmisartan plus HCTZ. Patients were enrolled between September 2003 and April 2005. All studies were performed in accordance with international and local regulations. The protocol was approved by an independent ethics committee. All patients provided written informed consent to participate.
Population
Males or females aged between 18 and 80 years with mild to moderate essential hypertension, who were either untreated or had uncontrolled clinic BP (≥ 140/90 mmHg) on their current antihypertensive regimen were included. Patients were excluded if they had congestive heart failure, unstable angina, acute myocardial infarction, heart surgery or stroke within the previous 6 months, arrhythmia, angio-oedema associated with RAS blockade, advanced hepatic or renal impairment or any other condition that would not allow for safe completion of the protocol. Other exclusion criteria included chronic administration of oral anticoagulants or digoxin and known hypersensitivity to telmisartan or HCTZ. Pregnant, nursing or pre-menopausal women not using birth control were also excluded.
Treatments and BP monitoring
Patients with clinic BP ≥ 140/90 mmHg were prescribed telmisartan (40 or 80 mg) either alone or in combination with HCTZ (12.5 mg) based on the decision of the treating physician. Patients using current antihypertensive treatment could either switch medications or have these agents added to their regimen. Patients were instructed to take their medication once daily between 07:00 and 10:00 h.
During screening, all physicians were instructed to measure clinic BP using a mercury sphygmomanometer, and in accordance with guideline recommendations (Citation15,Citation16); measures were taken three times, at intervals of 2 min, and the mean was used. Patients with uncontrolled hypertension (≥ 140/ 90 mmHg) were then supplied with validated home BP monitors [according to British Hypertension Society guidelines (Citation17)], such as the OMRON 705 IT monitor with data transfer (Omron Healthcare Europe BV, Hoofddorp, The Netherlands). Devices were supplied by the study sponsor (Boehringer Ingelheim International, Ingelheim, Germany). Patients were instructed on how to use the devices by the treating physicians and nurses, who were following European Society of Hypertension (2003) recommendations (Citation16). Brachial artery home BP measurements were taken in the seated position, three times per day: after getting up (before taking antihypertensive medication), before lunch and before dinner on 2 days over 1 week. On each occasion, two measurements were performed with an interval of 3 min between them, and an overall mean was determined for morning, lunchtime and evening home BP. Patients were instructed to document the time and values of BP measurements (this was defined as the baseline home BP).
Within 1 week of performing home BP measurements, patients returned to the clinic. Clinic BP was measured as above (this was defined as baseline clinic BP). Patients with clinic BP ≥ 140/90 mmHg were treated with telmisartan and/or HCTZ as described. Patients could then attend the clinic after 4 weeks (optional visit) where medication could be adjusted according to clinic BP control (< 140/90 mmHg or ≥ 140/90 mmHg). Home BP measurement was performed again by the patient after 8 weeks of treatment and clinic BP was measured during week 8 ().
Calculations and reference values
Considering that approximately one third of patients would be previously untreated, and one quarter of these previously untreated patients would receive the telmisartan/HCTZ combination, and around half of these patients would have data available, for at least one endpoint, for analysis at the final visit, a total sample size of 25,000 patients would therefore result in approximately 1000 previously untreated patients in the smallest, telmisartan/HCTZ group with available data. Furthermore, only one quarter (250) of these patients would have valid data available for all relevant endpoints (i.e. in-clinic BP, morning, lunchtime, evening HBP) in this smallest group of interest. With an intra-individual standard deviation of 20 mmHg for systolic blood pressure (SBP), the total sample size of 25000 patients guaranteed a power of 90% to detect a reduction of 5 mmHg at a two-sided alpha-level of 0.01 in any of the subgroups of interest.
The change from mean baseline morning SBP and diastolic BP (DBP) home BP (06:00–11:59) and the degree of morning home BP control (defined as the percentage of patients with home BP < 135/85 mmHg at baseline and during week 8) were reported. Lunchtime (12:00–14:00) and evening (18:00–22:00) home BP control (home BP < 135/85 mmHg at baseline and during week 8), clinic BP control (<140/90 mmHg at baseline and during week 8) and the mean SBP/DBP reductions during these periods were also reported. The response rates (defined as percentage of patients with > 10 mmHg reduction in SBP or DBP from baseline HBP or baseline clinic BP) to telmisartan-based treatments were also documented.
Tolerability was assessed by adverse events, which were collected, documented and reported in case report forms, and patients were asked to rate the tolerability and efficacy of telmisartan either alone or in combination with HCTZ, based on a scale of “very good” to “bad”. Change from baseline was analysed using the Student's t-test for paired data. All patients who received telmisartan either alone or in combination with HCTZ with no other therapy at final visit, and had morning home BP data available at week 8, were included in the analyses.
Results
Population
A total of 25,882 subjects were included; mean age was 55 years (previously untreated patients) and 59 years (previously treated patients), 48.0% of previously untreated patients were male and 42.7% of previously treated patients were male. The patient characteristics are shown in . The patients were from Canada (50%; n = 12,952/25,882), Mexico (26.5%; n = 6848/25,882), Belgium (8.1%; n = 2096/25,882), Lebanon (4.7%; n = 1212/ 25,882), Columbia (4.6%; n = 1182/25,882), Czech Republic (3%; n = 769/25,882), Jordan (1.9%; n = 496/25,882), Indonesia (< 1%; n = 189/25,882), Venezuela (< 1%; n = 79/25,882) and Yemen (< 1%; n = 59/25,882). CV risk factors were documented. The most common risk factor was hypercholesterolaemia [26.7% (previously untreated); 40.0% (previously treated)]. Diabetes was present in 14.3% of previously untreated patients and in 24.5% of previously treated patients, and electrocardiogram-based diagnosis of left ventricular hypertrophy was present in 2.2% of previously untreated patients and in 8.7% of previously treated patients. The majority of patients (71%; n = 18,364/25,882) were taking at least one antihypertensive; the most commonly prescribed were angiotensin-converting enzyme inhibitors (36.7%) and diuretics (32.4%).
Table I. Mean (standard deviation)a baseline characteristics according to previous therapy.
Initially, 70.8% (n = 18,319/25,882) of patients were given telmisartan and 25.8% (n = 6688/25,882) were given telmisartan plus HCTZ (drug choice was not recorded for 3.4% [n = 875/25,882] of patients). At final visit, 61.9% (n = 16,039/25,882) were receiving telmisartan and 31.7% (n = 8193/25,882) were receiving telmisartan plus HCTZ (drug choice was not recorded for 6.4% [n = 1650/25,882] of patients. At final visit, 64.8% (n = 4872/7518) of previously untreated patients were receiving telmisartan and 20.6% (n = 1547/7518) of previously untreated patients were receiving telmisartan plus HCTZ and no other therapy. In previously treated patients, 37.3% (n = 6851/18,364) received telmisartan and 22.6% (n = 4144/18,364) received telmisartan plus HCTZ and no other therapy at final visit (). Only patients who received telmisartan or telmisartan plus HCTZ and no other therapy were included in the final analyses.
Table II. Treatment allocation at final visit (n = 25,882).
Overall, a total of 1552 patients discontinued treatment. Patients could withdraw for more than one reason. The main reasons were insufficient decrease of BP (n = 251) and no further contact with the patient (n = 234). Patients who discontinued treatment were not included in the final analyses. A total of 16,589 patients had morning home SBP measures available at final visit and 16,560 had morning home DBP measures available at final visit. A total of 10,121 patients had clinic SBP measures available at final visit and 10,110 had clinic DBP measures available at final visit ().
Reduction in home and clinic BP
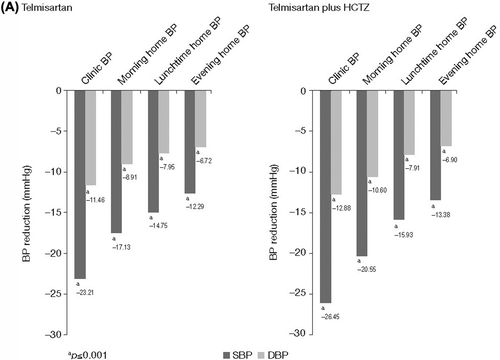
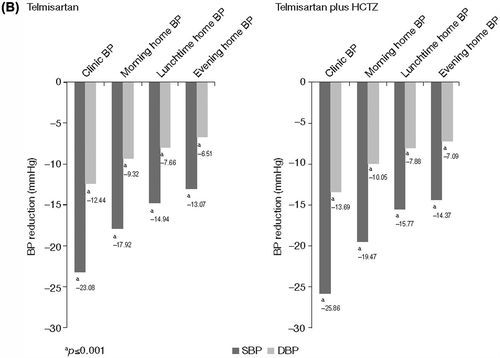
Statistically significant (all p < 0.001) reductions in mean morning, lunchtime and evening home BP were observed after 8 weeks of treatment with telmisartan either alone or in combination with HCTZ for both previously untreated and previously treated patients (). For previously untreated patients, the mean± standard deviation (SD) reduction in morning home SBP/DBP for telmisartan-treated patients was −17.13 ± 17.51/− 8.91 ± 10.22 mmHg; p < 0.001) and for telmisartan plus HCTZ treated patients it was −20.55 ± 19.39/ −10.60 ± 11.03 mmHg; p < 0.001). For previously treated patients, the mean ± SD reduction in morning home SBP/DBP for telmisartan-treated patients was −17.92 ± 17.35/− 9.32 ± 9.94 mmHg; p < 0.001 and for telmisartan plus HCTZ-treated patients it was − 19.47 ± 19.04/− 10.05 ± 10.77 mmHg; p < 0.001. The overall mean ± SD reduction for morning home SBP/DBP was −18.24 ± 18.32/ −9.30 ± 10.31 mmHg; p < 0.001. The greatest BP reductions were observed for clinic BP; the mean± SD clinic SBP/DBP reduction was−23.43 ± 19.05/−11.99 ± 11.15 mmHg; p < 0.001 for all telmisartan and telmisartan plus HCTZ-treated patients.
BP control and response rates
The improvements in morning, lunchtime and evening home and clinic BP control in patients treated with telmisartan either alone or in combination with HCTZ are shown in . Morning home BP control increased from 10.6–19.8% to 51.1–64.6%, lunchtime home BP increased from 20.6–26.0% to 57.7–70.5% and evening home BP increased from 21.3–31.4% to 59.0–68.8%, which indicates that BP control remained consistent throughout the day across all treatment groups. Clinic BP control also increased from 5.4–10.8% to 63.5–72.1% following telmisartan treatment. The response rates (DBP or SBP reduction > 10 mmHg assessed by either clinic or HBPM) were more than 48.8% in all patients following telmisartan-based treatment (). The morning home SBP response rate ranged from 62.6–67.5% and the morning DBP response rate ranged from 81.4–87.0%. The clinic SBP response rate ranged from 76.7–82.0%, and the clinic DBP response rate ranged from 77.4–80.2%.
Table III. Blood pressure (BP) control rates (%) initially and after 8 weeks of treatment with telmisartan either alone or in combination with HCTZ.
Table IV. Response rates (%) after 8 weeks of treatment with telmisartan either alone or in combination with HCTZ.
Tolerability
The tolerability of telmisartan either alone or in combination with HCTZ was rated as “very good” in the majority of patients (63.6%; n = 16,472/ 25,882); as “good” in 14.0% (n = 3612/25,882) and as “moderate” in 1.1% (n = 291/25,882). Less than 1% (n = 246/25,882) rated the tolerability as “bad”. It was not assessable in < 1% (n = 212/25,882) and data were missing for 19.5% (n = 5049/25,882) patients. Adverse events were reported by 1.2% of patients (n = 301/25,882). Overall, the most frequently reported adverse events were headache, dizziness and diarrhoea (all 0.2%) as shown in .
Table V. Most frequently reported (>1%) adverse events (n = 25,882).
Discussion
This practice-based SURGE 2 study, which was conducted in more than 25,000 patients, showed that telmisartan monotherapy or in combination with HCTZ, given to previously untreated or treated patients with uncontrolled hypertension, significantly reduced SBP and DBP in the morning hours as evaluated by HBPM. Between 51.1% and 64.6% of all patients achieved morning home BP control (< 135/85 mmHg) compared with 10.6–19.8% at baseline. Similar findings were observed for lunchtime and evening home BP control. Clinic BP control also increased from 5.4–10.8% to 63.5–72.1% following addition of telmisartan either alone or with HCTZ. At final visit, the majority of previously untreated patients were taking telmisartan only (64.8%), and a number of previously treated patients had switched to either telmisartan only (37.3%) or telmisartan plus HCTZ (22.6%). The findings also show that telmisartan-based regimens were well tolerated; the tolerability was rated as “very good” by 63.6% of patients and only 1.2% of patients reported adverse events.
The daytime home BP profile obtained (shown by home BP control > 50% during morning, lunchtime and evening measures) with telmisartan either alone or with HCTZ in both previously untreated and previously treated patients firstly indicates that the morning BP surge was partially blunted by telmisartan/telmisartan plus HCTZ treatments either alone or when added to other antihypertensive therapy. Secondly, it is an indication of the reduction in daytime BP variability, which is also known to contribute to CV risk. The impact of variability on organ damage and on the prognosis of long-term outcomes has been described previously (Citation18,Citation19). Even considering the relative small number of home BP values obtained during the daytime period (e.g. n = 1050 for home BP values obtained at lunchtime in previously untreated patients []), the reduction in variability achieved with telmisartan/telmisartan plus HCTZ in our study may reduce long-term CV risk. The Jichi Morning Hypertension Research (J-MORE) study, which assessed 969 treated hypertensive patients, showed a difference between morning and evening BP control; the average difference was 7.9 mmHg, and this difference was related to age, beta-blocker use and regular alcohol consumption (Citation20). Agents such as telmisartan that are able to maintain a smooth 24-h BP control (Citation21) may reduce the impact of other factors such as age and diet on BP variability. A recent meta-analysis of 24-h ambulatory BP data obtained in 4392 patients showed that the smoothness index, a measure of BP homogeneity, and the effectiveness of treatments over 24 h was highest for telmisartan 80 mg (SBP, 1.13; DBP, 0.97) and lowest for losartan 50 mg (SBP, 0.66; DBP, 0.57). These results indeed support the hypothesis that telmisartan reduces BP variability (Citation22).
Several other issues also need to be discussed in relation to our findings. A number of previous studies have demonstrated some discrepancy between home-based and clinic BP measurements; this may, in part, be attributable to the use of agents that cannot maintain smooth BP control throughout the 24 h in treated hypertensive patients. The Finn-HOME study, which assessed 2051 subjects from the adult Finnish population, showed that the mean difference between home and clinic BP was 7.7/3.4 mmHg, which indicates a discrepancy between the two measures (Citation23). In the Analysis of the Control of blood pressure using AMbulatory blood Pressure monitoring (ACAMPA) study, more than 50% of 240 treated patients with clinic BP control (BP < 140/90 mmHg) still had elevated morning home BP measurements (Citation24). These observations strongly emphasize the importance of regularly implementing home BP measurements in routine clinical practice, aimed at improving BP control in the hypertensive population.
Our findings are consistent with previous telmisartan-based studies, including the MICARDIS® Community Access Trial (MICCAT-2), which show that telmisartan 80 mg either alone or in combination with HCTZ maintains morning and smooth 24-h SBP and DBP control compared with other agents (Citation25). There is evidence that a reduction in home BP of 2–10 mmHg significantly reduces the risk of CV events (Citation26,Citation27). In the current study, the percentage of patients achieving a reduction in home SBP or DBP > 10 mmHg was high, with more than 77% of patients achieving a DBP reduction > 10 mmHg, and more than 48% of patients achieving a SBP reduction > 10 mmHg. These significant reductions in SBP and DBP achieved with telmisartan-based treatment may favourably affect patients’ prognosis, an issue that will be further addressed in the ongoing Multicenter PROBE Study Comparing the Effects of Angiotensin II Type-1 Receptor Blockers on Self-Monitored Home Blood Pressure in Patients with Morning Hypertension (MUSCAT) study (Citation28). Although our study was of a short duration and did not directly assess CV outcomes, it showed the “real-world” effectiveness of treatments.
An important and favourable feature of our study was the use of home monitoring, which has a number of advantages, including the assessment of morning BP (Citation29). However, HBPM does have some limitations; devices are not always validated, reliability can be limited and patient training is needed. Furthermore, there are no consistent references or values for target home BP threshold following treatment. These potential problems were carefully considered in the present study, by using validated devices, by training patients and by referring to the usual control threshold for HBPM (< 135/85 mmHg), which is now recommended by the updated European Society of Hypertension/European Society of Cardiology guidelines (Citation30,Citation31). The practice-based, open-label nature of this study could also be considered a potential limitation.
In conclusion, systematic use of HBPM in a general practice setting was overall well accepted in this practice-based study, and showed that telmisartan either alone or with HCTZ improved morning home BP control and maintained a smooth home BP profile throughout the day in both previously treated and previously untreated patients. The reduction in daytime BP variability following treatment with telmisartan either alone or with HCTZ represented an additional favourable feature of this type of treatment.
Acknowledgements
The authors would like to thank all investigators comprising the SURGE Steering Committee for their participation, in terms of patient involvement, data collection and analyses. Writing and editorial assistance was provided by PAREXEL, which was contracted by Boehringer Ingelheim International GmbH for these services. Data analysis was provided by QUINTILES. The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors and were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. The author received no compensation related to the development of the manuscript. The SURGE series of trials was sponsored by Boehringer Ingelheim International GmbH.
Declaration of interest: Josep Redon, Grzegorz Bilo and Gianfranco Parati declare no conflict of interest. No remuneration was received by investigators for participation in this study.
References
- Mancia G. Effective ambulatory blood pressure control in medical practice. Good news to be taken with caution. Hypertension. 2007;49:17–18.
- Parati G. Blood pressure variability: Its measurement and significance in hypertension. J Hypertens. 2005;23 Suppl 1: S19–S25.
- Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, et al. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–476.
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Eng J Med. 1985;313: 1315–1322.
- Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68.
- White WB. Relevance of blood pressure variation in the circadian onset of cardiovascular events. J Hypertens. 2003;21 Suppl:S9–S15.
- Omvik P, Gerhardsen G. The Norwegian office-, home-, and ambulatory blood pressure study (NOHA). Blood Press. 2003;12:211–219.
- Shimada K, Fujita T, Ito S, Naritomi H, Ogihara T, Shimamoto K, et al. The importance of home blood pressure measurement for preventing stroke and cardiovascular disease in hypertensive patients: A sub-analysis of the Japan Hypertension Evaluation with Angiotensin II Antagonist Losartan Therapy (J-HEALTH) study, a prospective nationwide observational study. Hypertens Res. 2008;31: 1903–1911.
- Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Inoue R, et al. Prevalence of masked uncontrolled and treated white-coat hypertension defined according to the average of morning and evening home blood pressure values: From the Japan Home versus Office Measurement Evaluation Study. Blood Press Monit. 2005;10:311–316.
- Banegas JR, Segura J, Sobrino J, Rodríguez-Artalejo F, de la Sierra A, de la Cruz JJ, et al; Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Effectiveness of blood pressure control outside the medical setting. Hypertension. 2007;49:62–68.
- Tanaka Y, Daida H, Imai Y, Miyauchi K, Sato Y, Hiwatari M, et al. Morning home blood pressure may be a significant marker of nephropathy in Japanese patients with type2 diabetes: ADVANCED-J study 1. Hypertens Res. 2009;32: 770–774.
- Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645.
- Galzerano D, Tammaro P, Cerciello A, Breglio R, Mallardo M, Lama D, et al. Freehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: A multicentre study. J Hum Hypertens. 2004;18:53–59.
- Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Eng J Med. 2004;351:1952–1961.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC7 Report. JAMA. 2003;289:2560–2572.
- O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21: 821–848.
- British Hypertension Society. Available at: http://www.bhsoc.org/blood_pressure_list.stm. Last accessed 19 July 2010.
- Parati G, Valentini M. Prognostic relevance of blood pressure variability. Hypertension. 2006;47:137–138.
- Parati G, Bilo G. Clinical relevance of day-by-day blood pressure and heart rate variability: New information from home self-measurements. Hypertension. 2008;52: 1006–1008.
- Ishikawa J, Kario K, Hoshide S, Eguchi K, Morinari M, Kaneda R, et al; J-MORE Study Group. Determinants of exaggerated difference in morning and evening blood pressure measured by self-measured blood pressure monitoring in medicated hypertensive patients: Jichi Morning Hypertension Research (J-MORE) Study. Am J Hypertens. 2005;18: 958–965.
- Giles TD. Circadian rhythm of blood pressure and the relation to cardiovascular events. J Hypertens. 2006;24 Suppl: S11–S16.
- Parati G, Schumacher H, Mancia G. Evaluating 24-hour antihypertensive efficacy by the smoothness index: A meta-analysis of an ambulatory blood pressure monitoring database [abstract]. J Hypertens. 2006;24 Suppl 4:S301.
- Niiranen TJ, Jula AM, Kantola IM, Reunanen A. Comparison of agreement between clinic and home-measured blood pressure in the Finnish population: The Finn-HOME Study. J Hypertens. 2006;24:1549–1555.
- Redon J, Roca-Cusachs A, Mora-Macia J. Uncontrolled early morning blood pressure in medicated patients: The ACAMPA study. Analysis of the Control of BLOOD PRESSURE using Ambulatory Blood Pressure Monitoring. Blood Press Monit. 2002;7:111–116.
- White WB, Weber MA, Davidai G, Neutel JM, Bakris GL, Giles T. Ambulatory blood pressure monitoring in the primary care setting: Assessment of therapy on the circadian variation of blood pressure from the MICCAT-2 trial. Blood Press Monit. 2005;10:157–163.
- Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population. Follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) Study. Circulation. 2005;111:1777–1183.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913.
- Uchida H, Nakamura Y, Kaihara M, Norii H, Hanayama Y, Makino H. The MUSCAT study: A multicenter PROBE study comparing the effects of angiotensin type-1 receptor blockers on self-monitored home blood pressure in patients with morning hypertension: Study design and background characteristics. Hypertens Res. 2008;31:51–58.
- Ho PM, Rumsfeld JS. Beyond inpatient and outpatient care: Alternative model for hypertension management. BMC Public Health. 2006;6:257.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: A summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526.