Abstract
Aim. To determine if persistence of electrocardiographic (ECG) left ventricular hypertrophy (LVH) during aggressive systolic blood pressure (SBP) lowering would identify patients at increased risk. Methods and results. Adjudicated outcomes were examined in relation to the presence of LVH by mean in-treatment Cornell product (CP) in 463 hypertensive patients with mean in-treatment SBP ≤ 130 mmHg randomly assigned to losartan- or atenolol-based treatment. During mean follow-up of 4.4 ± 1.3 years, persistence of mean CP > 2440 mm ms in 211 patients (45.6%) was associated with significantly higher 4-year rates of cardiovascular death (8.9% vs 3.4%, p = 0.003), myocardial infarction (7.0% vs 3.3%, p = 0.010), stroke (8.5% vs 2.1%, p = 0.002), the composite endpoint of these events (20.0% vs 7.0%, p < 0.001) and all-cause mortality (14.9% vs 10.0%, p = 0.015). In multivariate Cox analyses, adjusting for a propensity score for CP LVH, randomized treatment and Framingham risk score entered as standard covariates and in-treatment diastolic BP and Sokolow–Lyon voltage LVH entered as time-varying covariates, persistence of CP LVH remained associated with statistically significant increased risks of cardiovascular death (hazard ratio, HR = 2.51, 95% CI 1.10–5.70), stroke (HR = 2.63, 95% CI 1.03–6.97) and the composite endpoint (HR = 2.46, 95% CI 1.36–4.45). Conclusions. These findings suggest that persistence of LVH in a subset of these patients may in part explain the lack of benefit found in hypertensive patients despite treatment to lower SBP.
Key Words::
Introduction
It is well established that high blood pressure (BP) is strongly associated with increased cardiovascular (CV) risk and both CV and all-cause mortality (Citation1) and that, in patients with hypertension, reduction of BP improves outcomes (Citation2). Based on prior studies, current hypertension guidelines recommend treatment to reduce systolic (SBP) and diastolic (DBP) pressure to < 140/90 mmHg and to < 130/80 in diabetic hypertensive patients (Citation3–5). However, whether to lower BP more aggressively in hypertensive patients both with and without diabetes remains unclear (Citation6).
Randomized treatment to lower SBP targets in diabetic hypertensives has not reduced CV outcomes (Citation7,Citation8), and has been associated with higher rates of adverse events attributable to antihypertensive treatment (Citation7) and with a 15% higher risk of all-cause mortality in extended follow-up (Citation8). In the general population of hypertensive patients, findings have been mixed (Citation9–13). The Italian Study on Cardiovascular Effects of Systolic Blood Pressure Control (Cardio-Sis) of 1111 non-diabetic patients (Citation9) found a more aggressive SBP target (< 130 mmHg) was superior to a less aggressive target (< 140 mmHg) in reducing the primary endpoint of the prevalence of electrocardiographic (ECG) left ventricular hypertrophy (LVH) at 2 years follow-up. In contrast, stricter control of SBP was not associated with improved outcomes in elderly hypertensive patients (Citation10,Citation11), black hypertensive patients with chronic kidney disease (Citation12) and in the Losartan Intervention For Endpoint reduction (LIFE) study population of hypertensive patients with ECG LVH (Citation13). Indeed, achievement of SBP ≤ 130 mmHg among LIFE study patients with ECG LVH at baseline was associated with a significantly increased risk of death and a trend towards higher CV mortality (Citation13). However, it remains unclear why treatment to lower SBP has not improved outcomes.
It is well recognized that hypertensive patients with ECG LVH are at increased risk of CV morbidity and mortality (Citation14) and that regression of ECG LVH confers a decreased risk of CV morbidity and CV and all-cause mortality in patients with hypertension, whereas failure to regress ECG LVH is associated with increased risk (Citation15–19). In part because achieved SBP ≤ 130 mmHg in LIFE was not associated with greater reduction in ECG LVH than higher in-treatment SBP (Citation13), we hypothesized that failure to regress ECG LVH despite lower achieved SBP would identify a subset of LIFE study patients at higher risk of CV morbidity and CV and all-cause mortality. Therefore, the purpose of the present study was to examine whether persistence of ECG LVH by Cornell product criteria was associated with increased risk of CV morbidity and CV and all-cause mortality in patients with mean SBP ≤ 130 mmHg during antihypertensive treatment.
Methods
Subjects
The LIFE study (Citation20,Citation21) enrolled hypertensive patients with ECG LVH by Cornell product (Citation22) and/or Sokolow–Lyon voltage criteria (Citation23) on a screening ECG in a prospective, double-blind study large enough (n = 9193) to demonstrate an appreciable reduction in mortality and morbid events with use of losartan as opposed to atenolol (Citation20). Eligible patients were men and women aged 55–80 with previously untreated or treated essential hypertension with mean BP in the range 160–200/95–115 mmHg after 1 and 2 weeks on placebo. The present study included the 463 patients with mean in-treatment SBP ≤ 130 mmHg (213 women, 250 men, mean age 65 ± 7 years).
Treatment regimens
Blinded treatment was begun with losartan 50 mg or atenolol 50 mg daily and matching placebo of the other agent with a target BP of 140/90 mmHg or lower. During clinic visits at frequent intervals for the first 6 months and at 6-month intervals thereafter, study therapy could be up-titrated by addition of hydrochlorothiazide 12.5 mg followed by increase in blinded losartan or atenolol to 100 mg per day. In patients whose BP was still not controlled, additional open-label upward titration of hydrochlorothiazide and, if necessary, institution of therapy with a calcium channel blocker or additional other medications (excluding beta-blockers, angiotensin-converting enzyme inhibitors, or AT1-receptor antagonists) was added to the double-blind treatment regimen (Citation21).
Electrocardiography
Hard-copy ECGs were interpreted at a core laboratory by experienced readers blinded to clinical information as previously reported in detail (Citation17–21). QRS duration was measured to the nearest 4 ms in all 12 leads and R-wave amplitudes in leads aVL, V5, and V6 and S-wave amplitudes in leads V1 and V3 were measured to the nearest 0.5 mm (0.05 mV). The product of QRS duration times the Cornell voltage combination [RaVL+SV3, with 6 mm (0.6 mV) added in women (Citation17,Citation22)] > 2440 mm ms or Sokolow–Lyon voltage (SV1 + RV5/6)> 38 mm (Citation17,Citation23) were used to identify ECG LVH. For the present analyses, persistence of Cornell product LVH during treatment was defined by the mean of all Cornell product determinations during treatment (after baseline) being > 2440 mm ms.
Endpoint determination
The LIFE trial used a composite endpoint of CV death, non-fatal MI, or non-fatal stroke and the individual components according to previously defined criteria (Citation21). These potential endpoints and all-cause mortality were ascertained and then verified by an expert endpoint committee as previously described (Citation20,Citation21).
Statistical methods
Data management and analysis were performed with SPSS version 19.0 software. Data are presented as mean± SD for continuous variables and proportions for categorical variables. Patients were classified into two groups according to the presence of LVH by mean in-treatment Cornell product criteria > 2440 mm ms. Differences in prevalence between groups were compared using χ2 analyses and mean values of continuous variables were compared using unpaired Student's t-test.
Event rates were calculated and plotted according to the Kaplan–Meier product limit method and statistical significance tested using the log-rank statistic. The relation of outcomes to persistence of Cornell product LVH was assessed using Cox proportional hazards models. Partial residuals were plotted against survival times and visually examined to check the proportional hazards assumption. Because the relatively small number of events precludes using a multivariable Cox model which includes all baseline clinical and demographic characteristics that differ between patients with and without persistent LVH and may also be potential risk markers for the outcomes being assessed, a propensity score for persistent in-treatment mean Cornell product LVH was calculated using logistic regression analysis in which persistent Cornell product LVH was the dependent variable and the variables listed in and baseline variables in were the independent variables. To test the independence of the relationship of outcomes to persistence of Cornell product LVH, persistence of LVH was entered into a multivariable Cox model that also included as covariates the propensity score for persistent Cornell product LVH, randomized treatment allocation and baseline Framingham risk score as standard covariates and baseline and subsequent determinations of DBP and Sokolow–Lyon voltage as a time-varying covariates.
Table I. Demographic and clinical characteristics in relation to persistence of mean Cornell product left ventricular hypertrophy during treatment.
Table II. Baseline and change from baseline to last in-study measurement of blood pressure and electrocardiographic left ventricular hypertrophy in relation to persistence of mean Cornell product left ventricular hypertrophy during treatment.
Cox analyses for the prediction of the composite endpoint were repeated stratifying the population by treatment group, sex, age and by the presence or absence of LVH by Sokolow–Lyon voltage on the baseline ECG. Interaction between persistence of mean Cornell product LVH and these variables was formally tested by adding cross-product terms of persistence of LVH and these variables into the models of the total population. For all tests, a two-tailed p-value < 0.05 was required for statistical significance.
Results
There were 211 patients (45.6%) with persistence of ECG LVH defined by mean in-treatment Cornell product > 2440 mm ms and 252 patients (54.4%) whose mean Cornell product was ≤ 2440 mm ms. Baseline clinical and demographic characteristics of patients in relationship to mean Cornell product during treatment are shown in . Patients with persistence of ECG LVH during treatment were older, more likely to be female, have a history of ischemic heart disease, myocardial infarction, stroke and heart failure, and had higher baseline body mass indexes, but were similar with respect to treatment randomization and other baseline characteristics.
BP and ECG LVH measurements at baseline and changes in these measurements between baseline and last in-study determination in relation to in-treatment persistence of Cornell product LVH are shown in . Patients with in-treatment mean Cornell products > 2440 mm ms had similar baseline SBP and DBP and similar decreases in these measurements between baseline and last in-study determination. Patients with persistent LVH by Cornell product criteria had more severe baseline ECG LVH by Cornell product criteria but less severe baseline ECG LVH by Sokolow–Lyon voltage, whereas regression of both Cornell product and Sokolow–Lyon voltage was significantly lower in patients with persistence of Cornell product LVH.
During a mean follow-up of 4.4 ± 1.3 years, CV death occurred in 31 patients (6.7%), myocardial infarction in 26 (4.3%), stroke in 24 (5.2%), the LIFE composite endpoint of CV death, myocardial infarction or stroke in 63 (13.6%) and 71 patients (15.3%) died from any cause. The relationship of all outcomes to persistent LVH by Cornell product criteria is shown in and –. Persistence of mean Cornell product > 2440 mm ms was associated with significantly higher 4-year rates of CV death (8.9% vs 3.4%, p = 0.003), myocardial infarction (7.0% vs 3.3%, p = 0.010), stroke (8.5% vs 2.1%, p = 0.002), the composite endpoint of these events (20.0% vs 7.0%, p < 0.001) and all-cause mortality (14.9% vs 10.0%, p = 0.015). In univariate Cox analyses (), compared with mean in-treatment Cornell product ≤ 2440 mm ms, mean in-treatment Cornell product > 2440 mm ms identified patients with statistically significant 71–281% higher risks of all events. In multivariable Cox analyses adjusting for randomized treatment allocation, Framingham risk score and the propensity score for persistent Cornell product LVH entered as standard covariates and baseline and in-treatment DBP and Sokolow–Lyon voltage as time-varying covariates, persistence of Cornell product LVH during treatment remained associated with significantly increased risks of CV death, stroke and the LIFE composite endpoint, but was not a significant predictor of myocardial infarction or all-cause mortality. Of note, replacement of in-treatment Sokolow–Lyon voltage with change in Sokolow–Lyon voltage, also included as a time-varying covariate, did not change these results (data not shown).
Figure 1. Kaplan–Meier survival curves illustrating the rate of myocardial infarction according to the presence of left ventricular hypertrophy (LVH) by mean in-treatment Cornell product > 2440 mm ms.
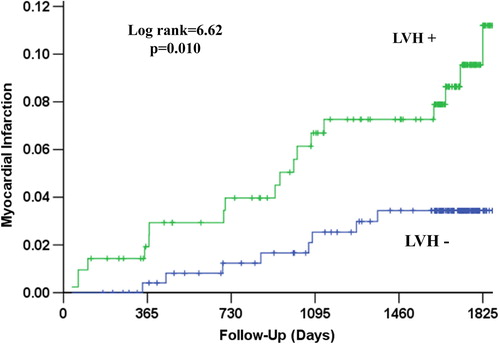
Figure 2. Kaplan–Meier survival curves illustrating the rate of cardiovascular mortality according to the presence of left ventricular hypertrophy (LVH) by mean in-treatment Cornell product > 2440 mm ms.
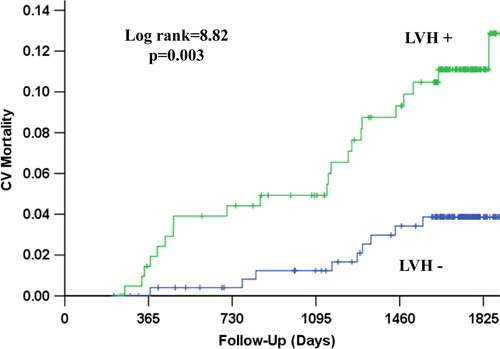
Figure 3. Kaplan–Meier survival curves illustrating the rate of stroke according to the presence of left ventricular hypertrophy (LVH) by mean in-treatment Cornell product > 2440 mm ms.
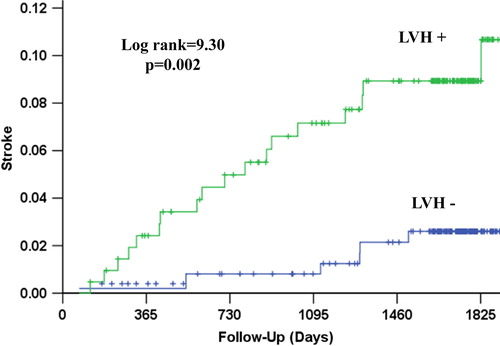
Figure 4. Kaplan–Meier survival curves illustrating the rate of the composite endpoint of myocardial infarction, cardiovascular mortality or stroke according to the presence of left ventricular hypertrophy (LVH) by mean in-treatment Cornell product > 2440 mm ms.
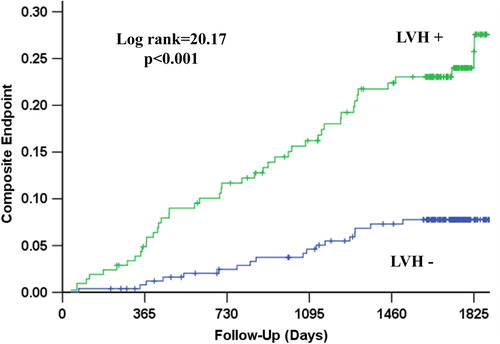
Figure 5. Kaplan–Meier survival curves illustrating the rate of all-cause mortality according to the presence of left ventricular hypertrophy (LVH) by mean in-treatment Cornell product > 2440 mm ms.
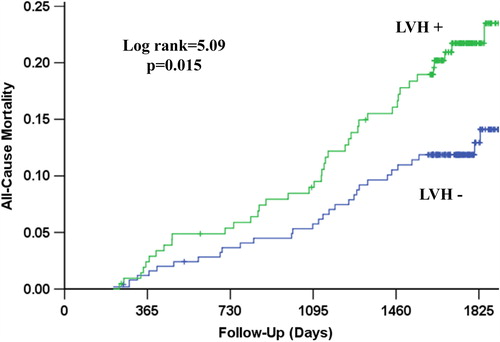
Table III. Four-year event rates, univariate and multivariable Cox regression analyses to assess the relation of outcomes to persistence of mean Cornell product left ventricular hypertrophy during treatment.
The predictive value of persistence of mean in-treatment Cornell product LVH for the composite endpoint in relevant subsets of the population is examined in . The association between persistence of Cornell product LVH and increased risk of the composite endpoint was similar in both treatment arms, men and women, and in patients with and without LVH on their baseline ECG by Sokolow–Lyon voltage criteria, with non-significant interaction terms for these variables. There was a trend towards a significant interaction of persistent Cornell product LVH with age, with patients < 65 years old having a higher risk of the composite endpoint associated with persistence of LVH than older patients ().
Table IV. Univariate Cox analyses to assess the predictive value of persistence of mean Cornell product left ventricular hypertrophy during treatment for the composite endpoint in relevant subgroups of the study population.
Discussion
Among hypertensive patients with ECG LVH at baseline who achieve mean in-treatment SBP ≤ 130 mmHg during antihypertensive treatment, persistence of Cornell product LVH was associated with significantly higher rates of CV death, myocardial infarction, stroke, the composite endpoint of these three outcomes and all-cause mortality. After controlling for randomized treatment to losartan vs atenolol, baseline Framingham risk score, a propensity score for the presence of mean Cornell product LVH during treatment that takes into account differences in risk factors between patients with and without persistence of LVH, in-treatment DBP and Sokolow–Lyon voltage, persistence of Cornell product LVH remained associated with statistically significant increased adjusted risks of CV mortality, stroke and the LIFE study composite endpoint. These findings suggest that the previously demonstrated lack of benefit and potential hazard observed in hypertensive patients despite treatment to lower SBP (Citation13) may be in part explained by the greater CV risk associated with failure to regress LVH in a subset of these patients with lower achieved SBP during antihypertensive treatment.
Previous studies have clearly demonstrated the strong association between higher BP and increased risk and that BP reduction can reduce this risk in patients with hypertension (Citation1–4). However, the target level BP to which patients should be treated remains unclear. Current hypertension guidelines recommend treatment to reduce BP to < 130/80 in hypertensive patients with diabetes (Citation3,Citation4) to further reduce risk, but studies examining treating to lower SBP targets in diabetic hypertensive patients have not supported these recommendations (Citation7,Citation8). In the ACCORD BP trial (Citation7), randomized treatment to achieve a SBP < 120 mmHg, compared with < 140 mmHg, failed to reduce the composite outcome of fatal and non-fatal CV events and was associated with a greater risk of serious adverse events from antihypertensive treatment. Similarly, tight SBP control did not improve CV outcomes in diabetic, hypertensive patients with coronary disease in the INVEST study and, in extended follow-up, was associated with a 15% increased risk of all-cause mortality (Citation8).
Treatment to lower achieved BP levels has, for the most part, also not been associated with improved prognosis in the general population of hypertensive patients (Citation9–13,Citation24). Studies examining elderly hypertensive patients (Citation10,Citation11), black hypertensive patients with chronic kidney disease (Citation12) and a meta-analysis of over 22,000 patients comparing different DBP targets (Citation24) found that stricter BP control did not reduce morbidity or mortality. Intriguingly, randomized treatment to a more aggressive SBP target (< 130 mmHg) was superior to a less aggressive SBP target (< 140 mmHg) in reducing the primary endpoint of the prevalence of ECG LVH at 2 years follow-up in 1111 non-diabetic hypertensive patients with a baseline SBP ≥ 150 mmHg (hazard ratio, HR = 0.50, 95% CI 0.31–0.79, p = 0.003) (Citation9). Unfortunately, this study was not powered to assess whether treatment to lower achieved SBP was associated with reduction of hard end-points and, if so, whether differences in outcome could be even in part explained by the lower prevalence of ECG LVH among the more aggressively treated group (Citation9).
A recent analysis of the overall LIFE study population from which the current study group was drawn further supports the general lack of benefit observed with achievement of lower SBP (Citation13). In the total LIFE study population of hypertensive patients with ECG LVH on a screening ECG prior to study enrollment, compared with in-treatment SBP ≥ 142 mmHg, in-treatment achievement of a SBP ≤ 130 mmHg was not associated with a significantly decreased risk of CV morbidity or mortality but did identify a subgroup of patients who had increased all-cause mortality risk. In contrast, patients with in-treatment SBP between 131 and 141 mmHg had significantly lower risks of myocardial infarction, stroke and the LIFE composite endpoint. These findings were independent of the possible impact of standard CV risk factors, randomized treatment assignment, baseline and in-treatment DBP and of the previously demonstrated prognostic value of in-treatment ECG LVH and heart rate in this population (Citation17,Citation25). However, both in this and other studies, possible reasons why treatment to lower SBP levels has been associated with lack of improvement and potential hazard remain unclear.
Findings from the ACCORD diabetes arm (Citation26,Citation27) provide a theoretical framework for examining this issue. In the ACCORD diabetes arm (Citation26), randomized treatment comparing an intensive glycemic treatment strategy with a standard strategy was terminated early due to an unexpected increased mortality in the intensive treatment arm, despite lower median glycated hemoglobin (HbA1c) levels. However, subsequent analyses demonstrated that the minority subgroup of patients with HbA1c > 7% accounted for the excess mortality risk associated with the intensive treatment arm (Citation27,Citation28). Further analyses demonstrated that higher risk in the intensive treatment arm was associated with less reduction in HbA1c from baseline levels during the first 4–12 months of treatment (Citation27,Citation28). Consistent with this construct, additional analyses in the current study demonstrate that patients with higher risk as manifested by having one of the composite endpoints had significantly lower reductions in Cornell product LVH between baseline and year 1 of the study (− 35 ± 751 vs − 264 ± 649 mm ms, p = 0.021). This suggests that it is the lack of improvement in end-organ damage as manifested by lesser regression and persistence of Cornell product LVH in response to lower achieved SBP that may be behind the increased CV morbidity in this patient subgroup.
There are several other potential explanations for the current findings. First, it is possible that persistence of LVH identifies a subset of hypertensive patients in whom excessive decreases in BP may be associated with an increase in morbidity and mortality at lower BP levels due to hypoperfusion (Citation29–32), the so-called J-curve phenomenon. However, a recent editorial (Citation33) urges caution with respect to the interpretation of these data and suggests the need for a correctly designed randomized trial to better address this question. It is interesting to note that the results of one of the large meta-analyses regarding this topic identified patients with achieved SBP of 131–140 mmHg to have the lowest age- and sex-adjusted death rates (Citation30), mirroring subsequent data in the overall LIFE study population (Citation13). Second, failure to regress LVH despite achievement of lower SBP may identify a subgroup of patients with a higher burden of risk factors or undetected underlying disease, which could lead to increased CV risk (Citation29,Citation30). Although this concept is partially supported by the greater prevalences of ischemic heart disease, prior myocardial infarction, stroke and heart failure among patients with persistent LVH in the current study (), persistent LVH remained associated with increased CV risk in this study even after adjusting for the propensity score for persistent LVH and other risk factors in the multivariate Cox models, making this less likely.
Study limitations
Several limitations of the present study warrant review. First, this is a post hoc analysis of a previously conducted randomized clinical trial that did not randomize patients to different levels of ECG LVH control. This could lead to possible sources of confounding due to differences between the achieved ECG LVH groups both at baseline and during the trial. Although we control for known, measured differences between groups, the propensity score for persistent Cornell product LVH and for the possible effects of treatment and in-treatment DBP, and Sokolow–Lyon LVH on outcome, multivariable analyses may not fully adjust for these differences and cannot adjust for other potential factors that were not measured. As a consequence, whether persistence of Cornell product LVH may be a marker of existing disease as opposed to causative of increased mortality risk cannot be definitively addressed using this approach. Second, use of ECG LVH criteria to select patients for LIFE increased the baseline risk of the population, suggesting that caution should be used in generalizing these findings to hypertensive patients at lower risk. However, it has been estimated that nearly 8 million patients in the first 15 member nations of the EU would meet LIFE entry criteria, with similar numbers in the remainder of Europe and the USA, indicating that the present results are applicable to a substantial patient population (Citation34).
Implications
The present findings suggest that persistence of Cornell product LVH during treatment of hypertension to an achieved SBP ≤ 130 mmHg is associated with significantly higher risk of CV mortality, stroke and the LIFE composite endpoint. These findings suggest a similar failure to regress LVH in subsets of patients with lower achieved SBP during treatment may in part explain the lack of prognostic benefit of treating to lower SBP goals found in previous studies of hypertensive patients (Citation7,Citation8,Citation10–13). These results suggest that it may be necessary to track hypertensive end-organ damage, in this case as manifested by ECG LVH, in addition to BP to fully assess response to treatment in hypertensive patients. Further study will be necessary to determine whether treatment to lower SBP goals should be reconsidered in the presence of persistent ECG LVH and whether treatment to specifically regress ECG LVH should take precedence over therapy aimed at further lowering BP once adequate BP control has been achieved.
Funding sources
Supported in part by grant COZ-368 and an Investigator Initiated Study grant from Merck & Co., Inc., West Point, PA, USA.
Disclosures
Dr. Okin has received grant support from Merck & Co., Inc. and Novartis, serves on a medical advisory board for GE Medical Systems and as a consultant to Novartis. Ms. Hille is employed by Merck & Co., Inc. and owns stock or stock options in Merck & Co., Inc. Dr. Kjeldsen receives grant support from Pronova and Astra-Zeneca, receives honoraria from Astra-Zeneca, Takeda, Medtronic and Nycomed, serves as a consultant to Bayer, Medtronic, Serodeus and Takeda, and receives royalties from Gyldendal. Dr. Dahlöf has received honoraria, support for travel to meetings, fees for participating in monitoring boards and administrative support from MSD, served on boards for Novartis and Boehringer-Ingelheim, been a consultant for Boryung, served on speakers bureaus for Novartis, Boehringer-Ingelheim, Pfizer, MSD, Krka, Vicore Pharma and Lundbeck and owns stock or stock options in Mintage Scientific AB. Dr. Devereux has received grant support, consulting fees and support for travel to meetings from Merck & Co., Inc.
Clinical Trials Registration: http://clinicaltrials.gov/ct/show/NCT00338260?order = 1
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913.
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: Results of prospectively designed overviews of randomised trials. Lancet. 2000; 355:1955–1964.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762.
- Zanchetti A, Mancia G, Black HR, Oparil S, Waeber B, Schmieder RE, et al. Facts of fallacies of blood pressure control in recent trials: Implications in the management of patients with hypertension. J Hypertens. 2009;27:673–679.
- The ACCORD Study Group. Effects of intensive blood- pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
- Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010; 304:61–68.
- Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): An open-label randomised trial. Lancet. 2009;374:525–533.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan Hypertension in the Elderly Isolated Systolic Hypertension Study. Hypertension. 2010;56:196–202.
- Appel LJ, Wright JT, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010; 363:918–929.
- Okin PM, Hille DA, Kjeldsen SE, Dahlöf B, Devereux RB. Impact of lower achieved blood pressure on outcomes in hypertensive patients. J Hypertens. 2012;30:802–810.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 1998;31:383–390.
- Verdecchia P, Reboldi G, Angeli F, Avanzini F, de Simone G, Pede S, et al. Prognostic value of serial electrocardiographic voltage and repolarization changes in essential hypertension: The HEART Survey Study. Am J Hypertens. 2007;20: 997–1004.
- Fagard RH, Staessen JA, Thijs L, Celis H, Birkenhäger WH, Bulpitt CJ, et al. Prognostic significance of electrocardiographic voltages and their serial changes in elderly with systolic hypertension. Hypertension. 2004;44:459–464.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and prediction of major cardiovascular events: The LIFE Study. JAMA. 2004;292:2343–2349.
- Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, et al. Reduction of electrocardiographic left ventricular hypertrophy is associated with decreased heart failure hospitalization in hypertensive patients. Ann Intern Med. 2007;147:311–319.
- Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new- onset atrial fibrillation: The LIFE Study. JAMA. 2006; 296:1242–1248.
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003.
- Dahlöf B, Devereux R, de Faire U, Fyhrquist F, Hedner T, Ibsen H, et al. The Losartan Intervention For Endpoint Reduction (LIFE) in Hypertension Study: Rationale, design, and methods. Am J Hypertens. 1997;10:705–713.
- Okin PM, Roman MJ, Devereux RB, Kligfield P. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995; 25:417–423.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186.
- Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension (Review). Cochrane Database Syst Rev.2009(3): CD004349.
- Okin PM, Kjeldsen SE, Julius S, Hille DA, Dahlöf B, Edelman JM, et al. All-cause and cardiovascular mortality in relation to changing heart rate during treatment of hypertension: The LIFE Study. Eur Heart J. 2010;31:2271–2279.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559.
- Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–990.
- Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the action to control cardiovascular risk in diabetes (ACCORD) trial. Circulation. 2010;122:844–846.
- Pastor-Barriuso R, Banegas JR, Damián J, Appel LJ, Guallar E. Systolic blood pressure, diastolic blood pressure, and pulse pressure: An evaluation of their joint effect on mortality. Ann Intern Med. 2003;139:731–739.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP, for the INDANA Project Steering Committee. J-shaped relationship between blood pressure and mortality in hypertensive patients: New insights from a meta-analysis of individual-patient data. Ann Intern Med. 2002;136:438–448.
- Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary disease be dangerous?. Ann Intern Med. 2006:144: 884–893.
- Bangalore S, Messerli FH, Wun C-C, Zuckerman AL, DeMicco D, Kostis JB, et al. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) trial. Eur Heart J. 2010;31:2897–2908.
- Zanchetti A. Blood pressure targets of antihypertensive treatment: Up and down the J-shaped curve. Eur Heart J. 2010; 31:2837–2840.
- Dahlöf B, Burke TA, Krobot K, Carides GW, Edelman JM, Devereux RB, et al. Population impact of losartan use on stroke in the European Union (EU): Projections from the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. J Human Hypertens. 2004;18:367–373.

