Abstract
Aim. Diastolic dysfunction related to hypertensive left ventricular hypertrophy (LVH) has been shown to affect right-sided cardiac morphology and haemodynamics. As left atrial enlargement (LAE) is a marker of chronically elevated left ventricular (LV) filling pressure and diastolic dysfunction, we investigated the relationship between LAE and right ventricular hypertrophy (RVH) in systemic hypertension. Methods. A total of 330 essential hypertensives, categorized according to tertiles of left atrial (LA) diameter indexed to body surface area were considered for the analysis. All subjects underwent a quantitative echocardiographic examination as well as extensive clinical and laboratory investigations. RVH was defined as anterior right ventricular (RV) wall thickness ≥ 6.0/5.5 mm in men and women, respectively, and LVH as LV mass index ≥ 51/47 g/m2.7 in men and women, respectively. Results. The prevalence of LVH increased across LA diameter tertiles from 21.0% to 50% and that of RVH from 26.3% to 41.8% (p < 0.01for both). This was also the case for biventricular hypertrophy (from 10.0% to 26.0%, p < 0.01). Differences in both LV and RV structure across LA diameter tertiles remained significant after adjusting for age, office systolic/diastolic blood pressure and duration of hypertension. Similar results were obtained when study population was divided according to absolute LA diameter tertiles. Conclusions. Our findings provide further evidence of an interaction between left and right chambers in systemic hypertension by showing that LAE is associated with RVH. The clinical and prognostic implications of such observation remain be evaluated in future prospective studies.
Introduction
Left ventricular hypertrophy (LVH) is the major manifestation of hypertensive heart disease reflecting an adaptive response to chronic elevation of systemic blood pressure (BP) aimed to counterbalance left ventricular (LV) wall stress (Citation1,Citation2). A variety of other structural and functional alterations of the heart such as myocardial fibrosis, left atrial enlargement (LAE), aortic dilatation, right ventricular hypertrophy (RVH), systolic/diastolic dysfunction, impaired coronary reserve, arrhythmias may also occur in hypertensive patients as a consequence of haemodynamic and non-haemodynamic factors (Citation3,Citation4).
Two key pathological processes may affect myocardial structure in hypertensive LVH: myocyte hypertrophy and accumulation of fibrous tissue within cardiac interstitium (Citation5,Citation6). These changes result in a distortion of tissue texture, increased myocardial stiffness leading to diastolic dysfunction and LAE (Citation7). In hypertensive heart disease, LAE is a reliable marker of chronically elevated LV filling pressure and diastolic dysfunction, in the absence of mitral valve diseases; the increase in left atrial (LA) size, indeed, tends to counterbalance the impairment of LV compliance and the progression of LV diastolic dysfunction in the hypertrophied ventricle (Citation8,Citation9). LV diastolic dysfunction related to hypertensive LVH markedly also affects right-sided cardiac morphology and haemodynamics as pulmonary venous hypertension secondary to elevated left-sided pressures is a powerful determinant of RVH and functional impairment (Citation10–12). Numerous experimental and clinical studies have described a parallel increase in LV and RV wall thickness associated with deterioration of diastolic parameters in both chambers (Citation13,Citation14). Since LAE in the setting of systemic hypertension is strongly related to diastolic dysfunction and LVH, which in turn is responsible of venous pulmonary hypertension and RV pressure overload, we hypothesized that LA size is a marker of a parallel involvement of both ventricles. Thus, the primary aim of this study was to investigate the relationship between LA size and RV wall thickness as well as the prevalence of RVH and biventricular hypertrophy in a large group of essential hypertensive patients free of overt cardiovascular and pulmonary diseases.
Methods
Study population
Three hundred and thirty consecutive treated and untreated essential hypertensive patients, mostly referred to our hypertension clinic by general practitioners during a 6-month period from January to June 2007, were included in the study.
High BP was defined as systolic BP (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg in untreated subjects; treated hypertensives were included regardless of BP values. Main exclusion criteria were history or evidence of congestive heart failure, atrial fibrillation, previous stroke, significant cardiac valve disease (regurgitation > 1 + at Doppler examination, stenosis of any degree or presence of prosthesis), previous myocardial infarction or coronary bypass, secondary causes of hypertension, lung disease and pulmonary arterial hypertension. After an informed consent had been obtained during the initial visit, all patients underwent the following procedures within a 1–2-week interval: medical history and physical examination, clinic BP measurement, blood and urine sampling, standard 12-lead electrocardiogram, M-mode, two-dimensional and Doppler echocardiographic examination. In all subjects, laboratory tests for secondary hypertension were performed when considered appropriate on clinical grounds. The study protocol was approved by the Ethic Committee of one of the Institutions involved (Istituto Auxologico Italiano).
Office BP measurement
BP was measured by a physician during two visits at the outpatient clinic using a mercury sphygmomanometer and taking the first and fifth phases of Koroktoff sounds to identify systolic and diastolic values, respectively. At each visit, three measurements were taken at 1-min interval after the subjects had rested for 5 min in the sitting position; the average value was used to define office SBP and DBP.
Echocardiography
Left ventricle. Technical details have been reported previously (Citation14). Briefly, end-diastolic and end- systolic left ventricular internal diameter (LVIDd, LVIDs), interventricular septum thickness (IVST) and posterior wall thickness (PWT) were measured on two-dimensionally guided M-mode tracings during at least five cycles according to the Penn Convention. Left ventricular mass (LVM) was calculated by Devereux's formula (Citation15) and normalized to body height2.7 (Citation16). Relative wall thickness was calculated as the ratio between PWT plus IVST and LV diastolic internal diameter. Patterns of abnormal left ventricular geometry were defined as follows: (i) LV concentric remodelling (normal LVM index combined with relative wall thickness ≥ 0.43); (ii) eccentric LVH (increased LVM index combined with relative wall thickness < 0.43); concentric LVH (increased LVM index combined with relative wall thickness ≥ 0.43 (Citation17).
LV filling was assessed by recording mitral flow by standard pulsed Doppler technique in apical four-chamber view; the following parameters were considered: early diastolic peak flow velocity (Em), late diastolic flow velocity (Am), (E/A)m ratio and Em wave deceleration time (from peak Em-wave to baseline). LV myocardial systolic function was assessed as the midwall circumferential shortening and calculated by a two-shell cylindrical model (Citation18).
Right ventricle. RV internal end-diastolic diameter and RV end-diastolic thickness were measured on two-dimensionally guided M-mode tracings in the parasternal long-axis view (anterior wall) at the outflow tract level as well as in the subcostal view at the tips level of the tricuspid valve. RV filling was assessed by recording tricuspid flow by standard pulsed Doppler technique in apical four-chamber view and the following parameters were considered: early diastolic peak flow velocity (Et), late diastolic flow velocity (At), (E/A)t ratio and Et wave deceleration time (from peak Et-wave to baseline). RV SBP was assessed only in a subset of 80 patients with minimal/mild tricuspid regurgitation (≤ 1+) by measuring the peak velocity of the regurgitant jet in the right atrium (data not shown).
Left and right atrium. LA diameter was assessed by the parasternal long-axis view using a leading edge-to-leading edge measurement of the maximal distance between posterior aortic root wall and posterior left atrial wall at end systole (Citation17).
Right atrial longitudinal diameter was measured in apical four-chamber view at ventricular end-systole.
Definition of cardiac phenotypes
LVH was defined by LVM index equal or greater than 51 g/m2.7 in men and 47 g/m2.7 in women (Citation16) and RVH by RV anterior thickness ≥ 6.0 mm/m2 in men and ≥ 5.5 mm/m2 in women (Citation19). RVH thresholds correspond to the 95th percentile in a group of 90 healthy normotensive adults evaluated in our echo-lab (Citation14). Biventricular hypertrophy was defined when both criteria were fulfilled. LAE was defined as absolute LA diameter > 41 mm in men and > 37 mm in women (Citation20).
Statistical analysis
Statistical analysis was performed by the SAS system (version 6.12; SAS Institute Inc., Cary, North Carolina, USA). Values were expressed as means± SD or percentages. Continuous variables were compared by analysis of variance (ANOVA), using the Student's t-test for dual comparison. Analysis of categorical data was carried out by the χ2 test or Fischer's exact test when appropriate. Simple Pearson's correlations were used to assess the bivariate association of RV anterior thickness with clinical (age, body size measures, clinic BP, duration of hypertension), laboratory [plasma glucose, low-density lipoprotein (LDL)-cholesterol, triglycerides, and serum creatinine] and echocardiographic data. Independent correlates of RV anterior thickness were assessed by means of standard multiple linear regression analyses. The candidate explanatory variables were selected on the basis of univariate correlations and pathophysiological associations with the dependent variable. Variables significantly correlated (p < 0.05) with the dependent variable of interest were included in the multiple regression models. The limit of statistical significance was set at p < 0.05.
Results
Of the 330 hypertensive patients examined, 204 were males (62%). Mean age was 58 ± 12 years, mean SBP and DBP were 139 ± 14 and 88 ± 10 mmHg, respectively; 90% of patients were on antihypertensive treatment (26% on mono-therapy and 74% on two or more drugs). Clinic BP values were ≥ 140 and/or ≥ 90 mmHg in 65% of the study sample. Current smokers were 14%; metabolic syndrome (MS), as defined by ATP III report amended criteria, was present in 45%, obesity (body mass index ≥ 30 kg/m2) in 17% and type 2 diabetes mellitus in 10% of the study sample. Overall, LVH criteria (i.e. LVM index ≥ 51/47 g/m2.7) were fulfilled in 114 patients (35%) and RVH criteria (i.e. anterior RVWT ≥ 6.0/5.5 mm) in 111 (34%). Biventricular hypertrophy was detected in 59 patients (18%).
shows demographic and clinical characteristics of patients divided according to tertiles of LA diameter indexed to body surface area.
Table I. Demographic and clinical characteristics of the study population categorized according to tertiles of left atrial diameter indexed to body surface area.
A progressive increase in mean age, prevalence of female gender and MS as well as the fraction of patients taking two or more antihypertensive drugs occurred across LA diameter tertiles. An opposite trend was observed for body surface area, body mass index and abdominal circumference as a likely consequence of the increase in female/male ratio from the lowest to the highest tertile. Office DBP was lower in the highest tertile, while SBP did not substantially differ among the three groups.
Echocardiographic parameters are shown in . Absolute LA diameter, LVM index and mitral deceleration time showed a progressive increase across the groups, whereas (E/A)m values displayed the opposite trend.
Table II. Echocardiographic parameters of the left and right chambers in the study population categorized according to tertiles of left atrial diameter indexed to body surface area.
With regard to right chambers, the following parameters were different among the groups: RA diameter and (E/A)t ratio. No differences were found in RV diameter and RV anterior wall thickness.
LVH and RVH prevalence
LVH increased significantly across the tertiles of LA diameter index from 21.0% to 50.0% (p < 0.01) and RVH from 26.3% to 41.8% (p < 0.01). This was also the case for biventricular hypertrophy, as defined under the Methods (from 10.0% to 26.0%, p < 0.01) (). The differences in both LV and RV structure across LA diameter tertiles remained significant after adjustment for age, gender, office SBP/DBP and duration of hypertension.
Figure 1. Prevalence rates of biventricular hypertrophy in hypertensive subjects divided according to tertiles of left atrial antero-posterior diameter indexed to body surface area. p < 0.01, III vs I.
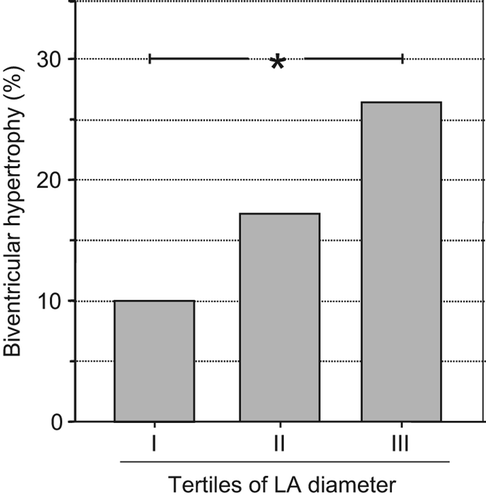
Similar results were obtained when the study population was divided according to absolute LA diameter tertiles (data not shown). Finally, a progressive increase in LA diameter occurred across the ventricular structural classes, namely from normal (35 ± 4 mm), to isolated RVH (36 ± 4 mm), isolated LVH (38 ± 4 mm) and biventricular hypertrophy (39 ± 4 mm) (p < 0.01).
Correlation analyses
reports univariate correlations between RV anterior wall thickness and demographic/ clinical characteristics in the whole study population. Age, abdominal circumference, office SBP, LVM index, LA diameter and Et deceleration time exhibited a significant direct correlation with RV wall thickness, whereas (E/A)m ratio and mid-wall shortening showed an inverse correlation. When these variables were tested in multiple regression analyses, LVM index, abdominal circumference, age and mid-wall shortening but not LA diameter were independently correlated with RV wall thickness (). Only after removing LV mass index from the model, LA diameter became an independent correlate of RV anterior wall thickness (beta = 0.221, p < 0.001).
Table IIIa. Univariate correlations between right anterior wall thickness and demographic/clinical characteristics in the study population (n = 330).
Table IIIb. Multivariate regression analysis of independent variables associated with right anterior wall thickness in the study population (n = 330).
Discussion
The present study assessed the relationship between LAE and RVH in a large sample of hypertensive patients unbiased by pre-selection criteria for LVH and referred to a single hypertension outpatient clinic. The main findings of our work can be summarized as follows: (i) the prevalence of LVH, RVH and biventricular hypertrophy increased from the lowest to the highest LA diameter tertile by 2.3-,1.6-, and 2.6-fold, respectively; (ii) the differences in both LV and RV structure across LA diameter tertiles remained significant after adjustment for several confounders including age, BP, duration of hypertension and anti-hypertensive treatment; (iii) a progressive increase in LA diameter occurred when patients were categorized in four groups according to their ventricular characteristics (i.e. normal structure, isolated RVH, isolated LVH and biventricular hypertrophy). Several aspects of these results deserve to be commented upon.
The data of the present study, in keeping with previous reports, support the view that LV structural alterations induced by systemic hypertension are paralleled by similar changes in RV chamber: biventricular hypertrophy, indeed, was present in about one fifth of the entire sample; moreover, RVH occurred in an additional 15% of the patients, without LVH. Overall, RVH was found approximately in one third of our series. In previous echocardiographic studies, the frequency of RVH ranged from 17% to 80% depending on the criteria of RVH and clinical characteristics of the patients. The highest RVH prevalence was found by Gottdiener et al. (Citation21) in 65 patients with LV pressure overload (49 with systemic hypertension and 16 with aortic valve stenosis) compared to 20 normal subjects. These authors reported that RV anterior wall thickness was increased (> 5 mm) in 80% and 63% of the patients with hypertension and aortic stenosis, respectively. This finding may be explained by the fact that Gottdiener and coworkers examined hypertensive patients with a severe degree of LVH, as clearly reflected by the mean LVM value (445 ± 113 g). In our series, mean LVM was markedly lower (195 ± 45 g) and LVH was detected only in one third of the patients. A recent meta-analysis by our group, based on a pooled population of 712 normotensive and hypertensive participants, showed that RV anterior wall was directly related with LV posterior wall thickness (r = 0.62, p < 0.01) (Citation22), thus supporting the view that RV structure in patients with systemic hypertension is strongly related to the extent of LVH. Haemodynamic, anatomical and humoral factors likely contribute to modulate RV structure and function in essential hypertension, in particular ventricular interdependence, loading conditions (i.e. increased LV filling pressure), venous pulmonary hypertension, renin–angiotensin–aldosterone and sympathetic activation (Citation23–25). The time relationship between RV alterations and the development of LVH and venous pulmonary hypertension remains to be clarified.
This study for the first time, to our knowledge, investigated the relationship of LA dimension, a sensitive marker of diastolic function, with RV wall thickness, a widely accepted index of RVH (Citation26). We found that hypertensive patients with LAE exhibited a higher prevalence of increased RV anterior wall thickness (i.e. isolated RVH or biventricular hypertrophy) than their counterparts with normal LA dimensions; the difference persisted after adjusting for demographic and clinical correlates such as age, gender, BP and use of antihypertensive drugs. It is worth noting that in multivariable analyses the correlation between LA diameter and RV wall thickness achieved statistical significance only when LVM was removed from the model, suggesting that the relation between LAE and RVH is mediated by LVH. This observation is in line with the concept that LAE in hypertensive patients, in the absence of other pathological conditions such as mitral valve diseases, is an adaptation process of the atrial chamber to a pressure overload driven by impaired LV filling and relaxation secondary to increased LV mass. Data provided by a recent meta-analysis in 9354 hypertensive patients reported that LVH prevalence was significantly higher in patients with LAE than in their counterparts (OR = 2.97, 95% CI 2.68–3.29, p < 0.01) (Citation27).
In previous studies focusing on RV structure in systemic hypertension, the relationship between LA size and RV wall thickness or RVH has not been specifically investigated. A rough evidence about this issue is provided by Tadic et al. (Citation28), who showed that non-dipper hypertensives had a higher RV anterior wall thickness (4.7 ± 0.87 mm vs 4.2 ± 0.84 mm) and LA antero-posterior diameter (38.7 ± 3.8 mm vs 35.3 ± 3.3 mm) than dippers (p < 0.001 for both); unfortunately, these differences were unadjusted for confounders.
Limitations of the study
Our study has several limitations. First, LAE was detected on the basis of a linear measurement of antero-posterior diameter in spite of the fact that numerous observations have shown that this approach may underestimate LA three-dimensional structure because of LA irregular geometry and LA expansion may occur along the longitudinal diameter. Nonetheless, LAE estimation based on this linear method is mostly used in research/clinical applications and has a prognostic value (Citation29). Second, systolic pulmonary pressure was estimated only in less than a quarter of the study population. A sub-analysis investigating the correlation of this parameter with LV and RV structure or LA size did not provide significant results (data not shown). Third, diastolic function was assessed by conventional Doppler, as colour flow propagation velocity and tissue Doppler analysis were not part of our echocardiographic protocol. Thus, the relation between RV structure and newer indexes of diastolic function was not investigated. Fourth, RV structure was determined by a single linear measurement of RV anterior wall from the parasternal long axis view by two-dimensionally guided M-mode tracings. The complex morphology of RV that can be hardly assimilated to a geometric model, at difference from the conical shape of LV, represents a major methodological limitation for a comprehensive assessment of RV chamber in echocardiographic studies.
Conclusions
Our findings provide a new piece of information about the interaction between left and right chambers in systemic hypertension by showing that LAE is associated with RVH and biventricular hypertrophy. The clinical and prognostic implications of this observation remain to be evaluated; in particular, future studies are needed to explore of the role of RV structure and function in cardiovascular risk classification and therapeutic strategies of hypertensive patients.
Conflicts of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens. 2008;21:500–506.
- Diez J, Frohlich E. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1–8.
- Raman SV. The hypertensive heart. An integrated understanding informed by imaging. J Am Coll Cardiol. 2010;55: 91–96.
- Schmieder R. The role of non-haemodynamic factors of the genesis of LVH. Nephron Dial Transplant. 2005;20:20–23.
- Cuspidi C, Ciulla M, Zanchetti A. Hypertensive myocardial fibrosis. Nephron Dial Transplant. 2006;21:2610–2612
- Frohlich E, Gonzales A, Diez J. Hypertensive left ventricular hypertrophy risk: Beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011;29:17–26.
- Cioffi G, Mureddu GF, Stefenelli C, de Simone G. Relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J Hypertens. 2004;22:1589–1596.
- Gerdts E, Oikarinen L, Palmieri V, Otterstad JE, Wachtell K, Boman K, Dahlof B, Devereux RB. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy. The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. Hypertension. 2002;39:739–743.
- Cuspidi C, Meani S, Fusi V, Valerio C, Catini E, Sala C, Sampieri L, Magrini F, Zanchetti A. Prevalence and correlates of left atrial enlargement in essential hypertension: Role of ventricular geometry and the metabolic syndrome. J Hypertens. 2005;23:875–882.
- Goncalvesova E, Luknar M, Lesny P. ECG signs of right ventricular hypertrophy may help to distinguish pulmonary arterial hypertension from pulmonary hypertension due to left ventricular diastolic dysfunction. Bratisl Lek Listy. 2011; 112:614–618.
- Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4: 257–265.
- Pedrinelli R, Dell’Omo G, Talini E, Canale ML, Di Bello V. Systemic hypertension and the right-sided cardiovascular system: A review of the available evidence. J Cardiovasc Med. 2009;10:115–121.
- Cicala S, Galderisi M, Caso P, Petrocelli A, D’Errico A, de Divitiis O, Calabrò R. Right ventricular diastolic dysfunction in arterial systemic hypertension: Analysis by pulsed tissue Doppler. Eur J Echocardiography. 2001;3:135–142.
- Cuspidi C, Negri F, Giudici V, Valerio C, Meani S, Sala C, et al. Prevalence and correlates of right ventricular hypertrophy in essential hypertension. J Hypertens. 2009;27: 854–860.
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass; Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MN, Laragh JH. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–1451.
- Cuspidi C, Valerio C, Sala C, Negri F, Esposito A, Masaidi M, et al. Metabolic syndrome and biventricular hypertrophy in essential hypertension. J Hum Hypertens. 2009;23:168–173.
- Cuspidi C, Meani S, Fusi V, Valerio C, Catini E, Sala C, et al. Prevalence and correlates of left atrial enlargement in essential hypertension: Role of ventricular geometry and the metabolic syndrome. J Hypertens. 2005;23:875–882.
- Gottdiener JS, Gay JA, Maron BJ, Flectcher RD. Increased right ventricular pressure overload: Echocardiographic determination of hypertrophic response of the “nonstressed” ventricle. J Am Coll Cardiol. 1985;6:550–555.
- Cuspidi C, Sala C, Muiesan ML, De Luca N, Schillaci G. Right ventricular hypertrophy in systemic hypertension: An updated review of clinical studies. J Hypertens. 2013; in press.
- Mirsky I, Laks M. Time course of changes in the mechanical properties of the canine right and left ventricle during hypertrophy caused by pressure over-load. Circ Res. 1980;46: 530–542.
- Guazzi MD, De Cesare N, Fiorentini C, Galli C, Montorsi P, Pepi M, Tamborini G. Pulmonary vascular super-sensitivity to catecholamines in systemic high blood pressure. J Am Coll Cardiol. 1986;8:1137–1144.
- Ventetuolo CE, Lima JA, Barr RG, Bristow MR, Bagiella E, Chahal H, et al. The renin–angiotensin system and the right ventricular structure and function: The MESA-Right Ventricle Study. Pulm Circ. 2012;2:379–386.
- Jurcut R,Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU. The echocardiographic assessment of the right ventricle: What to do in. 2010?Eur J Echocardiogr. 2010; 11:81–96.
- Cuspidi C, Rescaldani M, Sala C. Prevalence of echocardiographic left atrial enlargement in hypertension: A systematic review of recent clinical studies. Am J Hypertens. 2013 Feb 6. [Epub ahead of print]
- Tadic MV, Ivanovic VA, Celic VP. Does nondipping impact on right ventricle in hypertensive patients ?. Blood Press Monit. 2012;17:47–54.
- Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS). Am Heart J. 2006;151:412–418.

