?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The study aim was to investigate the relationship between blood pressure (BP) profile of ambulatory BP monitoring (ABPM) and brachial–ankle pulse wave velocity (baPWV). A total of 196 untreated hypertensive subjects (103 women, age 54.7 ± 12.4 years) who underwent both baPWV measurement and ABPM were analyzed. Systolic dipping of < 10% was defined as a non-dipper. Eighty subjects (40.8%) were non-dippers. The baPWV values were similar between dippers and non-dippers (1609 ± 293 vs 1539 ± 240, p = 0.070). In multiple regression analyses, after controlling age, height and heart rate, daytime systolic BP (SBP) (β = 0.346, p < 0.001) and night-time SBP (β = 0.244, p = 0.006) had significant positive associations with baPWV in women but not in men. Diastolic BP and dipping status were not associated with baPWV in either gender. Univariate analysis after further stratification of women according to postmenopausal status showed significant correlations of daytime SBP (β = 0.317, p = 0.007) and night-time SBP (β = 0.339, p = 0.004) with baPWV in postmenopausal women but not in premenopausal women. These results suggest that age and gender effects should be considered in the interpretation of the association between BP and arterial stiffness.
Introduction
Increased arterial stiffness reflecting vascular aging and damage, has been advocated to be associated with high cardiovascular morbidity and mortality (Citation1–4). Arterial stiffness can be estimated by measuring pulse wave velocity (PWV), which is a widely used tool in epidemiological and clinical studies. There have been several methods for the measurement of PWV. Among them, carotid–femoral PWV (cfPWV) is known to be the most reliable tool for PWV; however, it has some limitations in clinical use due to technical difficulty. Instead, brachial–ankle PWV (baPWV), the currently developed method, is simple and requires a short measurement time. In addition, baPWV is well correlated with cfPWV (Citation5) and arterial stiffness measured by an invasive study (Citation6). Moreover, the prognostic value of baPWV is well validated in various clinical settings (Citation7–10) and meta-analysis (Citation11). Therefore, baPWV is largely used for the measurement of arterial stiffness as a screening tool.
Ambulatory blood pressure monitoring (ABPM) allows blood pressure (BP) to be intermittently monitored, and provides information about BP during daily activities and sleep. Therefore, accurate BP is more accurately estimated by ABPM than BP measurement at office. In this context, ABPM is particularly useful for evaluating subjects with variable BP readings in office. Moreover, nocturnal BP readings by ABPM provide prognostic data. Therefore, ABPM is becoming increasingly recommended for routine clinical practice for the diagnosis of high BP (Citation12,Citation13).
Many studies have shown that BP is the main determinant of arterial stiffness (Citation14–16). However, these studies used office BP as a parameter for the analysis. Considering that ABPM is more accurate than office BP, studies on relationship between ambulatory BP and arterial stiffness would be more valuable. However, studies investigating arterial stiffness in context with ambulatory BP profile have been scarce.
Therefore, the aim of this study was to examine the correlations between BP components assessed by ABPM and baPWV.
Materials and methods
Study subjects
This retrospective single center study was performed at Boramae Medical Center (Seoul, Korea). Between September 2008 and July 2013, 337 consecutive subjects who underwent both baPWV and ABPM within 3 days were identified. Of these subjects, 196 hypertensive subjects who were untreated with anti-hypertensive drugs were finally enrolled in this study. A diagnosis of hypertension was based on a documented history of high BP (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg on at least three outpatient measurements), or confirmed by ABPM (daytime BP ≥ 135/85 mmHg or night-time BP ≥ 120/70 mmHg) (Citation17). There were no exclusion criteria. Approval of the study protocol was obtained from the Institutional Review Board of Boramae Medical Center (Seoul, Korea).
Data collection
Information on medical history of diabetes mellitus, coronary artery disease and stroke, smoking habits, and menopausal status was obtained through medical record reviews. Diabetes mellitus was defined by a fasting glucose of ≥ 126 mg/dl on more than two occasions or if subjects took oral hypoglycemic agents or insulin. Coronary artery disease was defined by a history of myocardial infarction or coronary revascularization. Laboratory tests were performed to obtain hemoglobin, total cholesterol, triglyceride, high- and low-density lipoprotein cholesterol, estimated glomerular filtration rate (eGFR), and C-reactive protein. In addition, eGFR was calculated using the following formula (Citation18):
baPWV measurement
The baPWV measurement protocol has been previously described (Citation19). Medications in current use were allowed at the day of examination. Subjects were examined in the supine position at room temperature after ≥ 5 min of sedentary period. baPWV were measured using a volume-plethysmographic apparatus (VP-1000; Colin Co. Ltd., Komaki, Japan). Occlusion and monitoring cuffs were placed around both arms (brachials) and ankles, and pressure waveforms of the brachial and posterior tibial arteries were recorded. Electrocardiogram, heart sound, BPs and heart rates were also simultaneously recorded. PWV was calculated by measuring the time for the pulse wave to travel between the brachial and posterior tibial arteries (velocity = distance/time). Distance between brachialis and posterior tibial arteries were estimated based on the height of the subject, and time interval between them was calculated by the time difference between wave front of them. The mean of left and right baPWV values was used for study analysis. All measurements were made by the same experienced operator who was blinded to subjects’ information.
ABPM measurement
At 10:00 h, 24-h ABPM was performed on the non-dominant arm using an oscillometric device (Oscar 2; Sun Tech Medical, Inc, Morrisville, NC, USA). The device was clinically validated to internationally recognized standard (Citation20). The device was set to obtain BP readings every 30 min during daytime and night-time. ABPM measurements were made on working days, and normal daily activities were encouraged. Subjects were instructed to keep their arms wrapped with cuffs motionless and relaxed during BP measurement. Daytime and night-time periods were defined individually according to the subjects’ self-reported data. The percentages of systolic and diastolic dipping values were calculated using the following formula: [1−(mean night systolic BP (SBP)/mean day SBP)]× 100 for systolic dipping, and [1−(mean night diastolic BP (DBP)/mean day DBP)]× 100 for diastolic dipping, respectively. Those subjects with systolic dipping < 10% were defined as non-dippers.
Statistical analysis
Continuous variables are presented as mean± standard deviation (SD), and categorical variables are expressed as percentages. Continuous variables were compared using the Student's t-test, and categorical variables were compared using the chi-square test between dippers and non-dippers. In order to adjust the effect of BP, study subjects were stratified into tertiles according to their mean 24-h SBP levels, and value of baPWV between dipper and non-dippers were compared in each tertile group. Univariate and multivariable linear regression analyses were used to assess the associations between baPWV and the parameters of ABPM and office BP. Age, height and heart rate were adjusted during multivariable analyses. Linear associations were represented using scatter plots. A p value of < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS 18.0 (IBM Co., Armonk, NY, USA).
Results
Clinical characteristics
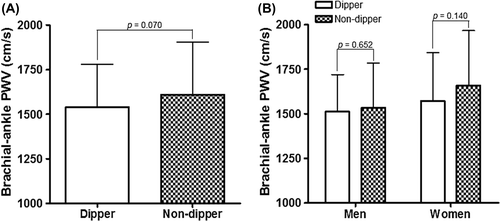
There were 80 non-dippers (40.8%). The clinical characteristics of study subjects according to dipping status (dipper vs non-dipper) categorized by ABPM are shown in . Non-dippers are female predominant than dippers (61.2% vs 46.6%, p = 0.043). Previous medical history and concomitant medications were not significantly different between the two groups. The results of major laboratory parameters except hemoglobin were similar between the two groups. The results of BP measurements according to dipping status are demonstrated in . SBP, DBP and heart rate measured at office were not significantly different between dippers and non-dippers (p > 0.05 for each). Regarding ABPM, both SBP and DBP values during daytime were significantly lower in non-dippers than in dippers (p < 0.001). Both SBP and DBP during night-time were higher in non-dippers than in dippers (p ≤ 0.001). Mean systolic and diastolic dipping values were 16.8 ± 4.3% and 19.2 ± 5.6% in dippers, respectively, and 4.6 ± 4.2% and 7.0 ± 5.9% in non-dippers, respectively (p < 0.001 for each).
Table I. Clinical characteristics of study subjects according to dipping pattern.
Table II. Blood pressure measurements.
baPWV in non-dippers and dippers
The baPWV values were not significantly different between dippers and non-dippers (1609 ± 293 vs 1539 ± 240 cm/s, p = 0.070) (). There was no gender difference for baPWV between the two groups (). There were also no significant differences in baPWV values between dipper and non-dipper in spite of further stratification of study subjects into their 24-h SBP tertiles (p > 0.05 for each tertile group).
Associations between baPWV and the parameters of ABPM and office BP
Correlations between baPWV and the parameters of ABPM are shown in . In all subjects, night-time SBP (β = 0.229, p = 0.001) and diastolic dipping (β = − 0.146, p = 0.043) had significant associations with baPWV. Daytime SBP (β = 0.258, p = 0.009) and night-time SBP (β = 0.312, p = 0.001) were significantly correlated with baPWV in women. These associations were not statistically significant in men. DBP and dipping status were not associated with baPWV in either gender. The associations of baPWV with daytime SBP (β = 0.253, p = 0.007) and night-time SBP (β = 0.250, p = 0.008) in women remained significant in multivariate linear regression analyses even after controlling confounders including age, height and heart rate. When we performed further stratification of women by menopausal status, there were significant correlations of baPWV with daytime SBP (β = 0.317, p = 0.007) and night-time SBP (β = 0.339, p = 0.004) in postmenopausal women but not in premenopausal women (). DBP and dipping patterns were not associated with baPWV in either gender, irrespective of their age (data not shown). When univariate and multiple regression analyses using office BP instead of ABPM BP were performed, there were significant correlations of baPWV with both office SBP and DBP in a whole subjects and both genders even after controlling confounders (p < 0.05 for each) ().
Figure 2. Linear correlations between brachial–ankle pulse wave velocity (PWV) and daytime and night-time systolic blood pressure according to menopausal status in women.
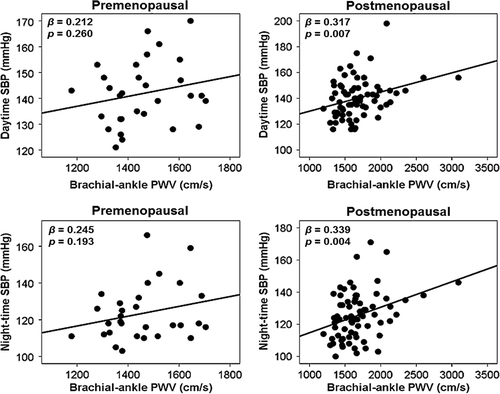
Table III. Univariate and multivariable analysis in the correlation of brachial–ankle pulse wave velocity (baPWV) with parameters of ambulatory blood pressure monitoring (ABPM).
Table IV. Univariate and multivariable analyses showing the correlations of brachial–ankle pulse wave velocity (baPWV) with office blood pressure (BP).
Discussion
This study demonstrated that, in untreated hypertensive subjects, baPWV had independent and positive correlations with daytime and night-time SBP in women but not in men. In women, linear correlations among these parameters were present in postmenopausal women, and absent in premenopausal women, suggesting that hormonal status is a contributing factor for the association between arterial stiffness and ambulatory SBP. Ambulatory DBP and dipping patterns were not associated with baPWV in either gender.
Associations between arterial stiffness and ambulatory BP
Many studies reported that a chronic increase in arterial BP accelerates arterial stiffening, and that BP is one of the major correlates of arterial stiffness (Citation14–16). However, office BP measurement was used for the analysis in those studies. Although ABPM estimates more accurate BP than office BP measurement, information on the association between arterial stiffness and ambulatory BP has not yet been well described. Zang et al. (Citation21) reported that arterial stiffness measured by ambulatory arterial stiffness index (AASI) is independently associated with daytime systolic BP of ABPM in hypertensive subjects aged over 60 years. Wei and colleagues (Citation22) investigated 1037 untreated subjects who underwent both aortic PWV by tonometry and ABPM, and demonstrated that 24-h SBP and mixed hypertension were independently associated with aortic PWV, whereas these associations were not observed in 24-h DBP and isolated diastolic hypertension. Recently, PWV measured using a radial artery tonometer has been shown to be negatively associated with mean 24-h BP in untreated mild to moderate hypertensive subjects in univariate analysis (Citation23). Our study also showed positive correlations of baPWV with ambulatory SBP during day and night after controlling confounders. We think that our data using ABPM may be more reliable than those studies (Citation14–16) because ABPM data more accurately reflect a patient's actual BP.
There has been conflicting data on which parameter between SBP and DBP is more influential to arterial stiffness. Several studies reported that PWV was found to be correlated with SBP but not DBP (Citation16,Citation21,Citation22). Others found that PWV was correlated with both SBP and DBP (Citation24). Augmentation index and PWV was correlated only to DBP in some other studies (Citation25,Citation26). Our ABPM result indicates that SBP appears to be more important than DBP in having association with arterial stiffness. However, when we analyzed the correlations between baPWV and office BP, both SBP and DBP had independent associations with baPWV in a whole subjects and both genders. These different results between ABPM and office BP emphasize the caution in the interpretation of the results on the association between arterial stiffness and BP, in terms of their method used for BP measurements.
Moreover, we observed a gender difference in the association between baPWV and ambulatory BP. To date, no information has been available on gender difference in the association between baPWV and ambulatory BP. Previous studies did not address gender specific changes of baPWV in relation to ambulatory BP (Citation21,Citation22,Citation23). Therefore, our study results showing the gender difference in the association baPWV and ambulatory BP seem to be meaningful.
Generally, it has been reported that women have lower PWV values than men (Citation27,Citation28), suggesting vascular protective effects of estrogen in women (Citation29,Citation30) and gender differences in aortic remodeling (Citation31). A longitudinal study also showed that, compared with women, men have steeper longitudinal increases in PWV with aging, leading to higher PWV values beyond their fifth decade (Citation16). These results are consistent with ours, indicating that women had lower baPWV than men (1503 ± 218 vs 1599 ± 295 cm/s, p = 0.001).
Although only univariate analysis was performed due to the small number of subjects, this study showed that the association between baPWV and ambulatory SBP was significant in postmenopausal women but not in premenopausal women. This age specific association in women may be related to changes in hormonal status. The impact of hormonal status on the association between arterial stiffness and ambulatory BP has not been fully understood. It has been documented that age-dependent changes in large artery properties in women are mainly affected by hormonal status. Sex hormones increase arterial compliance through modulation of vascular smooth muscle tone, perfusion of the vasa vasorum and extracellular matrix composition (Citation29,Citation32,Citation33). Menopause diminishes actions of ovarian hormones, and promotes more rapid deterioration of arterial elasticity. In contrast, it has been reported that hormonal replacement therapy restores arterial elasticity in postmenopausal women (Citation30,Citation34). Based on these data, we can postulate the important role of hormonal status in women not only on arterial compliance but also on its association with ambulatory BP.
Association between arterial stiffness and dipping status
Although there have been several clinical studies investigating the associations between arterial stiffness and dipping patterns, their results are still conflicting. A study performed by Asar et al. (Citation35) showed that PWV was an independent predictor of systolic dipping in 106 subjects with untreated hypertension. Lekakis et al. (Citation23) analyzed 72 subjects with untreated mild to moderate arterial hypertension, and showed higher aortic PWV values in non-dippers compared with dippers. Cicek et al. (Citation36) indicated that increased cfPWV is independently associated with nondipping status in untreated hypertensive subjects. Baumann et al. (Citation37) demonstrated, in a study of 120 subjects with or without hypertension who were evaluated for kidney donation, that arterial stiffness represented by AASI is correlated with a decline in nocturnal BP, and increased in non-dippers. In contrast, there have been some studies indicating insignificant results on the association between arterial stiffness and dipping patterns. Tsioufis et al. (Citation38) showed no difference in PWV between dippers and nondippers in 66 postmenopausal women with untreated hypertension. Similarly, Grandi et al. (Citation39) found no significant association between PWV and dipping status in 253 untreated hypertensive subjects. In line with those studies, ours also showed that baPWV values were not significantly different according to dipping patterns. Different study populations and methods for measuring APBM/arterial stiffness may contribute to the discrepancies among the studies.
Study limitations
Besides retrospective design, our study has several limitations. First, our study sample size is relatively small, which limits multiple regression analysis: only age, height and heart rate were adjusted, and multiple regression analysis could not be performed in a subgroup according to menopausal status. For a similar reason, there might be a possibility that pre-menopausal women did not reach statistical significance in the association between SBP and baPWV. Second, since this study was cross-sectional, it did not evaluate the causal relationship between baPWV and SBP. Third, although it was significant, correlation powers between two parameters were not strong in our study, therefore, drawing a hard conclusion was difficult. Lastly, since our study included only untreated hypertensive subjects, our results are difficult to generalize to other groups.
Conclusions
In untreated hypertensive subjects, daytime and night-time SBP are positively correlated with baPWV in postmenopausal women but not in premenopausal women or men. These results suggest that age and gender effects should be considered in the interpretation of the association between BP and arterial stiffness. Prospective studies with a larger sample size are needed to confirm our results.
Acknowledgement
The authors appreciate the help of Heesun Yu in data collection.
Declaration of interest:
This study was supported by grant no. 09-2012-2 from Boramae Medical Center Research Fund.
References
- Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670.
- Berard E, Bongard V, Ruidavets JB, Amar J, Ferrieres J. Pulse wave velocity, pulse pressure and number of carotid femoral plaques improve prediction of cardiovascular death in a population at low risk. J Hum Hypertens. 2013;27:529–534.
- Munakata M, Ito N, Nunokawa T, Yoshinaga K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens. 2003;16:653–657.
- Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial–ankle pulse wave velocity measurement. Hypertes Res. 2002;25:359–364.
- Washida N, Wakino S, Hayashi K, Kuwahara T, Itoh H. Brachial–ankle pulse wave velocity predicts silent cerebrovascular diseases in patients with end-stage renal diseases. J Atheroscler Thromb. 2010;17:165–172.
- Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, et al. Brachial–ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005;69:815–822.
- Nakamura M, Yamashita T, Yajima J, Oikawa Y, Sagara K, Koike A, et al. Brachial–ankle pulse wave velocity as a risk stratification index for the short-term prognosis of type 2 diabetic patients with coronary artery disease. Hypertens Res. 2010;33:1018–1024.
- Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, et al. Brachial–ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622.
- Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial–ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556–562.
- O’Brien E. Ambulatory blood pressure monitoring in the management of hypertension. Heart. 2003;89:571–576.
- Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: Executive summary: A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008; 52:1–9.
- Alecu C, Gueguen R, Aubry C, Salvi P, Perret-Guillaume C, Ducrocq X, et al. Determinants of arterial stiffness in an apparently healthy population over 60 years. J Hum Hypertens. 2006;20:749–756.
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “Establishing normal and reference values”. Eur Heart J. 2010;31:2338–2350.
- Alghatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Lee CS, Cha RH, Lim YH, Kim H, Song KH, Gu N, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25: 1616–1625.
- Kim HL, Im MS, Seo JB, Chung WY, Kim SH, Kim MA, et al. The association between arterial stiffness and left ventricular filling pressure in an apparently healthy Korean population. Cardiovasc Ultrasound. 2013;11:2.
- Goodwin J, Bilous M, Winship S, Finn P, Jones SC. Validation of the Oscar 2 oscillometric 24-h ambulatory blood pressure monitor according to the British Hypertension Society Protocol. Blood Press Monit. 2007;12:113–117.
- Zang XY, Zhang H, Cheng SL, Gao YJ, Cao YJ, Zhao Y, et al. Pivotal factors interfering in 24-hour blood pressure fluctuation and arterial stiffness in a community of Chinese elderly hypertensive patients. J Clin Nurs. 2013;22:379–388.
- Wei FF, Li Y, Zhan L, Xu TY, Ding FH, Staessen J, et al. Association of target organ damage with 24-hour systolic and diastolic blood pressure levels and hypertension subtypes in untreated Chinese. Hypertension 2014;63:222–228.
- Lekakis JP, Zakopoulos NA, Protogerou AD, Papaioannou TG, Kotsis VT, Pitiriga V, et al. Arterial stiffness assessed by pulse wave analysis in essential hypertension: Relation to 24-h blood pressure profile. Int J Cardiol. 2005;102:391–395.
- Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. Q J Med. 1999;92:595–600.
- Nurnberger J, Dammer S, Saez AO, Philipp T, Schafers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Human Hypertens. 2003;17:153–158.
- Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001; 38:1461–1466.
- Bae JS, Shin DH, Park PS, Choi BY, Kim MK, Shin MH, et al. The impact of serum uric acid level on arterial stiffness and carotid atherosclerosis: The Korean Multi-Rural Communities Cohort study. Atherosclerosis. 2013;231: 145–151.
- Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia. 2008;51:527–539.
- Fischer GM, Swain ML. Influence of contraceptive and other sex steroids on aortic collagen and elastin. Exp Mol Pathol. 1980;33:15–24.
- Miura S, Tanaka E, Mori A, Toya M, Takahashi K, Nakahara K, et al. Hormone replacement therapy improves arterial stiffness in normotensive postmenopausal women. Maturitas. 2003;45:293–298.
- Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, et al. Aortic root remodeling over the adult life course: Longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–890.
- Rosano GM, Chierchia SL, Leonardo F, Beale CM, Collins P. Cardioprotective effects of ovarian hormones. Eur Heart J. 1996;17 Suppl D:15–19.
- Glusa E, Graser T, Wagner S, Oettel M. Mechanisms of relaxation of rat aorta in response to progesterone and synthetic progestins. Maturitas. 1997;28:181–191.
- Rajkumar C, Kingwell BA, Cameron JD, Waddell T, Mehra R, Christophidis N, et al. Hormonal therapy increases arterial compliance in postmenopausal women. J Am Coll Cardiol. 1997;30:350–356.
- Asar R, Scuteri A, Topouchian J, Brisac AM, Maldonado J, Cloarec L, et al. Arterial distensibility and circadian blood pressure variability. Blood Press Monit. 1996;1: 333–338.
- Cicek Y, Durakoglugil ME, Kocaman SA, Cetin M, Erdogan T, Dogan S, et al. Non-dipping pattern in untreated hypertensive patients is related to increased pulse wave velocity independent of raised nocturnal blood pressure. Blood Press. 2013;22:34–38.
- Baumann M, Dan L, Nurnberger J, Heemann U, Witzke O. Association of ambulatory arterial stiffness index and brachial pulse pressure is restricted to dippers. J Hypertens. 2008; 26:210–214.
- Tsioufis C, Tzioumis K, Dimitriadis K, Chatzis D, Skiadas I, Michailidis A, et al. Nondipping status does not attenuate the conjugated estrogen-induced improvement in aortic stiffness in postmenopausal women with untreated hypertension. Am J Hypertens. 2005;18:607–611.
- Grandi AM, Broggi R, Jessula A, Laurita E, Cassinerio E, Piperno F, et al. Relation of extent of nocturnal blood pressure decrease to cardiovascular remodeling in never-treated patients with essential hypertension. Am J Cardiol. 2002;89:1193–1196.