Abstract
Aim. White coat hypertension (WCH) is hard to differentiate from sustained hypertension without the use of 24-h ambulatory blood pressure monitoring (ABPM). This invaluable procedure is nevertheless cumbersome and expensive. A simple test of deep breathing over 30 s (DBT) was proposed as a method to unveil WCH. Methods. Two hundred and fourteen outpatients referred for the evaluation of uncontrolled hypertension (blood pressure, BP > 140/90 mmHg despite therapy) were enrolled in a controlled clinical trial. The examinees were randomly divided in two groups: control (n = 108; sequential standard BP measurement only) and intervention (n = 106; the same+DBT), using ABPM as the reference standard. Results. The relative decrease in BP was significantly larger in the intervention group than in the control group, by 15/4 mmHg (p = 0.005). The best detection of WCH was obtained at ≥ 15% systolic BP reduction following DBT, with a positive predictive value of 94.0% (95% CI 72.0–100.0). BP reduction of ≤ 8% may rule WCH out with a negative predictive value of 78.4% (95% CI 64.0 – 85.9). Conclusion. DBT is a reliable, inexpensive and fast test for the detection of WCH in primary care.
Introduction
One of the reasons for misclassification of hypertensive patients is white coat hypertension (WCH) or isolated clinical hypertension (Citation1–3). It is due to the white coat effect, an alarm reaction to the medical office environment that leads to sympathetic overstimulation and temporary blood pressure (BP) elevation (Citation3–5). As this condition is often labeled uncontrolled or poorly controlled hypertension, it leads to further testing and to an increase in antihypertensive drug dosage and/or additional medications, which in turn elevates the likelihood of side-effects, negatively affecting the quality of life, and inflates the cost of treatment (Citation1–5). The prevalence of WCH varies, ranging between 35% and 73% (Citation5,Citation6). It is often associated with female sex, increasing age, mild hypertension and large body mass, and it occurs in both treated and untreated hypertensives, dominantly affecting systolic BP (Citation1,Citation3–9). The gold standard for detection of WCH is 24-h ambulatory BP monitoring (ABPM) (Citation1,Citation10,Citation11). This technique is both time-consuming and requires a considerable investment, including equipment maintenance, which is not affordable in a family medicine setting, particularly in a relatively poor, transitional country such as Croatia. WCH suspects are therefore referred for an outpatient consultation, incurring additional charges, time consumption and discomfort. Indeed, the quality of patient's life should not be neglected, as ABPM requires wearing of the instrument for 24 h and at least two office visits. The procedure, known as the Deep Breath Test (DBT) is reportedly an inexpensive, simple and fast method for WCH identification, suitable for both patients and physicians (Citation12–17). The patient is seated and breaths deeply over several respiratory cycles. This maneuver probably increases the baroreceptor reflex sensitivity to vagal stimulation, resulting in BP lowering (Citation14–17). Nevertheless, comparative studies on the efficacy of DBT and ABPM in WCH revealing are unexpectedly rare and equivocal (Citation18–20). Even though all these reports agree that DBT can be used in detection of vascular hyperreactors and WCH, this claim has not undergone broad verification and has not entered clinical practice, despite numerous potential benefits for daily family medicine/primary healthcare outpatient settings. This trial was designed to reassess the diagnostic value of DBT (sensitivity, specificity and predictive value) in detection of WCH in comparison with ABPM.
Subjects and methods
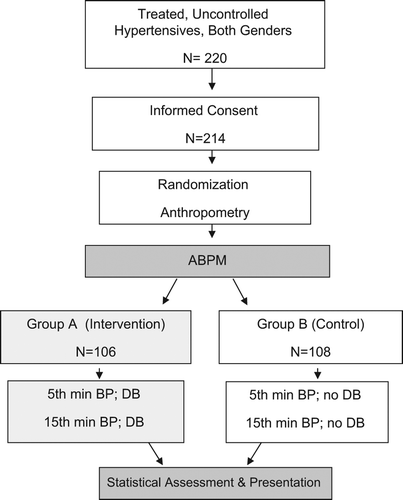
A consecutive sample of patients referred for ABPM monitoring was enrolled, between September 2011 and July 2012. The subjects were treated adult patients of either sex with poorly controlled arterial hypertension (BP ≥ 140/90 mmHg), confirmed at least 6 months beforehand. Individuals with suspected secondary hypertension, persistent atrial fibrillation, pregnant women and uncooperative patients were excluded. After signing the informed consent form, the subjects were randomly assigned to two groups, the intervention (A) and the control (B) group. Stratified block randomization was adopted with two gender and five age strata (≤ 39, 40–49, 50–59, 60–69 and ≥ 70 years), using a SealedEnvelope online service (https://www.sealedenvelope.com/simple-randomiser/v1/lists). Initial BP readings were taken after lounging of examinees for 5 min, in a sitting position, on the dominant arm, with a standard mercury sphygmomanometer, twice, 2 min apart. The same procedure was repeated after 15 min in the same visit. Following the 5- and 15-min readings, the patients in the intervention group were subjected to DBT, i.e. to breathe deeply and slowly for 30 s (three or four cycles) while being monitored by the observer. BP was recorded immediately after the test, and 2 min later, using the same equipment (). All BP measurements and DBTs were performed by the same observer (MT), unaware of the ABPM results, and recorded the following day in a nearby office by another investigator (VC), unaware of the sphygmomanometry results. ABPM was recorded with SL 90207 device (Spacelabs Healthcare, WA, USA). The readings were taken every 20 min during the day (07:00–22:00 h) and every 30 min during the night (22:00–07:00 h). The records containing ≥ 80% of the programmed readings were accepted, while others were repeated within 2 days. The subjects whose average daily BP on ABPM was > 10% lower than BP recorded in the clinical setting were considered vascular hyperreactors (confirmed WCH) (Citation1,Citation2). Socio-demographic and anthropometric data, the BP readings, and the ABPM results were entered in individual test lists. The cumulative data were processed using SPSS 17.0.1 (SPSS Inc.), for MS Windows 7. A number of parametric and non-parametric statistical tests were applied, as appropriate, and p < 0.05 was considered significant.
Results
This prospective, randomized trial was conducted on 214 uncontrolled hypertensives. There were no significant differences between the two groups in terms of sex, age or lifestyle (). The same is true for comorbidities, e.g. diabetes mellitus (12.3% vs 15.7%; p = 0.464) or hypercholesterolemia (30.2% vs 33.3%; p = 0.621). Body mass index did not differ between the groups either (median 28.4 with interquartile range, IQR, 24.9–30.9, vs 28.0 with IQR 25.4–32.1; p = 0.367).There were 59 (27.6%) subjects on monotherapy, 54 (25.2%) were taking two and 101 (47.2%) three or more antihypertensives. The mean heart rate was similar in both groups (70.0 with IQR 63–76, vs 69.0 with IQR 63–75; p = 0.838). The average daily BP registered on ABPM was 135.0 ± 13.6/82.0 ± 10.8 mmHg, while office readings were 160.0 ± 14.7/93.0 ± 10.6 mmHg at 5 min, and 153.0 ± 17.8/92.0 ± 11.5 mmHg at 15 min, with no significant differences between groups. The office BP (both at 5 and 15 min) was significantly higher than the ABPM daily BP (F = 605.157; p < 0.001; η2 = 0.741), with minimal differences between groups at 5 min (F < 0.001; p = 0.996 for systolic, and F = 0.551; p = 0.459 for diastolic BP) and at 15 min (F = 1.858; p = 0.176 systolic, and F = 0.064; p = 0.975 diastolic). BP in group A varied markedly through the study stages (). The daily ABPM results gave the lowest, and the 5-min office readings gave the highest figures. Following DBT, the BP values were significantly lower than at 5 min, but higher than on ABPM. All levels of systolic and diastolic BP after DBT decreased significantly (p < 0.001), except for the difference between the 5 and 15 min (p = 1.000). The average decline in systolic BP following DBT was 14.7 ± 11.7 mmHg (maximum 44, minimum 1 mmHg). Diastolic BP also decreased by some 4.3 ± 8.00 mmHg (minimum 1, maximum 32 mmHg). Both BPs after DBT at 5 min were significantly lower in group A than in group B (t = 6.475; p < 0.001 for systolic; t = 3.168; p = 0.001 for diastolic). The 15-min systolic BP in group A was markedly lower than in group B (t = 2.847; p = 0.005), which was not the case for diastolic BP (t = 1.477; p = 0.143). There were 168 (78.9%) vascular hyperreactors or WCHs identified with > 10% fall in systolic BP, and 116 (54.5%) with > 10% diastolic BP drop after DBT. Reductions in systolic BP after DBT were more consistent and distinct (t = 2.439; p = 0.018). There was no significant difference between the groups in the prevalence of WCH (systolic: χ2 = 0.37; p = 0.61; diastolic: χ2 = 0.36; p = 0.58). Hyperreactors in the intervention (A) group had significantly lower ABPM values and much higher clinic BP values than non- hyperreactors (). These subgroups did not differ in terms of BMI (mean 27.9, IQR 25.1–31.3, vs 28.7, IQR 25.5–30.6; p = 0.869); the hyperreactors had slightly lower heart rates (mean 69.0, IQR 62–76, vs 70, IQR 64–75; p = 0.472). The decrease in systolic BP was more pronounced among hyperreactors than among non-hyperreactors as well (F = 3.575; η2 = 0.054; p = 0.063). Hyperreactors in group A had significantly lower systolic (t = 6.018; p < 0.001) and diastolic BP (t = 2.432; p = 0.016) following DBT at 5 min than the hyperreactors in group B. Readings at 15 min showed significantly lower systolic BP among hyperreactors in group A (t = 2.638; p = 0.098) as well, with no marked difference in diastolic BP (t = 0.968; p = 0.336). A cut-off point for WCH set at a decrease in systolic BP by ≥ 10% after DBT was too high, with low predictivity (sensitivity 51%, specificity 61%, positive predictive value 82%, negative predictive value 26%). Moving the cut-off point to ≥ 12% following DBT (such a fall was observed in 44.1% of the examinees), the test specificity increases to 83% (95% CI 63–94%) and the positive predictive value rises to 89% (95% CI 76–96%). Decreasing the cut-off point further to ≥ 15%, as observed in 20% of our subjects, the specificity of DBT goes up to 96% (95% CI 79–100%), and positive predictive value to 94% (95% CI 72–100%). Further lowering of the cut-off point to, for instance ≥ 18%, may increase the DBT specificity to 99%, and its predictive value to 99.1%, but at an unacceptable sensitivity since there were only 3.4% such hyperreactors in this trial; an extreme decrease by 27.4% (44 mmHg) was observed only once. The opposite is true for small DBT decreases in systolic BP. With drops ≤ 8%, DBT sensitivity is exceedingly low (0.78%) with a high negative predictive value (78.4%; 95% CI 64.0–85.9), allowing for WCH exclusion. There were 50.2% such subjects in our study. These relationships between sensitivity and specificity of DBT are best illustrated on a receiver operating characteristic (ROC) curve (): the area under the ROC curve of systolic BP change following DBT was 0.637 (95% CI 0.511–0.764; p = 0.046). Side-effects attributable to DBT hyperventilation, such as dizziness, lightheadedness or even tetany, have not been registered in this trial.
Figure 2. Receiver-operator characteristic (ROC) curve confronting sensitivity and specificity of the Deep Breath Test (DBT) resulting from the trial.
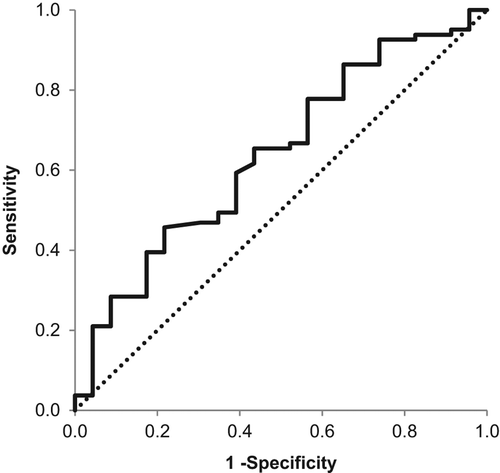
Table I. Relevant demographic data for the study participants (n = 214).
Table II. Blood pressure results in group A (n = 94).
Table III. Blood pressure differences between hyperreactors and non-hyperreactors according to the five measurements (in mmHg).
Discussion
ABPM is a very useful tool in disclosure and follow-up of hypertensive patients, as it records BP fluctuations during the day, at night and on waking up, which are clinically imperceptible. Its readings are better correlated to target organ damage than the office records, and represent the gold standard for WCH detection (Citation1,Citation2,Citation21–23). Another procedure for accurate evaluation of arterial hypertension is home BP monitoring (Citation21–24). Both methods have their specific downsides. ABPM requires considerable investment, is time-consuming and increases referral to consultants. The patient must carry the instrument for 24 h with many cuff inflations and attend at least two clinic visits. Home BP monitoring requires a considerable training of patients, good adherence (compliance) and adequate device selection. The correlation between the two methods is fair (Citation21,Citation24). This trial sought to assess the diagnostic value of DBT in detection of WCH in a sample of unsatisfactorily controlled hypertensives (Citation12–20), using ABPM as the gold standard. The observed prevalence of WCH (78.9% for systolic and 54.5% for diastolic BP) was higher than expected; the reported frequencies range between 35% and 73% (Citation5,Citation6) averaging some 40%. It was mainly attributed to the drop in systolic BP (vascular hyperreactivity was defined as a difference in systolic BP > 10% between clinical and daytime ABPM readings). Increasing the test specificity, i.e. setting the cut-off point at 15% and 20% drop, the percentage of hyperreactors decreased to 62% and 44%, respectively. Another explanation for the high prevalence of WCH could be the nature of our examinees: included were only patients with uncontrolled hypertension, and unrevealed WCH often lies behind poor BP control (Citation1,Citation4). Very similar results have previously published Augustovski et al. (Citation18) and Thalenberg et al. (Citation19). Yoshihara et al. (Citation20) concluded, however, that diastolic BP drop was better in predicting WCH. This discrepancy may be due to differences in executing DBT (five breaths in 60 s vs three or four breaths in 30 s), to the nature of the examinees (untreated vs treated hypertensives) or to some particular ethnic differences. We have shown that one DBT over 30 s after a 5-min stay in office is sufficient to differentiate WCHs from truly uncontrolled hypertensives: any longer deep breathing and additional intervention at 15 min, while more time consuming and inconvenient to the patient and the physician, gave almost identical results. It has also been found that diastolic BP fall is less sensitive than the systolic one, which further simplifies the test. The assumed cut-off point at 10% or even 12% decrease in BP following DBT was not specific enough to rule in the majority of true WCH patients. A drop in systolic BP by ≥ 15%, which was observed in 20% of our poorly controlled hypertensives, could find hyperreactors with 96% specificity (95% CI 79.0–100.0) and 94% positive predictive value (95% CI 72.0–100.0), achieving high clinical reliability. In other words, inadequately controlled hypertensives whose systolic BP following DBT decreases by ≥ 15% are very likely true hyperreactors. Alternatively, if the BP drop after DBT is ≤ 8%, the patient is unlikely a hyperreactor (negative predictive value 78.4%; 95% CI 64.0–85.9).
Other investigators have previously obtained similar results (Citation18–20). However, our study was conducted on a larger number of subjects [214 vs 73, 92 or even 30 (Citation18–20)], all treated with one to three antihypertensive agents, and with a control, matching group. Accordingly, we have shown that BP lowering in DBT is not due to a measurement peculiarity, such as the time lag or the examinees’ position. Differences in DBT execution may matter as well. We have used three or four deep breaths in 30 s, while others were using undetermined number of deep breaths in 30 s (Citation18), six deep breaths in 60 s (Citation19) or five deep breaths in 60 s (Citation20). Shorter deep breathing is more convenient to the examinees, is time sparing and stimulates a reliable fall in sympathetic tone. In addition, a single observer for both office BP measurement and DBT in our trial reduced the likelihood of inadvertent error and interindividual variability. The fitting of the ABPM instrument and reading of ABPM records was delegated to another blinded observer (VC), which contributed to objectivity of the results. Nevertheless, our results did not show better WCH predictability than the previous ones (Citation18–20). For instance, in the Augustovski et al. study (Citation18), the area under the ROC curve reflected a forecasting power of 69% (95% CI 57–81%), while our data were in the range of 64% (95% CI 51–76%).
Our study is subject to some limitations. The assessor physician (MT), aware of the expected DBT effects, could have biased the BP assessment. Unaware of the ABPM results, that bias could hardly affect the DBT reliability since all the readings could have been skewed in the same direction. Since the interpretation of the maneuver is subjective, there is room for interobserver variability in clinical practice. DBT has potential side-effects of hyperventilation, which were not observed among our 106 examinees following two challenges (the likelihood of such an event is low).
Obviously, DBT is not flawless, which is even true for ABPM, the gold standard in the field (Citation1,Citation2,Citation16–20); there will always be some false positive and some false negative findings. In order to rule in or rule out WCH with confidence, the level of systolic BP decrease should be set accordingly, lower in the former and higher in the latter case. Even though DBT was already recommended for detection of vascular hyperreactors (Citation18–20), the test was not accepted in practice, possibly due to the mentioned drawbacks. However, DBT is quick and cost-effective, requiring no additional equipment and causing minimum inconvenience and discomfort to patients, particularly if performed in the described way.
We do not assume that WCH is a benign condition that should be ignored. Nevertheless, it is less dangerous than persistent hypertension (Citation23). Prognostic implications of high BP depend on global vessels’ tension burden, in other words on the area under the pressure curve over time. Intuitively, within the WCH population there must be a range of severity, which currently is not recognized. However, the distinction between persistent and periodic hypertension is of major clinical importance.
According to our results, DBT is proposed as a valuable tool in daily practice. While strengthening the actually endangered doctor–patient relationship, it marginally increases the visit time. With DBT, the family physician may easily and reliably discern the bulk of WCHs from truly resistant hypertensives, contributing to more selective and rational use of ABPM. In addition, patients may be spared from further more complex, uncomfortable, long-lasting and expensive diagnostic procedures. Moreover, the test may be done in any office, even during a home visit, and could be performed by nurses and possibly by paramedics and family members as well.
Conclusions
WCH is an important issue in the management of arterial hypertension, with the prevalence well above 20%. Detection of WCH is based on ABPM (i.e. average daytime BP ≥ 10% lower than office BP).
DBT (three or four deep breaths in 30 s) markedly lowers alarm-related elevation in BP, on average by 14/5 mmHg, consistently more in its systolic component (p = 0.018).
DBT can reliably, with a positive predictive value approaching 95%, detect WCH among poorly controlled hypertensives when the cut-off point is set at the lowering of systolic BP by ≥ 15% vs office readings (e.g. from 160 to 136 mmHg or less; observed in about 20% of our examinees). On the other hand, DBT can rule out WCH, with a predictability of almost 80%, when systolic BP lowering in DBT is ≤ 8% (e.g. from 160 to ≥ 147 mmHg; seen in some 50% of our patients).
For the remaining 30% of the uncontrolled hypertensives the options are further monitoring with the assessment of compliance (e.g. urinary test for the selected antihypertensive agents and/or their metabolites), repeated DBT, referral to ABPM or to a hypertension specialist.
Acknowledgements
We are grateful for the expertise, suggestions and help in statistical evaluation of data to Maja Jeličić and Žarko Bajić from Biometrika Healthcare Research, Zagreb, Croatia.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Zaninelli A, Parati G, Cricelli C, Bignamini AA, Modesti PA, Pamparana F, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: The ‘Monitoraggio della pressione ARteriosa nella medicina Territoriale’ study. J Hypertens. 2010;28:910–917.
- Pickering TG, Coats A, Mallion JM, Mancia G, Verdecchia P. Blood Pressure Monitoring Task Force V: White-coat hypertension. Blood Press Monit. 1999;4:333–341.
- Fagard RH. Resistant hypertension. Heart. 2012; 98:254–261.
- Verberk WJ, Kroon AA, Thien T, Lenders JW, van Montfrans GA, Smit AJ, et al. Prevalence of the white-coat effect at multiple visits before and during treatment. J Hypertens. 2006;24:2357–2363.
- Myers MG, Oh PI, Reeves RA, Joyner CD. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591–597.
- Streitel KL, Graham JE, Pickering TG, Gerin W. Explaining gender differences in the white coat effect. Blood Press Monit. 2011;16:1–6.
- MacDonald MB, Laing GP, Wilson MP, Wilson TW. Prevalence and predictors of white-coat response in patients with treated hypertension. CMAJ. 1999;161:265–269.
- Ogedegbe G, Pickering TG, Clemow L, Chaplin W, Spruill TM, Albanese GM, et al. The misdiagnosis of hypertension: The role of patient anxiety. Arch Intern Med. 2008;168:2459–2465.
- Owens P, Atkins N, O’Brien E. Diagnosis of white coat hypertension by ambulatory blood pressure monitoring. Hypertension. 1999;34:267–272.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood- pressure monitoring. N Engl J Med. 2006;354:2368–2374.
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718.
- Fontana F, Bernardi P, Lanfranchi G, Pisati MS, Merlo Pich E. Blood pressure response to hyperventilation test reflects daytime pressor profile. Hypertension. 2003;41:244–248.
- Mori H, Yamamoto H, Kuwashima M, Saito S, Ukai H, Hirao K, et al. How does deep breathing affect office blood pressure and pulse rate? Hypertens Res. 2005;28:499–504.
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002;105:143–145.
- Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypothes. 2006;67:566–571.
- Pinheiro CH, Medeiros RA, Pinheiro DG, Marinho Mde J. Spontaneous respiratory modulation improves cardiovascular control in essential hypertension. Arq Bras Cardiol. 2007;88:651–659.
- Augustovski FA, Calvo CB, Deprati M, Waisman G. The deep-breath test as a diagnostic maneuver for white-coat effect in hypertensive patients. J Am Board Fam Pract. 2004;17:184–189.
- Thalenberg JM, Povoa RM, Bombig MT, Sa GA, Atallah AN, Luna Filho B. Slow breathing test increases the suspicion of white-coat hypertension in the office. Arq Bras Cardiol. 2008;91:243–249.
- Yoshihara K, Fukui T, Osawa H, Ishii Y, Morita H, Yamashiro S, et al. Deep breathing test (DBT) in predicting white coat hypertension. Fukuoka Igaku Zasshi. 1993;84:395–401.
- Pickering TG, White WB, Giles TD, Black HR, Izzo JL, Materson BJ, et al. When and how to use self (home) and ambulatory blood pressure monitoring. JASH. 2010; 4:56–61.
- Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: Follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783.
- Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19:801–807.
- Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: A summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008; 26:1505–1526.