Abstract
Objective. To evaluate relationships between fasting plasma glucose (FPG), other cardiovascular risk markers and left ventricular hypertrophy (LVH) as detected by electrocardiography. Methods. Subjects were selected randomly from groups defined by FPG. Traditional risk markers were assessed. LVH was defined by either Cornell voltage–duration product (CP) or Sokolow–Lyon voltage combination (SL), and univariate and multivariable regressions were performed in search of explanatory factors for the presence of LVH and the values of CP and SL. Results. Of the 1759 subjects included, 1007 had a history of cardiovascular disease and/or medical treatment, while 752 subjects appeared to be healthy. We found an independent association between FPG and LVH (odds ratio 1.152, p = 0.042] as well as continuous CP (beta = 0.126, p = 0.007) in healthy men. As expected, we found an association between systolic blood pressure and LVH (odds ratio 1.020, p < 0.001) among healthy subjects, but only in subjects with FPG < 6 mmol/l (p = 0.04 for interaction). Conclusions. We found an independent association between FPG and LVH in healthy men, and no potentiating effect by FPG on the impact of hypertension.
Introduction
Fasting plasma glucose (FPG) is an essential component in the detection and follow-up of glucometabolic disturbances, i.e. impaired fasting glucose and diabetes mellitus (DM) (Citation1). DM is an important risk factor for cardiovascular disease (CVD) (Citation2), possibly due to an almost toxic effect of hyperglycemia and/or hyperinsulinemia on the cardiovascular system, leading to maladaptive changes in cardiac structure and function (Citation3).
The electrocardiogram (ECG) is a simple and inexpensive method for recognizing cardiac overload in the form of left ventricular hypertrophy (LVH), although its sensitivity is relatively low (Citation4–6). The ECG is therefore recommended for the assessment of asymptomatic adults with hypertension or diabetes (Citation7). Hypertension is an important risk factor for the development of LVH (Citation8,Citation9), although LVH can also be present in normotensive subjects (Citation10). ECG- detected LVH is a well-known independent predictor of CVD (Citation4,Citation11,Citation12).
LVH is more prevalent in subjects with metabolic syndrome (Citation13–15) and DM (Citation16,Citation17) than in the general population. The mechanism of this is unclear, but possible explanations are higher rates of hypertension in subjects with DM (Citation9) or direct growth-stimulating effects of hyperglycemia and/or hyperinsulinemia on the myocardium. Furthermore, hyperglycemia and/or hyperinsulinemia may potentiate the hypertrophic effect of hypertension (Citation14).
In a random cohort of middle-aged and elderly individuals, stratified for health status and FPG, we set out to evaluate the relationship between FPG, other traditional cardiovascular risk markers and established ECG-derived risk markers.
Methods
Study population
The subjects were derived from the Malmö Preventive Project (1974–1992, n = 33,346), a population-based cohort study including inhabitants in Malmö, Sweden, with the purpose of screening for cardiovascular risk factors (Citation18). A re-examination study was conducted during 2002–2006 with 18,240 now middle-aged and elderly subjects. The participants answered a self-administered questionnaire on lifestyle and medical history, and blood samples were drawn after overnight fasting with whole blood stored in a biobank. Blood pressure and pulse rate were measured twice in the supine position after 5 min of rest. Moreover, height and weight as well as waist and hip circumferences were measured.
This study was a cross-sectional study comprising a subsample of 1792 participants from the re-examination study in which ECG recordings were performed and additional blood samples were drawn (Citation19). These subjects were randomly selected from groups defined by FPG with oversampling from groups with elevated FPG to ensure sufficient numbers of subjects studied from each group. Glucometabolic status was overall defined according to World Health Organization criteria (Citation1), but based on a single measurement: normal fasting glucose (NFG), FPG ≤ 6.0 mmol/l; impaired fasting glucose (IFG), FPG 6.1–6.9 mmol/l; established or new-onset DM, antidiabetic medical treatment or FPG ≥ 7.0 mmol/l. For the current study, we excluded subjects with missing ECG interpretation or blood pressure measurements. The remaining subjects were divided into an apparently healthy group consisting of subjects with no medical history of CVD (previous acute myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting, heart failure, atrial fibrillation or flutter, or stroke) and no use of prescribed cardiovascular, antidiabetic or lipid-lowering drugs, and a patient group consisting of subjects meeting any of these criteria. After exclusion of subjects with either missing ECG analysis or blood pressure measurements, 1759 subjects were left for analysis, comprising our study population. Of these, 37% had NFG, 28% IFG and 35% new-onset or established DM.
The ethics committee at Lund University, Sweden, approved both the Malmö Preventive Project and the re-examination study. The study complied with the Declaration of Helsinki. All participants signed an informed consent form.
Blood tests
Laboratory tests included FPG, serum (s) N-terminal prohormone of brain natriuretic peptide (NT-proBNP), s-total cholesterol, s-triglycerides and s-high-density lipoprotein (HDL) cholesterol. The low-density lipoprotein (LDL) cholesterol level was calculated using the Friedewald formula (Citation20). Additional analyses were run in 2013, including high-sensitivity cardiac troponin T (hs-TnT).
Electrocardiography
A standard 12-lead ECG was recorded at 25 mm/s and 1 mV/cm. Investigators with no knowledge of the clinical data analyzed the ECGs to detect LVH. LVH was defined by either Cornell voltage–duration product [CP; males: (RaVL + SV3) × QRS duration; females: (RaVL + SV3 + 8 mm) × QRS duration] > 2440 mm × ms or Sokolow–Lyon voltage combination [SL; SV1 + (RV5 or RV6)] > 38 mm (Citation21,Citation22).
Statistical analysis
Continuous variables are presented as means and standard deviations for approximately normally distributed variables and medians and interquartile ranges for non-normally distributed variables, whereas categorical variables are presented as frequencies and corresponding percentages. Group-wise comparisons between the three FPG groups were performed in strata split by health status, gender and median age, using one-way analysis of variance for continuous variables and Pearson's χ2 test or Fisher's exact test for categorical variables.
The associations between the dichotomous LVH categories and risk factors were assessed by binary logistic regression, whereas the analyses using the continuous ECG variables for LVH were performed using multiple linear regression.
To define potentially explanatory variables for LVH as well as continuous SL and CP, univariate regressions were performed separately for the patients and apparently healthy subjects, and separately for men and women. The univariate regressions were applied on the demographic and clinical variables as listed in and : gender, age, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), body mass index (BMI), waist/hip ratio, various plasma levels (triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol, LDL/HDL ratio, glucose, NT-proBNP and hs-TnT), smoking status, cardiovascular/lipid-lowering/antidiabetic medical treatment, previous major adverse cardiac event (acute myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft or heart failure), AF or stroke. The significance level was 5%. Statistically significant variables were included in the final multivariable binary logistic and linear regression models. NT-proBNP and hs-TnT were not included, because a possible elevation in these biomarkers would be expected to be secondary to the ECG findings. The FPG group was included in all models, since the subjects in the data set were selected according to FPG, and history of CVD was included in the patient analyses. The multivariable analyses were performed in groups stratified by patient status as well as by patient status and gender. In the multivariable analyses, backward selection was performed until a significance level of 10% was reached. Furthermore, the analyses were repeated in groups split by median age in order to consider possible survival bias in situations where the association was only present in the older group. Also, the analyses were repeated in the separate FPG groups, and tests for interaction were performed in order to consider cases where a variable remained significant in a specific FPG group only. All statistical analyses were carried out using IBM SPSS Statistics 22 (IBM, Armonk, NY, USA).
Table Ia. Characteristics of the male patients (with a history of cardiovascular event or cardiovascular, antidiabetic or lipid-lowering medical treatment).
Table Ib. Characteristics of the female patients (with a history of cardiovascular event or cardiovascular, antidiabetic or lipid-lowering medical treatment).
Table IIa. Characteristics of the healthy male subjects (without a history of cardiovascular event or cardiovascular, antidiabetic or lipid-lowering medical treatment).
Table IIb. Characteristics of the healthy female subjects (without a history of cardiovascular event or cardiovascular, anti-diabetic or lipid-lowering medical treatment).
Results
Patients’ characteristics
In total, 1007 subjects had a history of CVD (n = 289) and/or were treated with cardiovascular (n = 845), antidiabetic (n = 322) or lipid-lowering drugs (n = 451). These subjects comprised the patient group. Seventy-one per cent were male and the mean age was 68 ± 6 years. The mean systolic blood pressure was 147 ± 19 mmHg and the mean BMI was 29.0 ± 4.4 kg/m2. The prevalence of LVH was 15%, since 138 fulfilled the CP criterion, 16 fulfilled the SL criterion and six fulfilled both. According to the previously defined FPG groups, 27% of the patients had NFG, 24% had IFG and 48% had new-onset or established DM. The patient population was split by gender and FPG group, and the characteristics of this population are presented in and .
Patients with higher FPG or antidiabetic treatment tended to be male and more often had higher SBP, higher HR, higher BMI, higher waist/hip ratio, higher triglycerides, lower total, LDL and HDL cholesterol, and higher hs-TnT. In addition, the proportions of patients treated with cardiovascular and lipid-lowering drugs were significantly different between the groups, although with no clear trend. Among the male patients there was a tendency towards lower SL in the DM group.
Healthy population characteristics
The 752 subjects without a history of a cardiovascular event or cardiovascular, antidiabetic or lipid- lowering medical treatment comprised the healthy population. Seventy per cent were male, mean age was 65 ± 6 years, mean systolic blood pressure was 147 ± 20 mmHg and mean BMI was 27.3 ± 3.9 kg/m2. The prevalence of LVH was 12%, since 80 fulfilled the CP criterion, 13 fulfilled the SL criterion and six fulfilled both. 49% had NFG, 32% had IFG and the remaining 18% had new-onset DM. This apparently healthy population was also split by gender and FPG group, and the characteristics of this population are presented in and .
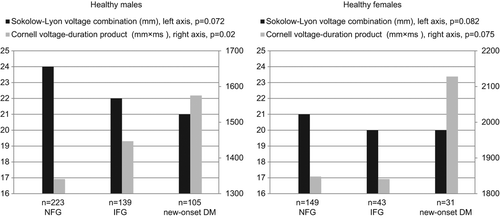
Apparently healthy subjects with higher FPG tended to be male and more often had higher SBP, higher DBP, higher HR, higher BMI, higher waist/hip ratio, higher triglycerides, lower HDL cholesterol and higher hs-TnT. Among the males there was a tendency towards higher CP with higher FPG ().
Figure 1. Differences between electrocardiographic markers of left ventricular hypertrophy among fasting plasma glucose groups. p, p value for difference between the groups analyzed using one-way analysis of variance; n, number; NFG, normal fasting glucose; IFG, impaired fasting glucose; DM, diabetes mellitus.
Independent associations in multivariable regression models
Left ventricular hypertrophy
Female gender and older age were independently associated with LVH in the patient group as well as in the healthy population (). Among healthy subjects, the association between LVH and female gender was lost when median age split was performed. Higher SBP was only associated with LVH in the healthy population. When the analyses were performed among men and women separately, it was apparent that fewer factors were independently associated with LVH in women than in men. In healthy men, higher FPG was independently associated with LVH (). This finding remained in younger men after separate analysis on both sides of the median age. When the analyses were performed separately in the FPG groups, the association between higher SBP and LVH was only present in the healthy NFG group (p = 0.04 for interaction), not among otherwise healthy subjects with IFG or new-onset DM.
Figure 2. Odds ratios (×) and 95% confidence intervals for the independent explanatory factors for the presence of electrocardiographically identified left ventricular hypertrophy in healthy males.
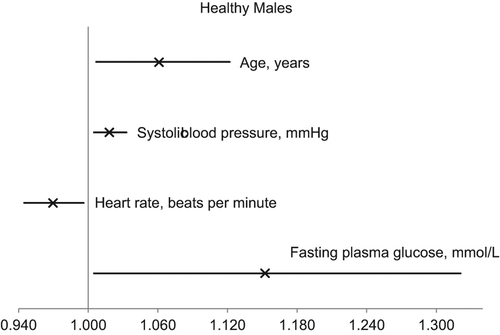
Table III. Independent explanatory factors for the presence of electrocardiographically identified left ventricular hypertrophy.
Sokolow–Lyon voltage combination
Higher SBP and lower HR and BMI were closely related to higher SL in both patients and healthy subjects (). Male gender was associated with higher SL in patients only, and the association was lost when median age split was performed. When the analyses were performed among men and women separately, it was apparent that the association between lower HR and higher SL was only present in men. Furthermore, among male patients, lower FPG was independently associated with higher SL. However, the association was lost when only the younger half was considered.
Table IV. Independent explanatory factors for Sokolow–Lyon voltage combination.
Cornell voltage–duration product
As shown in , female gender, older age and higher SBP were independently associated with higher CP in both patients and healthy subjects, and higher FPG was associated with higher CP in healthy subjects. When the analyses were performed among men and women separately, it was apparent that the associations between risk factors and CP were more established in men than in women. Furthermore, higher FPG was associated with higher CP in healthy men only, and this finding remained in younger men after separate analysis on both sides of the median age.
Table V. Independent explanatory factors for Cornell voltage–duration product.
Discussion
Main findings
The main findings in our study were as follows. (i) There was a high prevalence of LVH among both patients and healthy subjects. (ii) An independent association was found between higher FPG and ECG pattern of LVH as well as higher CP in healthy men. Higher FPG category was inversely associated with SL in male patients, which, however, may be due to survival bias, because the association was lost when only the younger half was considered. (iii) FPG did not potentiate the independent associations between SBP and LVH, CP and SL, and the association between SBP and LVH was only significant in healthy subjects with NFG. (iv) An independent association was found between female gender and CP in patients as well as in apparently healthy subjects, and between female gender and LVH in patients.
Prevalences of left ventricular hypertrophy
The prevalences of ECG-verified LVH in our study are comparable to findings from two similar population studies of healthy 60-year-olds: one study applied LVH criteria identical to ours and found prevalences of 3.9% and 5.0% for men and women, respectively (Citation23); and another study applying almost identical criteria found prevalences of 9.5% and 5.5%, respectively (Citation24). The prevalences of LVH in the younger half of our healthy population (mean age 60 years) were 6.0% and 5.5% for men and women, respectively. Using only the gender-specific Cornell voltage (Citation25) as the criterion for LVH in a large white population free of coronary heart disease and cardiac medication, Machado et al. found the age-adjusted prevalences of LVH to be 0.7% and 0.6% for men and women, respectively (Citation11). The Cornell voltage criterion is known, though, to have low sensitivity for LVH, especially in subjects with DM (Citation17). Applying the same criterion to our younger, healthy population, the corresponding prevalences were 1.0% and 1.8%. Our healthy population had a mean SBP of 147 mmHg and the mean BMI was 27 kg/m2. Thus, keeping in mind our selected population with high proportions of glucometabolic disturbances as well as hypertension and obesity, the prevalences of LVH in the current study are in agreement with previous reports.
Impact of fasting plasma glucose on electrocardiographic changes
Previous studies report contradictory findings regarding the association between glucometabolic disturbances and LVH. Supporting our finding of an independent association between FPG and LVH is a cross-sectional study in which FPG was associated with LVH verified by magnetic resonance imaging (MRI) (Citation16). However, the subjects of this study were younger than ours, and patients treated with blood-pressure reducing drugs were not excluded. Also in support of an association between FPG and LVH is a prospective study of healthy subjects over 60 years of age, in which baseline FPG independently predicted incident LVH verified by echocardiography after 4 years of follow-up (Citation8). The findings of both studies were independent of gender and remained consistent after adjustment for factors including body composition and SBP. The studies are strengthened by the fact that both echocardiography and MRI are more sensitive for LVH than ECG (Citation26,Citation27). Finally, also supporting our findings is a large cross-sectional population study of hypertensive middle-aged subjects that found FPG to be independently associated with the significantly larger prevalence of LVH in men with metabolic syndrome (Citation13).
Our finding that the higher FPG group was inversely associated with SL in male patients, independently of BMI, may be due to survival bias, but could also be explained by a study that found insulin resistance to be inversely associated with LVH identified by ECG voltages, yet positively associated with LVH verified by MRI (Citation28). Two additional studies found insulin resistance to be associated with LVH verified by MRI (Citation29) or echocardiography (Citation15), while Sciacqua et al. (Citation30) found glucose intolerance to be correlated with increasing left ventricular mass in hypertensive patients. All findings remained significant after adjustment for age, SBP and body composition. Finally, voltage criteria are indeed less sensitive in subjects with DM (Citation17).
Contrary to our findings of an independent association between FPG and LVH are studies applying MRI (Citation29) as well as echocardiography to assess left ventricular mass in both healthy (Citation15) and hypertensive subjects (Citation9). However, one of these studies found an independent association between insulin resistance and left ventricular mass (Citation15), and another found an independent association between FPG and the left ventricular mass/left ventricular end-diastolic volume ratio in men (Citation29). None of these studies excluded subjects treated with blood-pressure reducing drugs.
Factors associated with electrocardiographic changes
As expected (Citation8,Citation9,Citation13,Citation24), we found SBP to be associated with LVH in apparently healthy subjects and with continuous SL in patients as well as healthy subjects. Regarding continuous CP, the association was lost for the older male patients (> 69 years) and for all female patients, which can be explained by the fact that CP, compared to SL, is more closely associated with obesity and increased waist/hip ratio than with SBP (Citation6). The lack of an association between SBP and LVH in the patient population was probably due to use of blood-pressure reducing drugs in patients with hypertension, since we found an association when excluding patients receiving cardiovascular medication (data not shown). The association between SBP and LVH was not potentiated by elevated FPG, but was actually only significant in the NFG group with significant interaction between SBP and FPG. This suggests that hyperglycemia and/or associated hyperinsulinemia may induce LVH by themselves and not by potentiating the effect of SBP.
We found an independent association between female gender and continuous CP in patients as well as in apparently healthy subjects, and between female gender and LVH in patients, whereas continuous SL showed a tendency towards association with male gender in patients. This finding probably reflects both our choice of population and the ECG criteria used for LVH. The population was elderly with an oversampling of subjects with elevated FPG, resulting in a rather obese population with some degree of survival bias against men compared to women with LVH. Furthermore, untreated hypertension was rare. Owing to obesity and relatively low SBP, very few of the subjects with LVH met the SL criterion (15% of healthy subjects with LVH and 11% of patients with LVH), and SL paradoxically decreased with increasing BMI, preferring lean males over other subjects. This is in agreement with the SL criterion's low sensitivity (Citation25), which is especially low in obese subjects (Citation4), and the finding is in accordance with previous studies (Citation6,Citation28).
Instead, most of the subjects with LVH in our study were identified by the CP criterion, which primarily included women (16% of healthy women and 22% of female patients vs 9% and 11% in men). Disagreements exist as to whether the add-on to the voltage in CP in women should be lower than 8 mm, since it has been suspected of leading to female oversampling (Citation6). In our study, this did not seem to be the case in the younger half of the healthy population, but among the older subjects, more women than men fulfilled the CP criterion (19.6% vs 14.0%). In accordance with previous findings, we found continuous CP to be associated with female gender, higher BMI and older age (Citation6). The CP criterion has long been proclaimed as the most sensitive (Citation5), reaching sensitivities of 51% or 37% at matched specificities of > 95% when verified at autopsy (Citation31) or echocardiography (Citation21), respectively. Finally, the combination of the CP and SL criteria has been shown to heighten the sensitivity by > 10% without loss of specificity (Citation32). Nonetheless, other methods such as echocardiography (Citation26) and especially MRI (Citation27) outperform ECG in detection of LVH, but these methods remain costly, and thus ECG is still recommended for primary screening in patients without cardiac symptoms (Citation7).
Limitations
With the intention of investigating the effects of FPG on risk markers intended for primary cardiovascular risk stratification, our participants were chosen randomly from groups defined by levels of FPG, although retrospectively, groups defined by levels of hemoglobin A1c would have been preferable. Moreover, the cross-sectional nature of our study prevents us from making finite inferences about causality. In order to represent a population typically making contact with the health-care system, the population was rather old, with a high prevalence of medical treatment, creating a risk of survival bias.
Conclusion
In this cross-sectional study of an elderly, general population sample, we found LVH to be associated with elevated FPG in healthy men. However, FPG did not potentiate the effect of other cardiovascular risk factors such as SBP. On the contrary, in apparently healthy subjects higher SBP was only associated with LVH among subjects with NFG, suggesting that hyperglycemia and/or the associated hyperinsulinemia might overrule the impact of hypertension on LVH.
Declaration of interest: The authors declare no conflicts of interest. This research received no grant from any funding agency in public, commercial or not-for-profit sectors. There have been no previous presentations of the whole or part of the work presented in this article.
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53.
- Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8.
- Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: the Cardiovascular Health Study. Am Heart J. 1997;133:36–43.
- Antikainen RL, Grodzicki T, Beevers DG, Webster J, Jokelainen JJ, Bulpitt CJ. Left ventricular hypertrophy by Sokolow–Lyon voltage criterion predicts mortality in overweight hypertensive subjects. J Hum Hypertens. 2009;23: 20–6.
- Norman JE Jr, Levy D. Improved electrocardiographic detection of echocardiographic left ventricular hypertrophy: results of a correlated data base approach. J Am Coll Cardiol. 1995;26:1022–9.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Dahlof B. Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: the Losartan intervention for endpoint reduction (LIFE) in hypertension study. The Life Study Investigators. Hypertension. 2000;36:766–73.
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103.
- Lin TH, Chiu HC, Su HM, Voon WC, Liu HW, Lai WT, Sheu SH, et al. Association between fasting plasma glucose and left ventricular mass and left ventricular hypertrophy over 4 years in a healthy population aged 60 and older. J Am Geriatr Soc. 2007;55:717–24.
- Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, et al. Long-term risk of diabetes, hypertension and left ventricular hypertrophy associated with the metabolic syndrome in a general population. J Hypertens. 2008;26: 1602–11.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6.
- Machado DB, Crow RS, Boland LL, Hannan PJ, Taylor HA, Folsom AR. Electrocardiographic findings and incident coronary heart disease among participants in the atherosclerosis risk in communities (ARIC) study. Am J Cardiol. 2006;97:1176–81.
- Larsen CT, Dahlin J, Blackburn H, Scharling H, Appleyard M, Sigurd B, Schnohr P. Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T-wave – the Copenhagen City Heart Study. Eur Heart J. 2002;23:315–24.
- Cuspidi C, Sala C, Lonati L, Negri F, Rescaldani M, Re A, et al. Metabolic syndrome, left ventricular hypertrophy and carotid atherosclerosis in hypertension: a gender-based study. Blood Press. 2013;22:138–43.
- de Simone G, Okin PM, Gerdts E, Olsen MH, Wachtell K, Hille DA, et al. Clustered metabolic abnormalities blunt regression of hypertensive left ventricular hypertrophy: the LIFE study. Nutr Metab Cardiovasc Dis. 2009;19:634–40.
- Hwang YC, Jee JH, Kang M, Rhee EJ, Sung J, Lee MK. Metabolic syndrome and insulin resistance are associated with abnormal left ventricular diastolic function and structure independent of blood pressure and fasting plasma glucose level. Int J Cardiol. 2012;159:107–11.
- Rerkpattanapipat P, D’Agostino RB Jr, Link KM, Shahar E, Lima JA, Bluemke DA, et al. Location of arterial stiffening differs in those with impaired fasting glucose versus diabetes: implications for left ventricular hypertrophy from the Multi-Ethnic Study of Atherosclerosis. Diabetes. 2009;58:946–53.
- Somaratne JB, Whalley GA, Poppe KK, ter Baals MM, Wadams G, Pearl A, et al. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community. Cardiovasc Diabetol. 2011;10:29.
- Berglund G, Nilsson P, Eriksson KF, Nilsson JA, Hedblad B, Kristenson H, Lindgarde F. Long-term outcome of the Malmo preventive project: mortality and cardiovascular morbidity. J Intern Med. 2000;247:19–29.
- Leosdottir M, Willenheimer R, Plehn J, Borgquist R, Gudmundsson P, Harris TB, et al. Myocardial structure and function by echocardiography in relation to glucometabolic status in elderly subjects from 2 population-based cohorts: a cross-sectional study. Am Heart J. 2010;159:414–20.e4.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
- Okin PM, Roman MJ, Devereux RB, Kligfield P. Electrocardiographic identification of increased left ventricular mass by simple voltage–duration products. J Am Coll Cardiol. 1995; 25:417–23.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–86.
- Diederichsen SZ, Gerke O, Olsen MH, Lambrechtsen J, Sand NP, Norgaard BL, et al. Coronary artery calcification and ECG pattern of left ventricular hypertrophy or strain identify different healthy individuals at risk. J Hypertens. 2013;31:595–600.
- Halldin M, Fahlstadius P, de Faire U, Vikstrom M, Hellenius ML. The metabolic syndrome and left ventricular hypertrophy – the influence of gender and physical activity. Blood Press. 2012;21:153–60.
- Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75: 565–72.
- Reichek N, Devereux RB. Left ventricular hypertrophy: relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation. 1981;63:1391–8.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–8.
- Vernooij JW, Cramer MJ, Visseren FL, Korndewal MJ, Bots ML, Meijs MF, et al. Relation between abdominal obesity, insulin resistance and left ventricular hypertrophy diagnosed by electrocardiogram and magnetic resonance imaging in hypertensive patients. Am J Cardiol. 2012;110:227–33.
- Velagaleti RS, Gona P, Chuang ML, Salton CJ, Fox CS, Blease SJ, et al. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:257–63.
- Sciacqua A, Miceli S, Carullo G, Greco L, Succurro E, Arturi F, et al. One-hour postload plasma glucose levels and left ventricular mass in hypertensive patients. Diabetes Care. 2011;34:1406–11.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage–duration product. J Am Coll Cardiol. 1992;20:1180–6.
- Dahlof B, Devereux RB, Julius S, Kjeldsen SE, Beevers G, de Faire U, et al. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint Reduction in Hypertension. Hypertension. 1998;32:989–97.

