Abstract
The intestinal immune system is severely affected by HIV and circulating microbial products from the intestinal tract that provide an ongoing source of systemic inflammation and concomitant viral replication. In addition, HIV-infected individuals can have a deregulated immune response that may hamper the anti-viral capacity of the host. Various probiotic organisms and prebiotic agents have been shown to enhance intestinal epithelial barrier functions, reduce inflammation, and support effective Th-1 responses. As these characteristics may benefit HIV patients, this review aims to provide a theoretical framework for the development of probiotic and prebiotic interventions specifically for this population.
INTESTINAL DEFENSES AND HOMEOSTASIS
The gastrointestinal tract provides a range of habitats for microbes that have either co-evolved with the human species as symbionts or as potential pathogens. The different compartments of the tract host approximately 500 to 1000 bacterial species [Citation1], totaling 1013–1014 cells. Collectively, these organisms represent at least 100 times more genes than the human genome. This complex microbial population influences an estimated 10% of all metabolites in our body [Citation2], and could be regarded as “the neglected organ.” In various eukaryotic species, including humans, the relation between bacterial communities and their host is mutualistic and symbiotic in nature [Citation3]. The symbiotic benefits in humans include energy supply, nutrient metabolism, and prevention of colonization by opportunistic pathogens [Citation4].
In order to benefit from this symbiotic relationship, the immune system has to balance permissive, tolerogenic responses to food antigens and commensal microbes with potentially damaging, inflammatory responses to ward off pathogens. This delicate balance is maintained by the constant interplay between the microbiota, the intestinal barrier, and the mucosal immune system and is a prerequisite for normal gut homeostasis (). Imbalance of this system may lead to autoimmune inflammation or infectious pathology.
FIGURE 1 Normal mucosal defenses and homeostasis: The commensal microbiota induces a state of non-responsiveness through interaction with dendritic cells (DC) and subsequent induction of the T-regulatory phenotype and secretion of IL-10 and TGF-β. There is a limited uptake of bacterial antigens, such as lipopolysaccharide (LPS), polysaccharide-A (PSA), and DNA, that induce intestinal defense systems, such as the excretion of β-defensin and secretory IgA.
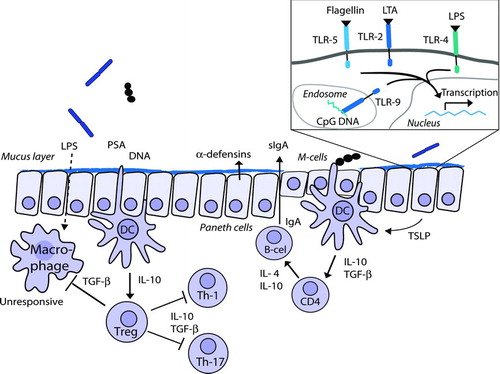
The first barrier against pathogenic infection and damaging inflammatory responses against commensal bacteria is a degree of physical separation between the intestinal bacteria and the host. Important components of this barrier are intestinal epithelial cells (IEC) that form a physical barrier on the body's largest surface area for interaction with microbes. The epithelium is also home to mucus-producing goblet cells and antimicrobial-peptide-producing Paneth cells [Citation1]. Collectively, these cells produce a mucus layer that selectively limits the contact between bacteria and host cells, a mechanism that is thought to limit damaging inflammatory responses [Citation5].
Despite this physical barrier, sampling and recognition of the intestinal content is a crucial function of the intestinal immune system that is necessary to mount appropriate immune responses. An important mechanism for IEC and immune cells to interact with commensal and pathogenic bacteria is the recognition of microbe-associated molecular patterns by germline-encoded pattern recognition receptors (PRR). The best described PRR are Toll-like receptors (TLR), which have been found on a wide range of cell types. The TLR detect various conserved microbial structures, such as lipoteichoic acid (TLR-2), lipopolysaccharide (LPS) (TLR-4), flagellin (TLR-5), and CpG DNA (TLR-9) (reviewed in [Citation6]). Interaction of commensal microbes with TLRs appears to be essential for IEC integrity [Citation7]. Other important PRR groups include the sugar-binding lectins and NOD protein families. NOD proteins are located in the cytoplasm of IEC and are activated upon invasion. While NOD-1 is located within all IEC, another variant, NOD-2, is only expressed in Paneth cells and plays a role in the synthesis of cryptidin and defensin [Citation8].
Specialized structures for sampling intestinal content are present in the gut-associated lymphoid tissues (GALT). These include Peyer's patches (PP) in the small intestine and lymphoid follicles in the colon, which are covered by follicle-associated epithelium containing non-mucus producing microfold (M-) cells. These cells are devoid of microvilli and are specialized in antigen transport into the PP, where the antigens are taken up by antigen presenting cells (APC) [Citation9]. Dendritic cells (DC), the main APCs in PP, interact with T- and B-lymphocytes to induce suitable adaptive immune responses, depending on the type of stimulus. Therefore, PPs are major inductive sites of mucosal adaptive immune responses (reviewed in [Citation10]). After activation, lymphocytes home to the lamina propria (LP) or intestinal epithelium to perform effector functions. The heterogeneous population of intestinal intra-epithelial lymphocytes (iIEL) is thought to regulate the intestinal epithelial barrier integrity and regeneration, and reduce damage due to local immune responses [Citation11]. Furthermore, the LP contains DCs that can sample luminal content by extending dendrites through the intracellular epithelial tight junctions, providing a mechanism to sample intestinal content outside of PP as well [Citation12]. DCs are central regulators of adaptive immune responses, initiating either effector responses or inducing tolerance. The many different DC subpopulations that are present within the mucosal immune system each have different functional characteristics (reviewed in [Citation13]).
In the absence of inflammatory signals, commensal microorganisms induce tolerogenic maturation of DCs, leading to the induction of various types of regulatory T-cells (Treg), including CD4+CD25+Foxp3+ lymphocytes [Citation14], or hyporesponsive T-cells [Citation15]. The maintenance of the Treg population are dependent on IL-2, IL-10, and TGF-β levels, which in turn are dependent on continuous background activation by commensal micro-organisms [Citation16]. In addition, intestinal DCs are potent inducers of IgA synthesis in B cells, which has anti-pathogenic effects but also prevents commensal bacteria from penetrating the host [Citation17]. IgA accounts for >70% of our body's total immunoglobulin production. Several grams of secretory IgA (sIgA) are secreted in the intestinal lumen daily, which exerts considerable immunological pressure on the intestinal microbiota [Citation18].
Reciprocally, the intestinal bacteria have a major influence on the immune system as well. Studies comparing germ-free mice with microbially-colonized mice have shown that the presence of microbes is crucial for the normal development of GALT as well as other secondary immune organs. In the GALT, the absence of bacteria leads to a multitude of effects, including limited development of Peyer's patches, reduced B-cell activation and IgA production, and reduced numbers of iIEL. Moreover, epithelial function is also affected, as evidenced by reductions in IEC turnover and changes in mucus production [Citation19].
The effects of commensal bacteria on the immune system are dualistic in nature. On the one hand, mechanisms are induced that maintain tolerance and/or prevent inflammation. This includes IgA production, β-defensin production in the epithelium [Citation20, 21], enhanced epithelial barrier integrity through TLR signaling [Citation7], Treg induction and even immunosuppressive effects [Citation22–24]. On the other hand, exposure to commensal bacteria induces the expansion of inflammatory lymphocyte populations, including cytotoxic iIEL and IL-17-producing CD4+ T-helper (Th17) cells [Citation25, 26]. Th17 and other IL-17 producing cells have been implicated in many inflammatory and autoimmune conditions [Citation27–29], however, they have also been shown to play important roles in protective mucosal responses against extra-cellular bacteria and fungi (reviewed in [Citation30]). It is interesting to note that the induction of Treg and Th17 populations share a dependency on TGF-β signaling. Furthermore, both populations are relatively abundant in the LP. Therefore, it is thought that both types of T-cells are induced by signals from the intestinal bacteria and the balance between these opposing cell types is determined by the specific host-microbe interactions [Citation31].
Commensal bacteria are able to influence the mucosal immune system, not only through cell-cell interactions, but also through the secretion of immune-modulatory molecules. These include: adenosine triphosphate (ATP), which enhances the polarization of Th-17 T-lymphocytes [Citation32]; polysaccharide A (PSA), which induces maturation of Th-17 cell populations [Citation33]; and DNA, which induces IFN-α syntheses and favors IEC integrity [Citation34]. Furthermore, the intestinal epithelium is also constantly exposed to inflammatory molecules, such as LPS and peptidoglycans. Despite this continuous exposure, the intestinal immune system is unique in its ability to maintain tolerance in the presence of a multitude of immune triggers while minimizing the risk of systemic infection.
In summary, the net effects of the interplay between the commensal microbiota and the mucosal immune system are enhanced mucosal defense mechanisms, balanced by an inhibition of potentially damaging, inflammatory immune responses. In the absence of pathogenic stimuli, virulence factors or chronic inflammation, the host-microbe interaction leads to a predominance of tolerogenic mechanisms and intestinal homeostasis.
THE RESULT OF HIV INFECTION ON THE HOST-MICROBE INTERFACE
HIV infection has a disruptive impact on the physiological interplay between the commensal microbiota and immune system: CD4+ cells associated with the mucosal immune system are rapidly depleted after HIV infection [Citation35, 36], including reduced numbers of DCs [Citation37], a change in the composition of iIEL [Citation38], and depletion and anergy of gamma-delta T-lymphocytes [Citation39]. These detrimental changes on the mucosal immune system have severe consequences for the (immunological) function of the intestine and are associated with compromised epithelial repair mechanisms and enhanced epithelial permeability [Citation40, 41]. The net result is an increased risk of gastrointestinal infections at all stages of HIV infection [Citation42] and a high prevalence of gastrointestinal disorders with unknown etiology [Citation43].
Chronic immune activation and inflammation have long been described as characteristic features of progressive HIV disease, while the source of inflammation has remained unidentified. Indeed, increased B-cell activation, increased T-cell turnover, and increased pro-inflammatory cytokines are observed with HIV infection. In this pro-inflammatory state, the replication of HIV is markedly enhanced [Citation44] and activation of the nuclear factor (NF) κB transcription factor plays a crucial role in this phenomenon [Citation45]. Strikingly, the degree of systemic immune activation, indicated by the expression of the immune activation marker CD38 on CD8+ cells, is a better predictor of HIV progression than viral load or CD4+ count alone [Citation46]. Recently, it has been suggested that the gut might be a source of chronic inflammation. The hypothesis is that dysfunction of the mucosal immune response due to preferential depletion of intestinal mucosal immune cells, including effector CD4+ cells and DCs [Citation36, 37, Citation47], may affect systemic immune activation through the increased translocation of microbes and bacterial products from the intestinal tract [Citation48]. The resultant pro-inflammatory environment [Citation40] may then cause further damage to the gut barrier function, augmenting bacterial translocation and subsequently fueling systemic inflammation. Indeed, evidence suggests that bacterial translocation affects the activation state of the immune system, and in turn HIV progression ().
Some HIV-infected individuals, termed “non-progressors” have a low HIV viral load even without treatment and maintain a low degree of systemic inflammation [Citation49, 50]. A mechanism that appears to contribute to the control of the virus is a capacity to maintain the integrity of the gut barrier and to mount an attenuated response to bacterial products and, thus, potentially reduce bacterial translocation [Citation40, Citation51]. In non-progressors, serum LPS has been shown to be lower than those with progressive HIV infection [Citation48]. One week of treatment with a “gut sterilizing” antibiotic regimen markedly reduced serum LPS levels in macaques, concomitant with a reduction of fecal Gram-negative bacteria and inflammatory markers. However, after two weeks of antibiotics, plasma LPS had increased again, apparently due to the growth of other bacterial species in the gut [Citation48]. Although anti-retroviral treatment (ART) has been shown to enhance epithelial barrier functions [Citation52], the efficiency of CD4+ recovery may still be compromised by bacterial translocation [Citation53]. Future studies will need to focus on the role of the epithelial barrier and the microbiota composition along with translocation in the progression of HIV.
TABLE 1 Evidence Implicating the Intestinal Microbiota and Epithelial Cell Barrier as a Factor in HIV Progression
The intestinal microbiota has been shown to play important roles in other disease conditions. For example, bacterial translocation occurs during surgery [Citation54], and plays a role in alcohol-induced liver cirrhosis [Citation55], in exacerbation of graft versus host disease [Citation56] and in inflammatory bowel disease (IBD) [Citation57, 58]. Furthermore, correlations between the microbiota composition and disease have been shown for IBD [Citation59] and obesity [Citation60]. Strikingly, the transplantation of gut microbes from obese mice (ob-/ob-) to bacteria-free mice resulted in obesity in the recipients [Citation61], suggesting that the microbiota may be a mediator of specific conditions. Lessons from such studies that focus on microbiota-disease interactions may help to provide more insight in the role of an aberrant microbiota in HIV-infected subjects.
The intestinal microbiota of HIV patients appears to contain higher levels of pathogens, such as Pseudomonas aeruginosa and Candida albicans [Citation62], and reduced or undetectable levels of Bifidobacterium and Lactobacillus species [Citation63]. In the macaque model, colitis is very common after simian immunodeficiency virus (SIV) infection, resulting in a reduced microbial diversity and an increased proportion of Campylobacteriaceae [Citation64]. If the aberrant microbiota among HIV patients more easily translocates and provides an inflammatory stimulus for HIV replication, therapeutic modification of the gut microbiota might have a beneficial impact on HIV progression.
Several lines of evidence suggest that HIV modulates systemic immunity by skewing the Th1/Th2 balance towards Th2 responses. Recently, it was suggested that the induction of T helper-2 (Th-2) cytokine synthesis [Citation65, 66] and the gradual increase of IL-4 and IgE [Citation67, 68] observed after HIV infection, might be due to a Th-2 response to viral proteins, such as gp-120, p24, and p17 [Citation69]. It is well known that HIV patients suffer from high rates of allergies [Citation70] and the ability of subgroups of HIV patients to maintain a vigorous Th-1 response and higher levels of IFN-γ is associated with increased survival [Citation71, 72]. An HIV-triggered Th-2-skewed state of the immune system could compromise immunological control of HIV replication and lead to reduced protection to opportunistic infections. This immune imbalance may also aggravate inflammation and barrier dysfunction in the gut, as the increase in IL-4 production can compromise the antimicrobial function of Th-17 cells [Citation73] that line the intestine.
PROBIOTICS
Probiotics, defined as “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host” [Citation74], have been studied in a myriad of conditions related to intestinal dysbiosis, including IBD, infectious diarrhea, allergy, and surgery. Relevant research from these fields and studies on probiotic interventions among people living with HIV and safety considerations are now discussed.
Effects on Gut Barrier Function
Supplementation of probiotic strains may enhance or restore the beneficial interactions between the commensal enteric flora and the host in both healthy and disease conditions, leading to an enhanced barrier function and reduced bacterial translocation (). Effects have been described in animal and human studies that may have relevance for HIV-infected subjects. For example, the Gram-negative probiotic strain Escherichia coli Nissle 1917 was shown to enhance the intestinal epithelial integrity via the induction of epithelial tight junctions proteins (ZO-1 and ZO-2) [Citation75, 76]. Prior administration of candidate probiotic strains Lactobacillus acidophilus (ATCC19258) and Streptococcus thermophilus (ATCC4356) to IEC has been shown to reduce the epithelial permeability induced by TNF-α and IFN-γ, suggesting that the impact of inflammation can be reduced [Citation77]. It appears that these various protective mechanisms occur through the production of as yet uncharacterized proteins [Citation78] or through direct interaction of microbes with IEC through TLR-4, TLR-5, and TLR-9 [Citation34]. That the enhancement of the epithelial barrier might also translate to a reduction in bacterial translocation was shown in a murine model of enterohemorrhagic shock. In this model, prior challenge with L. rhamnosus LMG P-22799 reduced bacterial translocation and systemic inflammation [Citation79], as did use of Bifidobacterium adolescentis in a murine model of burn wounds [Citation80]. Furthermore, allergy-associated intestinal hyper-permeability [Citation81] as well as alcohol-induced loss of gut barrier function have been found to be reversed by application of L. rhamnosus GG, resulting in less intestinal and liver inflammation in the latter [Citation82].
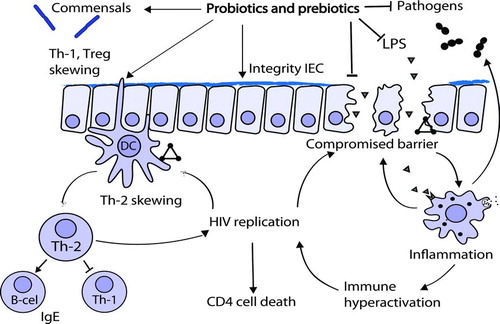
FIGURE 2 Potential benefits of probiotics and prebiotics in HIV-induced intestinal pathogenesis: HIV infection induces effects and positive feedback mechanisms that induce a loss of intestinal homeostasis and promote replication of the virus (triangles). Pro- and prebiotics may ameliorate the HIV-induced intestinal problems through effects on the microbiota and its metabolism, on various cells of the immune system (as represented by the arrow pointing at the sampling DC), and on intestinal epithelial cells.
In human clinical studies, probiotics have been applied to reduce bacterial translocation among different patient populations with varying degrees of success. A randomized controlled trial (RCT) among 65 critically-ill patients showed reduced rates of infections, sepsis, and mortality with a combination of probiotics and prebiotics (Synbiotic 2000 Forte) [Citation83]. In consecutive trials, the same product was shown to increase fecal IgA [Citation84], reduce the incidence of bacteremia [Citation85], and lower the rate of post-operative infections [Citation86]. Two other RCTs using different synbiotic preparations showed similar results. L. casei Shirota and L. breve Yakult given with galacto-oligosaccharides (GOS) supplementation (15 gr/day) before biliary cancer surgery, resulted in reduced inflammatory markers and post-operative complications [Citation87]. Another RCT with L. acidophilus La-5 and B. lactis Bb-12 combined with oligofructose (15 gr/day) resulted in a reduced incidence of bacterial translocation [Citation88]. However, the evidence is not conclusive as three other RCTs using L. acidophilus La-5 and B. lactis Bb-12 [Citation89, 90] or L. plantarum 299v [Citation91] reported no reduction in bacterial translocation. This suggests that there may be strain-specific effects or that prebiotics are needed for efficacy.
Effects on Mucosal and Systemic Immunity
The loss of tolerance in the intestine due to a defect in GALT homeostasis can have a detrimental impact on the gut barrier function, and specific probiotic strains have been shown to enhance the recovery of GALT homeostasis. For example, administration of Escherichia coli Nissle 1917 in wild type mice, but not in TLR-2 knock-out mice, ameliorated experimental colitis and reduced pro-inflammatory cytokine expression, suggesting a TLR-2-dependent pathway [Citation92]. This response can be mediated by specific components of probiotic organisms. DNA from probiotic organisms modulates TLR-9 and elicits a different response from immune and epithelial cells than DNA from pathogenic organisms [Citation93]. In HT-29 cells subjected to pro-inflammatory cytokines, challenge with DNA from the VSL#3 probiotic mixture could reduce the expression of the pro-inflammatory IL-8 cytokine and delayed NF-κB activation [Citation94]. In IL-10 deficient mice, the administration of the probiotic mixture VSL#3 led to a reduction in mucosal TNF-α and IFN-γ release and improved the histological disease in a TLR-9 dependent manner [Citation94].
Another way of restoring GALT homeostasis is through the induction of regulatory mechanisms to down-regulate inflammation. Induction of regulatory mechanisms by specific probiotics appears to be partly dependent on modulation of DCs [Citation95]. Some probiotic organisms induce regulatory T-cells (CD4+FoxP3+), mediated by DCs [Citation16, Citation96]. This induces tolerance that is partly mediated through IL-10 and TGF-β production. Recently, it was demonstrated that ingestion of a specific probiotic mixture (IRT-5) could induce CD4+FoxP3+ cells in mesenteric lymph nodes. Interestingly, probiotics alone, without the presence of DCs, could not induce this effect. Administration of the probiotic mixture also induced both T-cell and B-cell hypo responsiveness, down-regulated both Th-1 and Th-2 functions, and reduced the secretion of pro-inflammatory cytokines in GALT [Citation97]. The biological relevance of these changes was verified in an IBD model in which administration of probiotics was shown to enhance GALT homeostasis and reduce the severity of the disease. The therapeutic effects were associated with an enrichment of CD4+Foxp3+ T-cells in inflamed regions [Citation97]. O’Mahony et al. demonstrated that challenge with Lactobacillus salivarius or Bifidobacterium infantis of DCs from mesenteric lymph nodes induced secretion of IL-10. This was in contrast to a challenge with Salmonella strains, which induced the secretion of pro-inflammatory IL-12. Strikingly, DCs from peripheral blood did not show a differential response to lactobacilli or Salmonella strains [Citation98], suggesting that the response of DCs depends on their immunological compartment. In the intestine, tolerogenic effects of T-regulatory cells and anti-inflammatory effects may improve barrier function and intestinal homeostasis. Furthermore, a reduction in the chronic inflammatory state may reduce immune activation and potentially affect disease progression [Citation48].
Systemically, specific probiotics have also been shown to induce a T-regulatory phenotype and counter-balance a Th-1 or Th-2 dominant state in vivo and in vitro [Citation95, Citation99, Citation100]. The induction of a T-regulatory phenotype frequently occurred together with increased levels of anti-inflammatory IL-10 [Citation101, 102]. A prime example of the clinical effects of IL-10 induction comes from an RCT of 77 adults with an abnormal IL-10/IL-12 ratio and concurrent IBS. B. infantis 35624 was shown to normalize the IL-10/IL-12 ratio in parallel with a reduction in clinical symptoms [Citation103]. In Crohn's disease, a Th-1 mediated condition, a strain of L. rhamnosus was able to decrease both the syntheses of the Th-1 parameter IFN-γ, and IL-2, which is an essential survival and proliferation factor for effector T-cells [Citation104]. Remarkably, IL-4, a potent Th-2 cytokine, was also reduced with probiotic supplementation. Hence, the effects of probiotic supplementation were unlikely due to mere Th-1/Th-2 skewing and are best explained by the induction of a regulatory DC phenotype with the ability to induce a general hypo-responsiveness. In a study of children with atopic dermatitis, a Th-2-dominant condition, an up-regulation of IL-10 was noted after supplementation with L. rhamnosus GG [Citation105]. These findings might be valuable in relation to HIV management, as both the induction of T-regulatory cells and anti-inflammatory effects can potentially be of benefit to HIV patients.
In vitro evidence indicates that several probiotic candidate strains can down-regulate the production of Th-2 cytokines and chemokines [Citation106, 107] and modulate DCs to skew T-cell polarization toward a Th1-response [Citation108]. The ability of probiotics to skew the immune system away from a Th-2 dominant state has been studied to some extent in humans, albeit in HIV uninfected subjects. An RCT of 230 children with a cow's milk allergy showed that L. rhamnosus GG ingestion could reduce symptoms of atopic eczema and dermatitis [Citation109] and up-regulate IFN-γ, indicative of a more pronounced Th-1 response [Citation110, 111]. Moreover, L. rhamnosus GG was shown to up-regulate IL-6, involved in the mucosal response to stress [Citation112] but also epithelial IgA production and mucosal protein syntheses [Citation113], suggesting a direct effect of L. rhamnosus GG on IECs. Two other RCTs have shown similar results [Citation114, 115] but one study in children did not confirm this outcome [Citation116]. In addition to the ability of probiotics to improve barrier function and aspects of intestinal homeostasis, specific probiotic strains may, therefore, be able to skew away from an HIV-induced Th-2 predominance.
Effects on Intestinal Microbiota and Infections
Probiotics can interfere with the function and proliferation of pathogens in the gastrointestinal tract in various ways. They can enhance the secretion of pathogen-specific IgA [Citation117], induce β-defensin secretion [Citation118] or secrete bactericidal proteins [Citation119], and reduce the adhesion and invasion of pathogens [Citation120, 121]. Antibiotic-like compounds, such as reuterin produced by L. reuteri, exhibit broad spectrum effects against Gram-positive, Gram-negative bacteria as well as fungi, yeast, and protozoa [Citation119], while non-reuterin producing strains, such as L. reuteri RC-14, produce signaling molecules that down-regulate Staphylococcus aureus toxin production [Citation122]. These characteristics could be beneficial for acquired immune deficiency syndrome (AIDS) as L. reuteri was shown to prevent cryptosporidiosis in a murine AIDS model [Citation123].
Use of probiotics can lead to at least temporary modification of the intestinal microbiota. For example, in an RCT of 69 preterm babies, B. lactis Bb-12 significantly increased the levels of bifidobacteria and lactobacilli while reducing the numbers of enterobacteria and clostridia [Citation124]. In 36 adults receiving triple therapy for Helicobacter pylori infection, the addition of L. acidophilus CUL60 and CUL21 and Bifidobacterium spp. decreased the intestinal load of C. albicans, facultative anaerobes, and enterobacteria [Citation125]. Moreover, genomic and metabolic studies suggest that probiotic microbes change the behavior of the intestinal microbiota [Citation126].
The application of probiotics for the prevention and treatment of gastrointestinal infections has been well established and might be especially useful among people living with HIV. A Cochrane review concluded that probiotics are a useful adjunct to lower the occurrence and reduce the length of episodes of infectious diarrhea (reviewed in [Citation127]). Synbiotics (probiotics combined with prebiotics) have also been shown to reduce diarrhea associated with ART use [Citation128] but these findings could not be confirmed by a cross-over study [Citation129]. Although the application of probiotics to prevent gastrointestinal infections and the concomitant inflammatory state among HIV patients bears promise, no studies have so far been conducted to assess its potential.
Probiotic Interventions in HIV
A limited number of studies suggest that the probiotic benefits could be translated to people living with HIV. One RCT of 77 children in Brazil showed an increase of 118 CD4+ cells/μl among those receiving B. bifidum and S. thermophilus for two months compared to a decrease of 42 CD4+ cells/μl among the placebo group (p = 0.05) [Citation130]. An RCT of 24 HIV patients in Nigeria showed after four weeks of L. rhamnosus GR-1 and L. reuteri RC-14 an increase of 6.7 CD4+ cells/μl compared to a decrease of 2.2 CD4+ cells/μl among the placebo group (p < 0.05) [Citation131]. A large RCT in Malawi (n = 795) testing the effect of Synbiotic 2000 Forte on malnutrition also included a proportion of HIV-infected children (n = 361) [Citation132]. Although there was no improvement in nutritional cure, there was an overall reduction in outpatient mortality, including a trend towards a reduced mortality among the subgroup of HIV-infected children.
Safety
Among HIV patients, several studies have been conducted to assess the safety of probiotic interventions. When treated with L. reuteri SD2112, no safety concerns arose among moderately immune-compromised HIV patients (>350 CD4+ cells/μl) [Citation63]. In another study of severe immune compromised HIV patients (<200 CD4+ cells/μl), no safety concerns were detected with use of L. rhamnosus [Citation129]. To date, five case studies of lactobacillemia have been reported in end-stage AIDS patients. Of these, three patients were reported to have central venous catheters and one patient to have pneumonia, all of whom had extremely low CD4 counts (<55 CD4+ cells/μl) [Citation133–135]. Currently, no indication exists to avoid oral probiotic use in HIV populations, but close monitoring of safety parameters is recommended.
PREBIOTICS
Altered Microbe-Host Interaction
Prebiotics were defined most recently as “a non-viable food component that confers a health benefit on the host associated with modulation of the microbiota” [Citation136], although the term is often used less strictly for components that modify the composition or metabolism of the intestinal microbiota. Prebiotics can modify host-microbe interactions via the microbiota and its metabolism, host epithelial and other cells, as well as by modifying receptor expression and bacterial adhesion. As alluded to earlier, prebiotics are candidate agents to improve the intestinal homeostasis in HIV-infected individuals. Since prebiotics do not contain bacteria but provide substrate for the intestinal microbiota, their fermentation depends on the organisms present in the host. Prebiotic fructans and galacto-oligosaccharides (GOS) increase the percentage of “beneficial bacteria” through selective fermentation as shown in a variety of human target groups, including infants [Citation137, 138], healthy volunteers [Citation139], and seniors [Citation140]. The fermentation of fructans and GOS increases the production of short-chain fatty acids (SCFA), lactate, and other bacterial metabolites [Citation141, 142]. SCFA are known to have a plethora of effects on the intestinal milieu, epithelial cells as well as local immune cells. Various prebiotics induce differential effects on SCFA production and the ratios of butyrate, acetate, and propionate [Citation143]. The degree of specificity of prebiotic agents enables the potential development of specific prebiotics optimized to target HIV-specific issues. Relevant potential benefits for HIV patients are discussed in the next sections, focusing both on the effects of prebiotic intervention and on the effects of purified bacterial metabolites, such as SCFA.
Effects on Barrier Function
As described previously, decreased barrier function and increased bacterial translocation is observed in HIV-infected subjects. Prebiotics have been shown to influence barrier function via various mechanisms. A combination of fermentable fibers has been shown to significantly reduce endotoxemia over a 30-day intervention period in an RCT of 55 cirrhosis patients [Citation144]. Animal studies using alcohol-induced liver damage showed similar beneficial results preventing intestinal dysbiosis with an oat-based prebiotic intervention [Citation145].
There appear to be several mechanisms whereby prebiotics can enhance intestinal barrier function. Recently, Cani and coworkers [Citation146] have shown that a prebiotic-induced increase in glucagon-like peptide-2 (GLP-2) played an important role in the beneficial effects of prebiotic intervention in ob/ob mice on a high-fat diet. Increased intestinal barrier function and expression of tight-junction proteins were observed, leading to reductions of hepatic markers of oxidative stress and inflammation, as well as reduced levels of systemic inflammatory mediators and endotoxemia [Citation146]. The production of SCFA during fermentation of prebiotic agents can also lead to an improved barrier function. Butyrate, in particular, is an energy source for intestinal epithelial cells and, through the modulation of intestinal prostaglandins, it stimulates mucus production [Citation147]. Recently, butyrate was shown to enhance intestinal barrier function in vitro by regulating the assembly of tight junctions in Caco-2 cells [Citation148].
Effects on Gastrointestinal Infections
A few human studies have shown that prebiotics can reduce gastrointestinal infections, a functional characteristic that may potentially be used to counteract the HIV-increased prevalence of gastrointestinal infections [Citation42]. Fructan supplementation for 30 days reduced diarrhea relapse rates in an RCT among 140 patients with Clostridium difficile-associated diarrhea [Citation149]. In addition, fructan supplementation showed a tendency to reduce traveler's diarrhea in an RCT of 244 healthy volunteers [Citation150]. In a 12-month open-label intervention trial among 342 infants, a formula containing a specific combination of GOS and a long-chain fructan induced significant reductions in gastroenteritis and acute diarrhea [Citation151]. The same combination of oligosaccharides was shown to reduce the number of fecal pathogens and increase intestinal IgA production in infants—two mechanisms by which prebiotics could reduce intestinal infections [Citation152, 153].
SCFAs can contribute through acidification of the intestinal content and growth inhibition of acid-sensitive pathogens [Citation154]. Butyrate stimulates the production of antimicrobial peptides, such as cathelicidins, which are able to kill a variety of potential pathogenic bacteria [Citation155].
Prebiotic oligosaccharides can have anti-pathogenic effects that are independent of the intestinal microbiota and its metabolism. Human milk oligosaccharides are known to exhibit receptor-decoy functionality based on the molecular structure and sugar moieties of the oligosaccharides [Citation156], leading to binding of oligosaccharides to potential pathogens and preventing their adherence to the intestinal lining. Similarly, GOS and pectin-derived oligosaccharides can inhibit adherence of specific pathogens to epithelial cells in vitro, demonstrating that this mechanism is not limited to oligosaccharides of mammalian origin [Citation157, 158]. Studies on HIV-infected adults and infants are required to better determine the efficacy of prebiotics against diarrhea, especially in developing countries where such infections can be lethal.
Local Anti-Inflammatory and Immunomodulatory Effects
IBD is characterized by intestinal inflammation in which the microbiota plays an important role, a situation not dissimilar to the HIV-induced inflammation in the gut. Small-scale studies using fructan-based pre- and synbiotic intervention in ulcerative colitis patients have shown beneficial effects on histological inflammation scores, and on mRNA expression of inflammatory mediators in biopsy samples [Citation159, 160]. Similarly, a small, open-label study showed anti-inflammatory effects of fructan supplementation in moderate Crohn's disease patients. In that case, lamina propria biopsies showed that the intervention modulated the phenotype of intestinal DCs, enhancing IL-10 production and expression of TLR-2 and TLR-4 [Citation161]. This suggested that the anti-inflammatory effects are related to changes in microbe-host interactions.
Many preclinical data using prebiotics in IBD models show corresponding results (reviewed in [Citation162]). In mechanistic studies using different chemically-induced inflammation models, it was shown that the beneficial effects of prebiotic intervention could be reproduced in part or completely by infusing lactic acid bacteria intragastrically and/or SCFA into distal parts of the large intestine.
Recent animal studies confirm that the expression of PRRs in epithelial and immune cells can be modified by prebiotics and by butyrate in vitro [Citation163, 164]. However, the molecular mechanisms remain to be elucidated. Whereas butyrate was found to reduce LPS and TNF-α-induced NF-κB activation in a colonic epithelial cell line [Citation165, 166], these results were partly contradicted in a different colonic cell line [Citation163]. A recent study indicated that NF-κB may be modulated directly by unfermented oligosaccharides. Pectin-derived acidic oligosaccharides reduced NF-κB in vitro and reduced HIV-1 viral production in vitro [Citation167].
Another molecular target for SCFA-induced modulation of inflammation are the G-protein-coupled receptor 41 and 43 (GPR-43), that are most efficiently activated by acetate and propionate [Citation168–170]. GPR-43 is expressed mainly in innate immune cells and is critically important in the resolution or reduction of inflammation in a variety of mouse models. These mechanisms highlight the potential of prebiotic anti-inflammatory capacities, which could lead to an amelioration of chronic inflammation and possibly immune activation in HIV-infected subjects [Citation48].
Modulation of Systemic Immunity
In addition to local effects of prebiotics in the gut, systemic immunomodulatory effects of prebiotics have been described that are relevant for HIV-infected individuals. A 10-week cross-over study with GOS in 44 healthy, elderly subjects showed simultaneous bifidogenic and systemic immunomodulatory effects. The phagocytosis capacity and natural killer cell activity of circulating white blood cells was increased, whereas the production of inflammatory cytokines was reduced [Citation140]. Furthermore, specific prebiotic interventions have been shown to modulate the immunological balance, consistent with a shift away from a Th2-dominant state. For example, a specific combination of GOS and short-chain fructans was shown to reduce the incidence of atopic dermatitis and allergy-related symptoms in infants at risk for allergy [Citation171]. Correspondingly, changes in the antibody class and isotype ratios suggestive of a Th1 shift were detected [Citation172]. The ability to induce this shift is thought to be beneficial in HIV patients, as it might result in more effective anti-viral control and a better immunological defense against opportunistic pathogens [Citation69, Citation173].
Effects of the specific combination of GOS and long-chain fructans in multiple mouse models are also consistent with a shift from Th2 to Th1 responses, as allergic responses were reduced and Th1-dependent vaccine-specific DTH responses were enhanced [Citation174–176]. In addition to modifying the microbiota, prebiotics may also mediate effects via the carbohydrate structures on immune cells. Very low-level systemic bioavailability of short-chain fructans have been described in the urine of healthy volunteers [Citation177], suggesting the potential for direct systemic effects through interactions with lectins, galectins, or other sugar-binding molecules.
Prebiotic Intervention in HIV-Infected Individuals
A limited number of studies that have used prebiotics in HIV-infected individuals indicate that the benefits described above may be relevant for this population. Recently, a prebiotic intervention study was performed to investigate potential microbiological and immunological benefits among 57 HIV patients. A 12-week intervention with a specific mixture of GOS, long-chain fructans and pectin-derived oligosaccharides in ART-naïve HIV-1 infected individuals resulted in increased bifidobacterial levels and reduced numbers of the pathogenic Clostridium histolyticum cluster. In addition, reduced levels in the pathogenic E. rectale and C. coccoides cluster were found [Citation178]. The prebiotic intervention was associated with reduced CD4+ T-cell activation, measured as a percentage of CD4+/CD25+ T-cells. In addition, improved NK-cell cytotoxicity was observed [Citation179]. The beneficial effects of this pilot study were confirmed in a multi-center RCT, in which a one-year intervention was tested in HIV-infected individuals not on ART. A total of 340 participants were included in the trial and received the intervention product or an isocaloric, isonitrogenous control product. The intervention product consisted of the same mixture of prebiotic oligosaccharides in combination with bovine colostrum, omega-3 polyunsaturated fatty acids, and N-acetyl cysteine. The intervention significantly slowed down the decline of the CD4 count as the decrease in the intervention group (-28 cells/μl) was lower than the decrease in the control group (-68 cells/μl) [Citation180]. These findings are very promising and show the potential for nutrition-based strategies to become an integral part of disease management.
CONCLUSION AND FUTURE PROSPECTS
The interaction between the gastrointestinal microbiota and the human host plays a crucial role in intestinal homeostasis and the health status of the host. The gut-associated immune system tightly regulates this interaction and, under normal conditions, prevents damaging inflammatory reactions by maintaining a tolerogenic state. HIV infection has a disruptive impact on the intestinal homeostasis as it directly affects the host and indirectly affects the intestinal microbiota. The loss of intestinal CD4+ T-cells, epithelial function, and immune regulation, in combination with a pathogen-enriched microbiota composition, leads to an increase in intestinal permeability, bacterial translocation, and an inflammatory state.
Pro- and prebiotics are modulators of both microbiota and host factors, making them potential agents to ameliorate the intestinal problems induced by HIV. Various beneficial effects of pro- and prebiotic interventions have been demonstrated that may translate to applications in HIV. These effects include improved barrier function, reductions in the translocation of bacterial products, reductions in pathogenic load, local and systemic anti-inflammatory effects, and immunomodulatory effects to restore a proper Th1/Th2 balance.
Various therapeutic applications of pro- and prebiotics are conceivable, such as before initiation of ART, where they could potentially help reduce HIV-induced intestinal inflammation, intestinal infection, or diarrhea. In addition, by reducing systemic inflammation and associated immune activation, disease progression may be slowed down. Obviously, pro- and prebiotics should not be used as alternatives to ART but might have a role as conjoint therapy especially among immunological non-responders to ART.
An additional benefit of the pro- and prebiotic is the possible application in low-cost interventions of limited complexity, which might be especially useful in resource-limited countries [Citation181]. Depending on the target group, the oral application of pro- and prebiotics makes it possible to combine the intervention with specific (micro-) nutrients to prevent specific deficiencies or to aim at multiple targets simultaneously. Applied as interventions to improve intestinal homeostasis in HIV-infected individuals, pro- and prebiotics have potential to contribute beneficially to integrated disease management.
In recent years, promising initial studies encompassing pro- and prebiotic interventions have been performed in HIV-infected individuals, showing the potential for improvement of intestinal homeostasis and potentially a reduction in the decline in CD4 count. However, a clear need remains for additional well-designed double-blind, randomized studies to provide evidence for the efficacy of specific pro- and prebiotic interventions. and ,
ACKNOWLEDGMENTS
We are grateful for the careful review of our manuscript and knowledgeable feedback of Prof. Dr. Johan Garssen, Jaimie Hemsworth, and Wayne Miller. Support from NSERC and AFMnet is gratefully appreciated.
Declaration of Interest
As indicated in the affiliations, APV, BL, and KN are employed by Danone Research—Centre for Specialised Nutrition, which is part of the Danone group.
REFERENCES
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008;8:411–420.
- Wikoff WR, Anfora AT, Liu J, Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009;106:3698–3703.
- Backhed F, Ley RE, Sonnenburg JL, Host-bacterial mutualism in the human intestine. Science 2005;307:1915–1920.
- Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 2009;31:368–376.
- Johansson ME, Phillipson M, Petersson J, The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 2008;105:15064–15069.
- Wells JM, Loonen LM, Karczewski JM. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Int J Med Microbiol 2009;300:41–48.
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–241.
- Strober W, Murray PJ, Kitani A, Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 2006;6:9–20.
- Kraehenbuhl JP, Neutra MR. Epithelial M cells: Differentiation and function. Annu Rev Cell Dev Biol 2000;16:301–332.
- Sato A, Iwasaki A. Peyer's patch dendritic cells as regulators of mucosal adaptive immunity. Cell Mol Life Sci 2005;62:1333–1338.
- Roberts SJ, Smith AL, West AB, T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA 1996;93:11774–11779.
- Rescigno M, Urbano M, Valzasina B, Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–367.
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 2008;8:435–446.
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity 2009;31:401–411.
- Baba N, Samson S, Bourdet-Sicard R, Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol 2008;84:468–476.
- Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut 2005;54:317–320.
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004;303:1662–1665.
- Suzuki K, Meek B, Doi Y, Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA 2004;101:1981–1986.
- Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 2007;19:59–69.
- Ayabe T, Satchell DP, Wilson CL, Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 2000;1:113–118.
- Vaishnava S, Behrendt CL, Ismail AS, Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA 2008;105:20858–20863.
- Kelly D, Campbell JI, King TP, Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 2004;5:104–112.
- Neish AS, Gewirtz AT, Zeng H, Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 2000;289:1560–1563.
- Kelly D, Conway S, Aminov R. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol 2005;26:326–333.
- Niess JH, Leithauser F, Adler G, Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol 2008;180:559–568.
- Imaoka A, Matsumoto S, Setoyama H, Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur J Immunol 1996;26:945–948.
- Ahern PP, Izcue A, Maloy KJ, The interleukin-23 axis in intestinal inflammation. Immunol Rev 2008;226:147–159.
- Cua DJ, Sherlock J, Chen Y, Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003;421:744–748.
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–467.
- van de Veerdonk FL, Gresnigt MS, Kullberg BJ, Th17 responses and host defense against microorganisms: An overview. BMB Rep 2009;42:776–787.
- Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: A (co-)evolutionary perspective. Nat Rev Immunol 2009;9:883–889.
- Atarashi K, Nishimura J, Shima T, ATP drives lamina propria T(H)17 cell differentiation. Nature 2008;455:808–812.
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008;453:620–625.
- Katakura K, Lee J, Rachmilewitz D, Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest 2005;115:695–702.
- Kotler DP, Reka S, Borcich A, Detection, localization, and quantitation of HIV-associated antigens in intestinal biopsies from patients with HIV. Am J Pathol 1991;139:823–830.
- Mehandru S, Poles MA, Tenner-Racz K, Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004;200:761–770.
- Lim SG, Condez A, Poulter LW. Mucosal macrophage subsets of the gut in HIV: Decrease in antigen-presenting cell phenotype. Clin Exp Immunol 1993;92:442–447.
- Nilssen DE, Muller F, Oktedalen O, Intraepithelial gamma/delta T cells in duodenal mucosa are related to the immune state and survival time in AIDS. J Virol 1996;70:3545–3550.
- Poccia F, Piselli P, Vendetti S, Heat-shock protein expression on the membrane of T cells undergoing apoptosis. Immunology 1996;88:6–12.
- Sankaran S, Guadalupe M, Reay E, Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci USA 2005;102:9860–9865.
- Lim SG, Menzies IS, Lee CA, Intestinal permeability and function in patients infected with human immunodeficiency virus. A comparison with coeliac disease. Scand J Gastroenterol 1993;28:573–580.
- Kelly P, Todd J, Sianongo S, Susceptibility to intestinal infection and diarrhoea in Zambian adults in relation to HIV status and CD4 count. BMC Gastroenterol 2009;9:7.
- Knox TA, Spiegelman D, Skinner SC, Diarrhea and abnormalities of gastrointestinal function in a cohort of men and women with HIV infection. Am J Gastroenterol 2000;95:3482–3489.
- McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 2001;410:974–979.
- Duh EJ, Maury WJ, Folks TM, Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA 1989;86:5974–5978.
- Giorgi JV, Hultin LE, McKeating JA, Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999;179:859–870.
- Guadalupe M, Reay E, Sankaran S, Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003;77:11708–11717.
- Brenchley JM, Price DA, Schacker TW, Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371.
- Emu B, Sinclair E, Favre D, Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol 2005;79:14169–14178.
- Ferre AL, Hunt PW, Critchfield JW, Mucosal immune responses to HIV-1 in elite controllers: A potential correlate of immune control. Blood 2009;113:3978–3989.
- Maresca M, Mahfoud R, Garmy N, The virotoxin model of HIV-1 enteropathy: Involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J Biomed Sci 2003;10:156–166.
- George MD, Reay E, Sankaran S, Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol 2005;79:2709–2719.
- Marchetti G, Bellistri GM, Borghi E, Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008;22:2035– 2038.
- MacFie J, O’Boyle C, Mitchell CJ, Gut origin of sepsis: A prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 1999;45:223–228.
- Ersoz G, Aydin A, Erdem S, Intestinal permeability in liver cirrhosis. Eur J Gastroenterol Hepatol 1999;11:409–412.
- Cooke KR, Olkiewicz K, Erickson N, The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res 2002;8:441–448.
- Caradonna L, Amati L, Magrone T, Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: Biological and clinical significance. J Endotoxin Res 2000;6:205–214.
- Wyatt J, Vogelsang H, Hubl W, Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 1993;341:1437–1439.
- Frank DN, St Amand AL, Feldman RA, Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–13785.
- Turnbaugh PJ, Hamady M, Yatsunenko T, A core gut microbiome in obese and lean twins. Nature 2009;457:480–484.
- Turnbaugh PJ, Ley RE, Mahowald MA, An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027– 1031.
- Gori A, Tincati C, Rizzardini G, Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol 2008;46:757–758.
- Wolf BW, Wheeler KB, Ataya DG, Safety and tolerance of Lactobacillus reuteri supplementation to a population infected with the human immunodeficiency virus. Food Chem Toxicol 1998;36:1085–1094.
- McKenna P, Hoffmann C, Minkah N, The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog 2008;4:e20.
- Sindhu S, Toma E, Cordeiro P, Relationship of in vivo and ex vivo levels of TH1 and TH2 cytokines with viremia in HAART patients with and without opportunistic infections. J Med Virol 2006;78:431–439.
- Buonaguro L, Tornesello MS, Gallo RC, Th2 polarization in peripheral blood mononuclear cells from human immunodeficiency virus (HIV)-infected subjects, as activated by HIV virus-like particles. J Virol 2009;83:304–313.
- Israel-Biet D, Labrousse F, Tourani JM, Elevation of IgE in HIV-infected subjects: A marker of poor prognosis. J Allergy Clin Immunol 1992;89:68–75.
- Wright DN, Nelson Jr. RP, Ledford DK, Serum IgE and human immunodeficiency virus (HIV) infection. J Allergy Clin Immunol 1990;85:445–452.
- Becker Y. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers—Review and hypothesis. Virus Genes 2003;28:5–18.
- Rancinan C, Morlat P, Chene G, Prevalence of clinical manifestations of allergic reactions in HIV infection. Cross sectional study of 115 subjects. Rev Med Interne 1997;18:691–694.
- Harrer T, Harrer E, Kalams SA, Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses 1996;12:585–592.
- Ullum H, Cozzi Lepri A, Bendtzen K, Low production of interferon gamma is related to disease progression in HIV infection: Evidence from a cohort of 347 HIV-infected individuals. AIDS Res Hum Retroviruses 1997;13:1039– 1046.
- Harrington LE, Hatton RD, Mangan PR, Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–1132.
- Food and Agriculture Organization of the United Nations (FAO). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, 2001. Available at: www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed June 11, 2010).
- Ukena SN, Singh A, Dringenberg U, Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2007;2:e1308.
- Zyrek AA, Cichon C, Helms S, Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 2007;9:804–816.
- Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 2006;130:731–746.
- Yan F, Cao H, Cover TL, Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007;132:562–575.
- Luyer MD, Buurman WA, Hadfoune M, Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun 2005;73:3686–3692.
- Wang Z, Xiao G, Yao Y, The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma 2006;61:650–657.
- Isolauri E, Majamaa H, Arvola T, Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology 1993;105:1643–1650.
- Forsyth CB, Farhadi A, Jakate SM, Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009;43:163–172.
- Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Benefits of a synbiotic formula (Synbiotic 2000 Forte) in critically ill trauma patients: Early results of a randomized controlled trial. World J Surg 2006;30:1848– 1855.
- Alberda C, Gramlich L, Meddings J, Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2007;85:816–823.
- Shimizu K, Ogura H, Goto M, Synbiotics decrease the incidence of septic complications in patients with severe SIRS: A preliminary report. Dig Dis Sci 2009;54:1071–1078.
- Rayes N, Seehofer D, Theruvath T, Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation—A randomized, double-blind trial. Am J Transplant 2005;5:125–130.
- Sugawara G, Nagino M, Nishio H, Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: A randomized controlled trial. Ann Surg 2006;244:706–714.
- Reddy BS, Macfie J, Gatt M, Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg 2007;94:546–554.
- Jain PK, McNaught CE, Anderson AD, Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: A randomised controlled trial. Clin Nutr 2004;23:467–475.
- Anderson AD, McNaught CE, Jain PK, Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004;53:241–245.
- McNaught CE, Woodcock NP, MacFie J, A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut 2002;51:827–831.
- Grabig A, Paclik D, Guzy C, Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun 2006;74:4075–4082.
- Ewaschuk JB, Backer JL, Churchill TA, Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun 2007;75:2572–2579.
- Jijon H, Backer J, Diaz H, DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology 2004;126:1358–1373.
- Foligne B, Zoumpopoulou G, Dewulf J, A key role of dendritic cells in probiotic functionality. PLoS ONE 2007;2:e313.
- Di Giacinto C, Marinaro M, Sanchez M, Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 2005;174:3237–3246.
- Kwon HK, Lee CG, So JS, Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA 2010;107:2159–2164.
- O’Mahony L, O’Callaghan L, McCarthy J, Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am J Physiol Gastrointest Liver Physiol 2006;290:G839–G845.
- von der Weid T, Bulliard C, Schiffrin EJ. Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferative capacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol 2001;8:695–701.
- Baroja ML, Kirjavainen PV, Hekmat S, Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol 2007;149:470–479.
- Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun 1996;64:5403–5405.
- Sokol H, Pigneur B, Watterlot L, Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 2008;105:16731–16736.
- O’Mahony L, McCarthy J, Kelly P, Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005;128:541–551.
- Braat H, Van Den Brande J, van Tol E, Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr 2004;80:1618–1625.
- Pessi T, Sutas Y, Hurme M, Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy 2000;30:1804– 1808.
- Iwabuchi N, Takahashi N, Xiao JZ, Suppressive effects of Bifidobacterium longum on the production of Th2-attracting chemokines induced with T cell-antigen-presenting cell interactions. FEMS Immunol Med Microbiol 2009;55:324–334.
- Hougee S, Vriesema AJ, Wijering SC, Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: A bacterial strain comparative study. Int Arch Allergy Immunol 2009;151:107–117.
- Mohamadzadeh M, Olson S, Kalina WV, Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA 2005;102:2880–2885.
- Viljanen M, Savilahti E, Haahtela T, Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: A double-blind placebo-controlled trial. Allergy 2005;60:494–500.
- Viljanen M, Pohjavuori E, Haahtela T, Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J Allergy Clin Immunol 2005;115:1254–1259.
- Pohjavuori E, Viljanen M, Korpela R, Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy. J Allergy Clin Immunol 2004;114:131–136.
- Pritts T, Hungness E, Wang Q, Mucosal and enterocyte IL-6 production during sepsis and endotoxemia—Role of transcription factors and regulation by the stress response. Am J Surg 2002;183:372–383.
- Fujihashi K, McGhee JR, Lue C, Human appendix B cells naturally express receptors for and respond to interleukin 6 with selective IgA1 and IgA2 synthesis. J Clin Invest 1991;88:248–252.
- Isolauri E, Arvola T, Sutas Y, Probiotics in the management of atopic eczema. Clin Exp Allergy 2000;30:1604–1610.
- Rosenfeldt V, Benfeldt E, Nielsen SD, Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003;111:389–395.
- Brouwer ML, Wolt-Plompen SA, Dubois AE, No effects of probiotics on atopic dermatitis in infancy: A randomized placebo-controlled trial. Clin Exp Allergy 2006;36:899–906.
- Kaila M, Isolauri E, Soppi E, Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res 1992;32:141–144.
- Schlee M, Wehkamp J, Altenhoefer A, Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun 2007;75:2399–2407.
- Spinler JK, Taweechotipatr M, Rognerud CL, Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008;14:166–171.
- Heinemann C, van Hylckama Vlieg JE, Janssen DB, Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol Lett 2000;190:177–180.
- Frick JS, Fink K, Kahl F, Identification of commensal bacterial strains that modulate Yersinia enterocolitica and dextran sodium sulfate-induced inflammatory responses: Implications for the development of probiotics. Infect Immun 2007;75:3490–3497.
- Laughton JM, Devillard E, Heinrichs DE, Inhibition of expression of a staphylococcal superantigen-like protein by a soluble factor from Lactobacillus reuteri. Microbiology 2006;152:1155–1167.
- Alak JI, Wolf BW, Mdurvwa EG, Effect of Lactobacillus reuteri on intestinal resistance to Cryptosporidium parvum infection in a murine model of acquired immunodeficiency syndrome. J Infect Dis 1997;175:218–221.
- Mohan R, Koebnick C, Schildt J, Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J Clin Microbiol 2006;44:4025–4031.
- Plummer SF, Garaiova I, Sarvotham T, Effects of probiotics on the composition of the intestinal microbiota following antibiotic therapy. Int J Antimicrob Agents 2005;26:69–74.
- Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol 2006;4:e413.
- Allen SJ, Okoko B, Martinez E, Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev, CD003048, 2004.
- Heiser CR, Ernst JA, Barrett JT, Probiotics, soluble fiber, and L-glutamine (GLN) reduce nelfinavir (NFV)- or lopinavir/ritonavir (LPV/r)-related diarrhea. J Int Assoc Physicians AIDS Care (Chic Ill) 2004;3:121–129.
- Salminen MK, Tynkkynen S, Rautelin H, The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV patients on antiretroviral therapy: A randomized, placebo-controlled, crossover study. HIV Clin Trials 2004;5:183–191.
- Trois L, Cardoso EM, Miura E. Use of probiotics in HIV-infected children: A randomized double-blind controlled study. J Trop Pediatr 2008;54:19–24.
- Anukam KC, Osazuwa EO, Osadolor HB, Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol 2008;42:239–243.
- Kerac M, Bunn J, Seal A, Probiotics and prebiotics for severe acute malnutrition (PRONUT study): A double-blind efficacy randomised controlled trial in Malawi. Lancet 2009;374:136–144.
- Horwitch CA, Furseth HA, Larson AM, Lactobacillemia in three patients with AIDS. Clin Infect Dis 1995;21:1460–1462.
- Rogasi PG, Vigano S, Pecile P, Lactobacillus casei pneumonia and sepsis in a patient with AIDS. Case report and review of the literature. Ann Ital Med Int 1998;13:180–182.
- Abgrall S, Joly V, Derkinderen P, Lactobacillus casei infection in an AIDS patient. Eur J Clin Microbiol Infect Dis 1997;16:180–182.
- Pineiro M, Asp NG, Reid G, FAO Technical meeting on prebiotics. J Clin Gastroenterol 2008;42(Suppl 3 Pt 2):S156–S159.
- Knol J, Scholtens P, Kafka C, Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J Pediatr Gastroenterol Nutr 2005;40:36–42.
- Scholtens PA, Alles MS, Bindels JG, Bifidogenic effects of solid weaning foods with added prebiotic oligosaccharides: A randomised controlled clinical trial. J Pediatr Gastroenterol Nutr 2006;42:553–559.
- Ramirez-Farias C, Slezak K, Fuller Z, Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 2009;101:541–550.
- Vulevic J, Drakoularakou A, Yaqoob P, Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 2008;88:1438–1446.
- Rossi M, Corradini C, Amaretti A, Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Appl Environ Microbiol 2005;71:6150–6158.
- Kikuchi H, Andrieux C, Riottot M, Effect of two levels of transgalactosylated oligosaccharide intake in rats associated with human faecal microflora on bacterial glycolytic activity, end-products of fermentation and bacterial steroid transformation. J Appl Bacteriol 1996;80:439–446.
- Hernot DC, Boileau TW, Bauer LL, In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J Agric Food Chem 2009;57:1354–1361.
- Liu Q, Duan ZP, Ha DK, Synbiotic modulation of gut flora: Effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441–1449.
- Mutlu E, Keshavarzian A, Engen P, Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res 2009;33:1836–1846.
- Cani PD, Possemiers S, Van de Wiele T, Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–1103.
- Willemsen LE, Koetsier MA, van Deventer SJ, Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003;52:1442– 1447.
- Peng L, Li ZR, Green RS, Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009;139:1619–1625.
- Lewis S, Burmeister S, Brazier J. Effect of the prebiotic oligofructose on relapse of Clostridium difficile-associated diarrhea: A randomized, controlled study. Clin Gastroenterol Hepatol 2005;3:442–448.
- Cummings JH, Christie S, Cole TJ. A study of fructo oligosaccharides in the prevention of travellers’ diarrhoea. Aliment Pharmacol Ther 2001;15:1139– 1145.
- Bruzzese E, Volpicelli M, Squeglia V, A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin Nutr 2009;28:156–161.
- Knol J, Boehm G, Lidestri M, Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paediatr 2005;94(Suppl):31– 33.
- Scholtens PA, Alliet P, Raes M, Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J Nutr 2008;138:1141–1147.
- Gibson GR, McCartney AL, Rastall RA. Prebiotics and resistance to gastrointestinal infections. Br J Nutr 2005;93(Suppl 1):S31–S34.
- Schauber J, Dorschner RA, Yamasaki K, Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 2006;118:509–519.
- Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005;25:37–58.
- Tzortzis G, Goulas AK, Gee JM, A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J Nutr 2005;135:1726–1731.
- Guggenbichler JP, De Bettignies-Dutz A, Meissner P, Acidic oligosaccharides from natural sources block adherence of Escherichia coli on uroepithelial cells. Pharm Pharmacol Lett 1997;7:35–38.
- Furrie E, Macfarlane S, Kennedy A, Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005;54:242– 249.
- Welters CF, Heineman E, Thunnissen FB, Effect of dietary insulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum 2002;45:621–627.
- Lindsay JO, Whelan K, Stagg AJ, Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut 2006;55:348–355.
- Guarner F. Prebiotics in inflammatory bowel diseases. Br J Nutr 2007;98(Suppl 1):S85–S89.
- Trevisi P, De Filippi S, Minieri L, Effect of fructo-oligosaccharides and different doses of Bifidobacterium animalis in a weaning diet on bacterial translocation and Toll-like receptor gene expression in pigs. Nutrition 2008;24:1023–1029.
- Leung CH, Lam W, Ma DL, Butyrate mediates nucleotide-binding and oligomerisation domain 2-dependent mucosal immune responses against peptidoglycan. Eur J Immunol 2009;39:3529–3537.
- Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. J Biol Chem 2001;276:44641–44646.
- Schwab M, Reynders V, Loitsch S, Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signaling. Mol Immunol 2007;44:3625–3632.
- van Norren K, Dijk FJ, van't Land B, et al. NF-κB-mediated HIV-1 production is inhibited in vitro by pectin derived acidic oligosaccharides (pAOS). Abstract no. WEPEA095. 5th IAS Conference on HIV Pathogenesis and Treatment, Cape Town, July 19–22, 2009.
- Brown AJ, Goldsworthy SM, Barnes AA, The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278:11312–11319.
- Le Poul E, Loison C, Struyf S, Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 2003;278:25481–25489.
- Maslowski KM, Vieira AT, Ng A, Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009;461:1282–1286.
- Arslanoglu S, Moro GE, Schmitt J, Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr 2008;138:1091–1095.
- van Hoffen E, Ruiter B, Faber J, A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 2009;64:484–487.
- Clerici M, Shearer GM. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today 1993;14:107–111.
- Schouten B, van Esch BC, Hofman GA, Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J Nutr 2009;139:1398–1403.
- Vos AP, Haarman M, Buco A, A specific prebiotic oligosaccharide mixture stimulates delayed-type hypersensitivity in a murine influenza vaccination model. Int Immunopharmacol 2006;6:1277–1286.
- Vos AP, van Esch BC, Stahl B, Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int Immunopharmacol 2007;7:1582–1587.
- Molis C, Flourie B, Ouarne F, Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am J Clin Nutr 1996;64:324–328.
- Ben Amor K, Rizzardini G, Torti C, et al. Disturbed gut microbiota in HAART-naive HIV-1 positive adults: Effect of intervention with a specific prebiotic oligosaccharide mixture, Poster # 375b. 15th Conference on Retrovirus and Opportunistic Infections (CROI 2008), Boston, MA, February 3–6, 2008.
- van't Land B, Benlhassan-Chahour K, Rizzardini G, et al. A specific mixture of prebiotic oligosaccharides reduces hyper-immune activation and improves NK cell cytolytic activity in HAART-naive HIV positive adults, Poster # 723. 15th Conference on Retroviruses and Opportunistic Infections (CROI 2008), Boston, MA, February 3–6, 2008.
- Lange J, et al. Reduced CD4+ T cell decline and immune activation by NR100157, a specific multi-targeted nutritional intervention, in HIV-1 positive adults not on antiretroviral therapy (BITE), Abstract H-1230b. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2009). San Francisco, CA, September 12–15, 2009.
- Wenner M. A cultured response to HIV. Nat Med 2009;15:594–597.
